By Kenneth Wingerter
Progressive aquarists have every good reason to familiarize themselves with the biology of the Rotifaria. Firstly, these diverse and surprisingly complex creatures are quite interesting in their own right; however inconsequential their activities may appear to be, rotifers often exert significant influences on the ecosystems they populate. Further, advancements in the mass culture of certain rotifer species have played a considerable role in the rapid expansion of the mariculture industry over the last quarter century. Cultured rotifers offer an equal measure of promise for use as a feed for nonphotosynthetic corals and other filter feeding organisms. Additional species of rotifer are now being considered for use as live feed for increasingly specialized applications. Hence, an inclusive understanding of these unusual animals is of potentially great value; in the least, familiarity with the group’s natural history and culture procedure can give aquarists–from hobbyists to professional hatchery technicians–the requisite knowledge to produce and utilize them effectively.
This piece discusses the classification, etymology, and morphology of the Rotatoria. A following piece will discuss rotifer ecology, development and reproduction. A final piece will discuss rotifer culture. In all pieces, an emphasis is placed on certain members of the genus Brachionus, owing to their present importance in the aquarium industry.
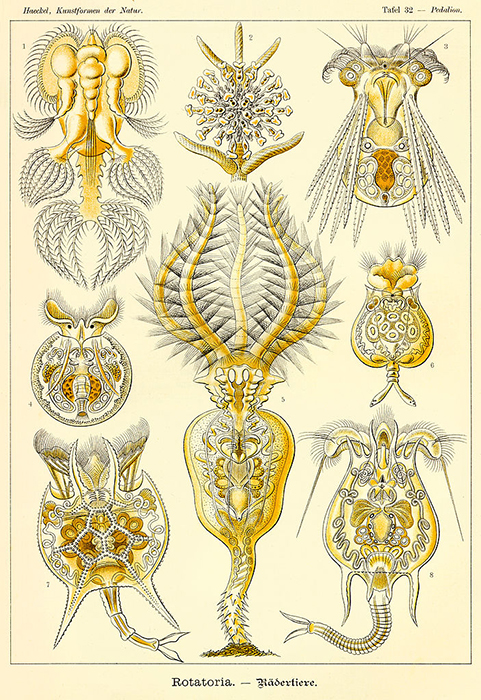
There is a considerable variety of body forms amongst the Rotatoria. (Illustration by Ernst Haeckel.)
Classification and etymology
Members of Phylum Rotifera (i.e., the Rotatoria) are among the smallest–though not necessarily the simplest–of the metazoans (Dhert, 1996). The name Rotifera (L. rota, wheel, + fera, those that bear) is derived from the characteristic ciliated feeding appendages that sometimes appear to rotate like wheels when beating (Hickman, Roberts and Larson, 1995; Hoff and Snell, 2008). In actual fact, the rings of cilia really only give the distinct impression of rotating wheels in a few species (Horne and Goldman, 1994).
The group consists of three classes and 120 genera (Hoff and Snell, 2008). While most authors estimate that there are 1,800 described rotifer species, others contend that there are anywhere from 1,000 to 2,200 species; it appears that much of this disagreement arises from confusion over whether or not certain varieties should be regarded as distinct species or rather as various strains and morphotypes (Campbell and Reece, 2002; Hoff and Snell, 2008; Dhert, 1996; Ohs, Cassiano and Rhodes, 2010).
Rotifers are conventionally classified by the characteristic shapes of their jaws and epidermises (Horne and Goldman, 1994). However, some authors group them according to their basic body shapes, which more or less correlate to various life modes. For example, worm-like forms are referred to as “creepers,” saccular and globular forms are referred to as “floaters,” elongate forms are referred to as “swimmers” and vase-like forms (some of which are colonial) are referred to as “sessile types” (Hickman, Roberts and Larson, 1995).
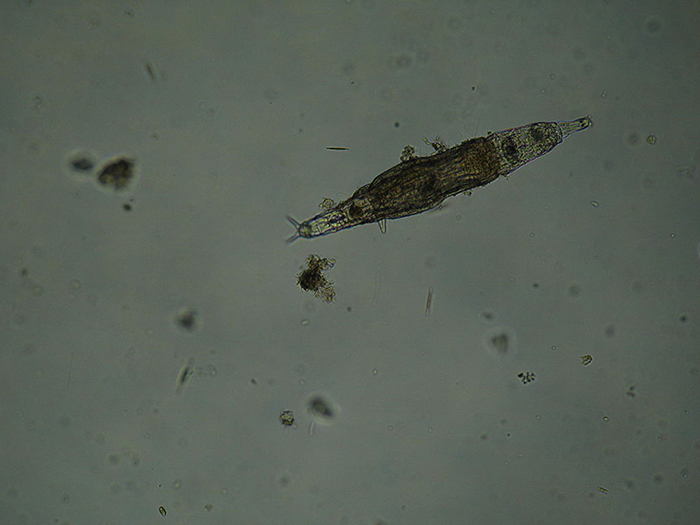
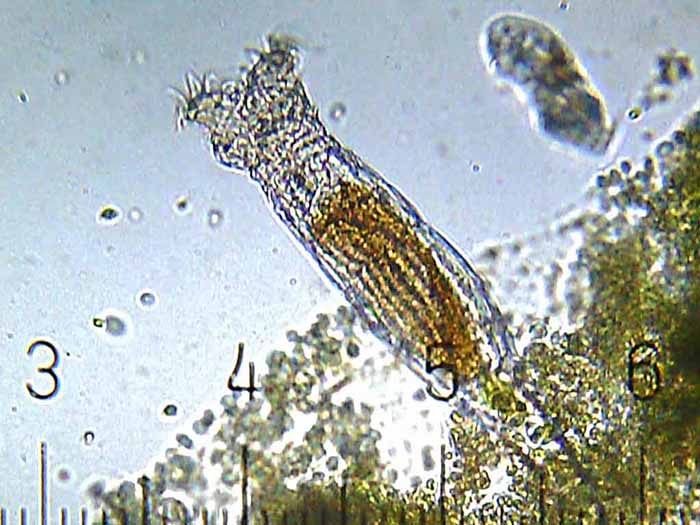
This unidentified freshwater rotifer serves as a good example of an elongate body form. (Photo by Gabriel Belfort Núñez.)
Class Seisonidea (Gr. seison, earthen vessel, + eidos, shape) is observed in elongate forms; it claims only a single genus with two species of rather unusual rotifers that occur only as epizoa on the gills of certain marine crustaceans. Class Bdelloidea (Gr. bdella, leach, + eidos, shape) is observed in either swimming or creeping forms; it claims about 350 freshwater and semi-terrestrial species–one of the largest groups of animals that reproduce exclusively by asexual means. Class Monogononta (Gr. monos, one, + gonos, primary sex gland) is observed in either swimming or sessile forms; it is by far the largest class of its phylum with about 1,500 freshwater species as well as a handful of brackish species, which includes the well-known genera Brachionus, Keratella and Notholca (Omori and Ikeda, 1984; Hickman, Roberts and Larson, 1995).
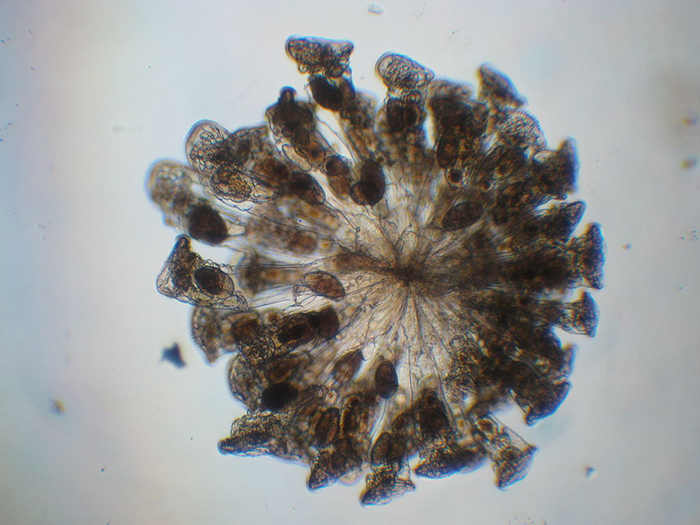
Most colonial rotifer species (such as Conochilius sp., shown here) belong to Class Monogononta. (Photo by Maurice J. Fox.)
Morphology
The Rotatoria range from 40 µm to 3 mm in length, though they rarely reach lengths of 2 mm. Most species are between 100 µm and 500 µm long (Hickman, Roberts and Larson, 1995; Dhert, 1996). Brachionus plicatilis and Brachionus rotundiformis (so-called large or L-type rotifers and small or S-type rotifers, respectively) are set apart as distinct species mainly (though not exclusively) on account of their differences in size; the former averages 239 µm in length, while the latter averages only 160 µm. An extra small strain of Brachionus rotundiformis (so-called super small or SS-type rotifer) has proven to be useful with tropical marine fish larvae (e.g., rabbitfish and groupers) that have mouth openings of <100 µm at first feeding. In many cases, rotifers may be smaller in size than some of the larger ciliated protozoa. Nevertheless, they are truly multicellular animals and have specialized organ systems (Horne and Goldman, 1994; Campbell and Reece, 2002).
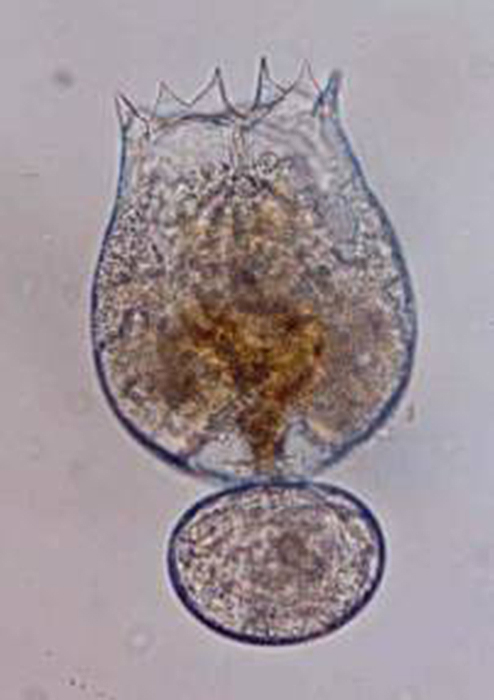
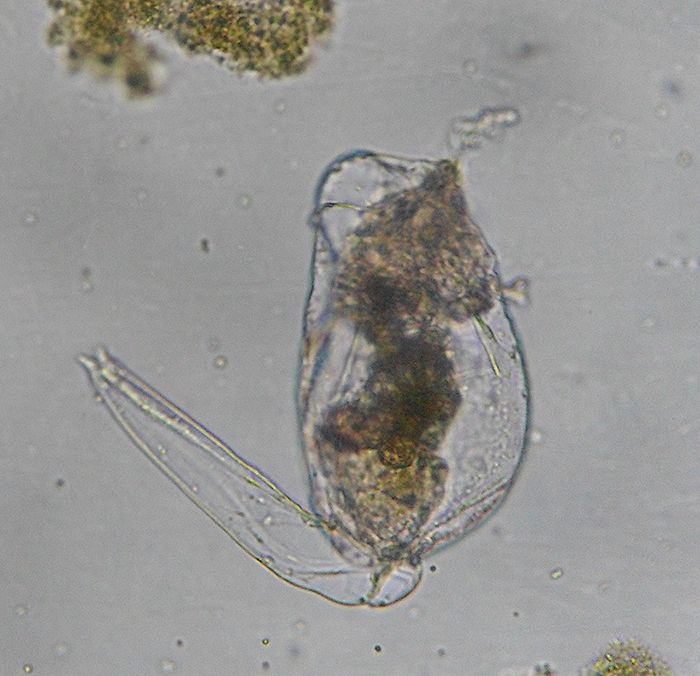
Bodies of brachionid rotifers (such as Brachionus rotundiformis, shown here)
are made up of approximately 1,000 cells. (Photo by Jung Min-Min.)
That having been said, the rotifer body should not be thought of as a multiplicity of discreet cells as with most other metazoans, but rather as a system of semi-closed plasma compartments, each with its own nucleus. This body structure (called a syncytium) confers greater efficiency for a tiny animal than would the typical conglomeration of cells with complete membranes. Rotifers may be partially or completely made up of syncytia. The body of each rotifer species is composed of a specific number of cells; the growth of an individual is the result not of mitosis but of increasing plasma volume (Dhert, 1996; Hyman, 1951).
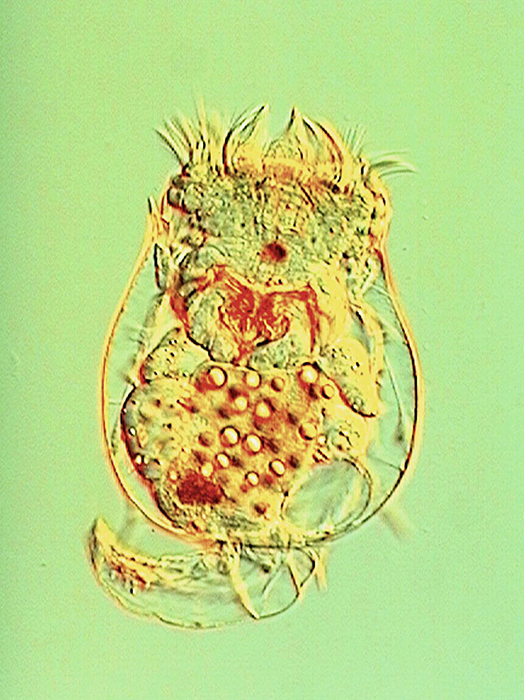
Much internal structure of Brachionus calyciflorus is displayed in this image using Nomarski illumination. (Photo by Takehito Yoshida and Randy O. Wayne.)
The body wall is formed from the cuticle, epidermis and subepidermal muscles. The cuticle may be ringed (especially in creeping forms). It does not contain chitin, though it may be hardened with densely packed keratin-like proteins to form a slightly flexible (and often uniquely ornamented) lorica. Loricate rotifers often are laterally or dorsoventrally flattened and may be edged with sharp spines or teeth. Though only some rotifer species bear a true secreted cuticle, all have a fibrous layer embedded within the epidermis. The rotifer epidermis is itself a syncytium that contains nuclei arranged in positions that are unique to each species. Rotifer musculature is present as an array of single muscles (both smooth and striated) rather than in discrete subepidermal layers. Visceral muscles are situated in the walls of the viscera; cutaneovisceral muscles connect the viscera (especially the digestive tract) to the body wall (Horne and Goldman, 1994; Dhert, 1996; Hyman, 1951).
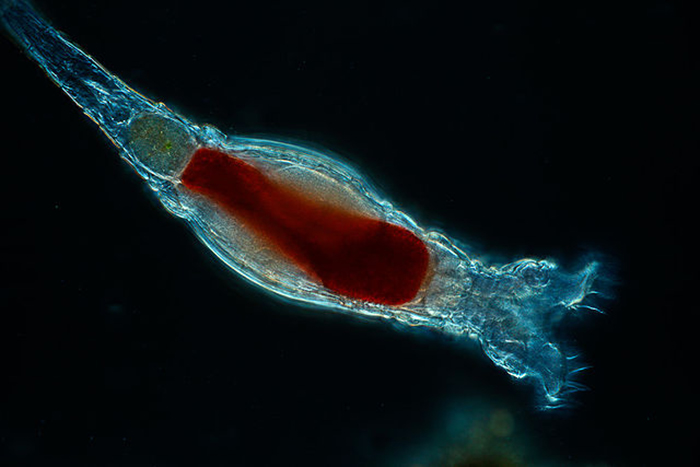
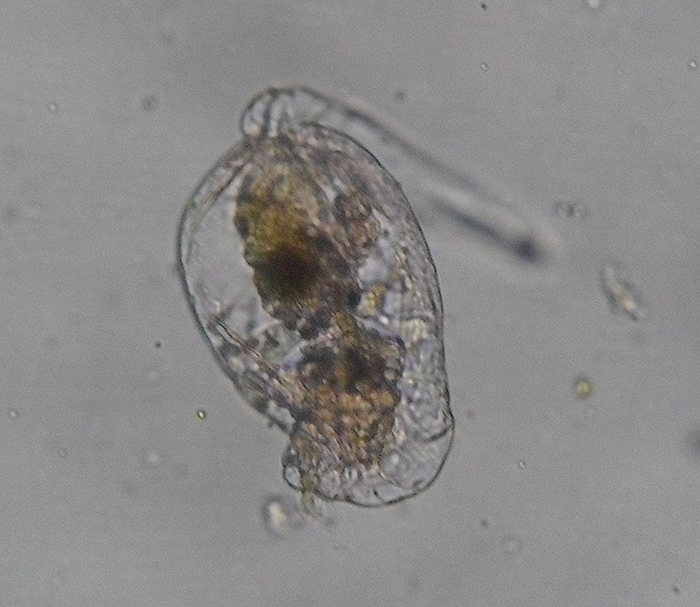
In this species (Brachionus quadridentatus), the corona takes form as a pair of discs. (Photo by Frank Fox.)
The bilaterally symmetric rotifer body is composed of three distinct parts, including a head bearing the ciliated rotatory organ (or corona), a trunk and a posterior tail (or foot) (Hickman, Roberts and Larson, 1995; Dhert, 1996). The overall appearance of the head depends largely upon which type of corona it has. The corona is typically either some sort of circlet or a pair of discs, though it is essentially vestigial in a few members (e.g., Class Seisonidea) (Hickman, Roberts and Larson, 1995). It occupies an area in the middle of the head that may also bear some number of papillae or sensory bristles. It may be retractable (Dhert, 1996). Coronal cilia beat in succession to create water currents that not only provide locomotion to the animal, but also facilitate the capture of minute food particles. They are capable of exercising some degree of selectivity, sorting out and casting off large or otherwise unsuitable particles (Hickman, Roberts and Larson, 1995; Dhert, 1996). The mouth is situated in a midventral area within the corona. In some species, a lower lip is formed from a coronal protrusion (Hickman, Roberts and Larson, 1995; Hyman, 1951). The entry to the digestive tract (or pharynx, or mastax) is situated just posterior to the mouth. A muscular portion of the mastax bears a pair of calcified jaw-like apparatuses (or trophi) that are used to suck in and macerate food particles (Hickman, Roberts and Larson, 1995; Campbell and Reece, 2002). A few raptorial predator species (e.g., Asplanchna spp.) have extendable trophi with sharp ends to help grasp and penetrate prey (Horne and Goldman, 1994). The brain is situated dorsally to the mastax. The nervous system is formed from a network of paired nerves that connect the bilobed brain to the mastax, muscles, viscera and sensory organs. Sensory organs may include papillae and sensory bristles, dorsal antennae, ciliated pits and, in some species, eyes. Eyes (or pigment spot ocelli) are single or paired red spots that are located on or near the head (Hickman, Roberts and Larson, 1995; Hyman, 1951).
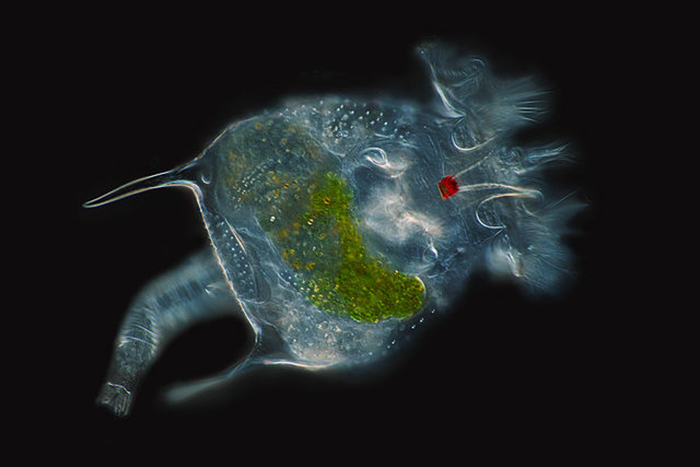
The eyespot of an unidentified rotifer is prominently displayed in this beautiful image. (Photo by Frank Fox.)
The trunk varies considerably in shape. It holds the visceral organs (i.e., digestive tract, excretory system and genital organs) and may bear sensory organs (Hickman, Roberts and Larson, 1995; Dhert, 1996). Viscera are contained within a body cavity that lacks an epithelial lining and mesenteries (or pseudocoelum). The relatively large rotatorian pseudocoelum lies in the space between the body wall and the viscera. Extensions from the epidermis to the pseudocoeleum (or epidermal cushions) contain nuclei, basal bodies and coronal cilia roots. The pseudocoeleum is lined with muscular bands, maintained by a network of branched, mesenchymal amoeboid cells and filled with fluids. These fluids provide a medium for the internal transport of nutrients and waste products as well as form a hydrostatic skeleton. Body movement redistributes fluids within the pseudocoelum, thereby conducting a crude circulatory system. (Hickman, Roberts and Larson, 1995; Campbell and Reece, 2002; Hyman, 1951). The digestive system is complete (that is, it includes a digestive tract with a mouth at one end and an **** at the other). Under the microscope, those parts collectively referred to as the “gut” appear as a dark area in the middle of the trunk. It is believed that salivary and gastric glands secrete extra-cellular digestive enzymes. Nutrients are absorbed in the stomach. Wastes are passed through the intestine. (Horne and Goldman, 1994; Hickman, Roberts and Larson, 1995).
Water is believed to pass not through the epidermis, but through the mouth. The excretory system includes a pair of protonephridial tubules that lead to a single bladder, which empties by pulsation into the cloaca. The cloaca, through which the intestine and oviducts also empty, is located middorsally near the point at which the trunk and the foot meet. The relatively high rate of pulsation (1-4 times per minute) suggests that the protonephridia play an important role in osmoregulation (Hickman, Roberts and Larson, 1995; Hyman, 1951).
The foot is primarily an attachment organ. As such, it is most developed in creeping and sessile forms. Though the foot is unsegmented, rings in its epidermis permit it to be telescopically retractable. It is much narrower than the trunk. It is tapered in some forms, but is abruptly cut off in others. The foot usually ends with one to four toes (or spurs). Adhesive compounds are secreted by pedal glands and excreted through ducts near the terminal end of the foot. Aided by the foot, some forms are able to move across surfaces by crawling in leech-like movements (Hickman, Roberts and Larson, 1995; Dhert, 1996).
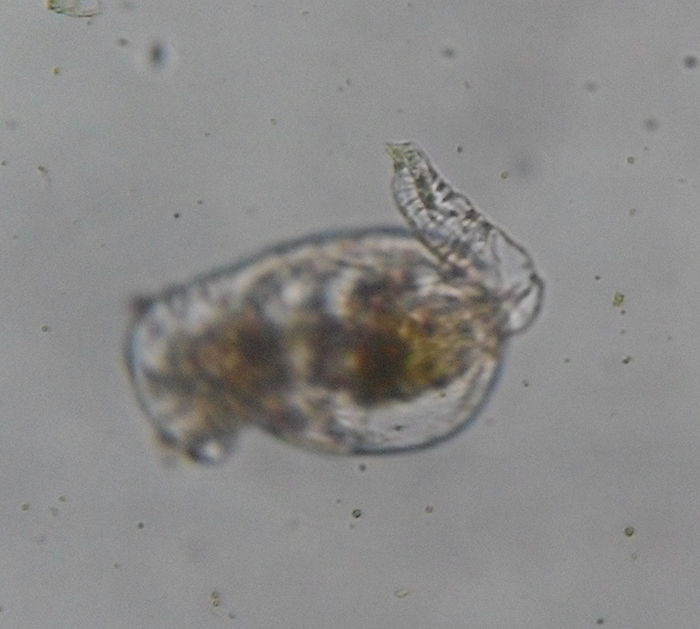
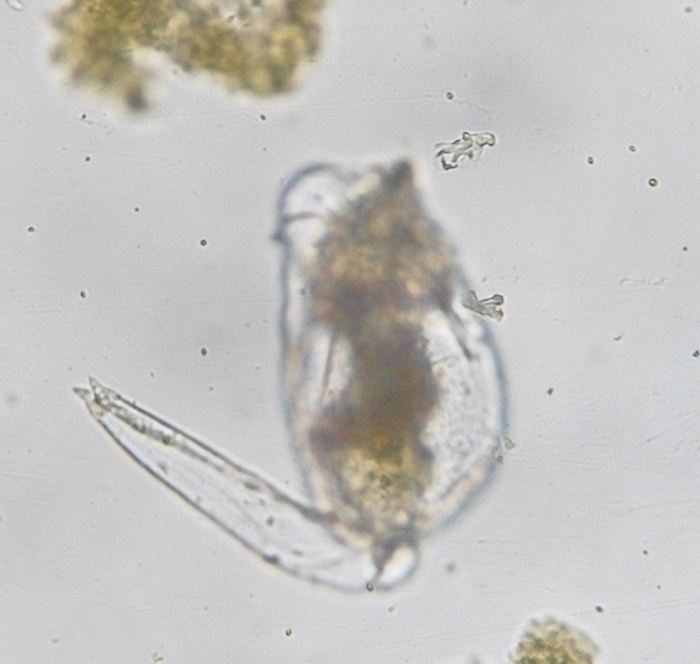
Brachionus plicatilis, unattached, with focus on the retracted foot (note conspicuous rings in cuticle). (Photo by Kenneth Wingerter.)
Rotatorian reproductive organs are (particularly for such minuscule animals) diverse and transmutable; reproductive biology of the Rotatoria is often complex. A more detailed treatment of rotatorian reproductive anatomy and life cycle will be presented in the following piece.
References
1. Campbell, Niel A. and Jane B. Reece. (2002). Biology (6th ed.). San Francisco, CA: Benjamin Cummings.
2. Dhert, Philippe. (1996). Manual on the Production and Use of Live Food for Aquaculture. FAO Fisheries Technical Papers, No. 361.
3. Hickman, Cleveland P. Jr., Larry S. Roberts and Allan Larson. (1995). Integrated Principles of Zoology (9th ed.). Dubuque, IA: Wm. C. Brown Publishers.
4. Horne, Alexander J. and Charles R. Goldman. (1994). Limnology (2nd ed.). New York, NY: McGraw-Hill, Inc.
5. Hoff, Frank A. and Terry W. Snell. (2008). Plankton Culture Manual (6th ed.). Dade City, FL: Florida Aqua Farms, Inc.
6. Hyman, L. H. (1951). The Invertebrates, Volume III: Acanthocephala, Aschelminthes and Entoprocta. New York, NY: McGraw-Hill, Inc.
7. Ohs, Cortney L., Eric J. Cassiano and Adelaide Rhodes. (2010). Choosing an Appropriate Live Feed for Larviculture of Marine Fish. University of Florida IFAS Extension Publication No. FA167.
8. Omori, Makoto and Tsutomu Ikeda. (1984). Methods in Marine Zooplankton Ecology. New York, NY: John Wiley & Sons Inc.



0 Comments