Probiotics may have a strong effect in improving larval fish development. Some methods for their action may include appetite stimulation, improved nutrition, and simply competitive exclusion (summarized in Irianto & Austin 2002). The method of action may not be well studied at this time, but positive outcomes from those interactions are certainly possible (Verschuere et al 2000). With a growing interest in preventing microbial disease outbreaks, the usage of probiotics may be the next step in large aquaculture production.
The authors are introducing these methods to the marine aquarium hobby. With a vast record of short-lived specimens, troubles with shipping, acclimation issues, and a condensed captive environment; the aquarium hobbyist is faced with countless challenges in keeping marine ornamentals. This project is designed to analyze the specific strains of bacteria used in a commercial probiotic formula. This particular probiotic compound could lead the way into the next generation of home aquaculture hobbyists.
Introduction
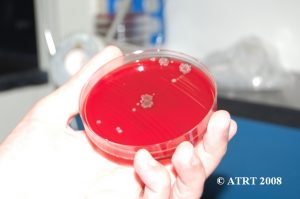
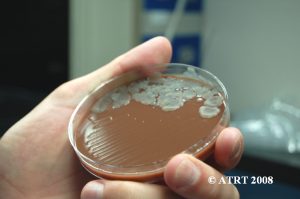
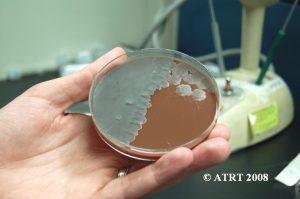
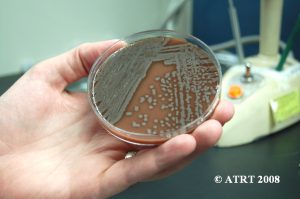
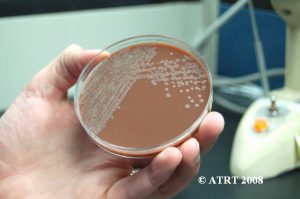
Following the Probiotic Analysis Part I (Blundell & Durrant 2009), we further isolated the bacteria recovered to produce pure subcultures. This was necessary to identify each type of bacteria present and to verify that each differing colony type was accounted for. Original cultures showed the best growth of bacteria on chocolate agar cultured in aerobic conditions. The original chocolate agar was used to provide starter colonies for subcultures.
Subculturing
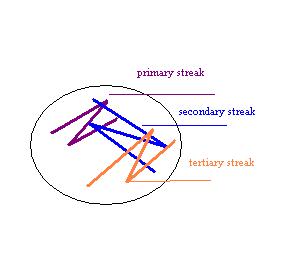
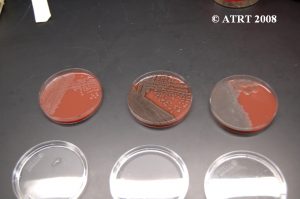
Subculturing is simply a way of isolating different colony types of bacteria. We take a culture plate with a mixture of bacteria and place the different colony types on separate plates (subcultures). This process is used in order to produce isolated and pure cultures.
Abbreviations
- SB
- Sheep Blood agar; an enriched media containing 5% sheep’s blood and other nutrients
- CHOC
- Chocolate agar; an enriched media containing lysed blood and other nutrients
- CNA
- Colistin Naladixic Acid agar; a selective media containing 5% sheep’s blood, the antibiotics colistin and naladixic acid, and other nutrients; selects for gram positive bacteria
- MAC
- MacConkey agar; a selective media that contains crystal violet and nutrients; selects for gram negative bacteria
- FB
- Fastidious Broth; an enriched liquid media that encourages robust growth of most bacteria
- BRUC
- an enriched anaerobic media containing blood, vitamin K, and hemin
- BBE/LKV
- Bacteroides Bile Esculin/Laked-Blood Kanamycin Vancomycin agar; a selective anaerobic bi-plate media; selects for gram negative bacteria
- PEA
- Phenylethylalcohol agar; a selective anaerobic media; selects for gram negative bacteria
- GS
- gram stain; a differential stain used to identify bacteria
Subculture Lab Report
Day 1 Plates read, subcultures made
- SB: 2+ very large gray wrinkly beta-hemolytic, #1
- 2+ med wet gray non-hemolytic, #2
- 2+ large rough fringy non-hemolytic, #3
- CHOC: 2+ very large dull gray mucoid, #1, GS: large boxy gram-positive rods
- 2+ medium wet umbilicate green-gray, #2, GS: med-large boxy gram-positive rods
- 2+ large pink rough fringy, #3, GS: long large boxy gram-positive rods
- CNA: 2+ pinpoint colonies
- MAC: No growth
- FB: Turbid, GS: large boxy gram-positive rods, some poorly-staining
- BRUC: 1+ pinpoint colonies
- BBE/LKV: No growth
- PEA: No growth
Day 2 Plates read
- SB: same
- CHOC: same
- CNA: 2+ large pink rough fringy
- MAC: No growth
- FB: same
- BRUC: 1+ medium rough, #3
- 1+ small wet, #1
- 1+ small flat green-gray, #2
- BBE/LKV: No growth
- PEA: No growth
Final Report
- Bacillus species, 3 colony types
| Report As | Primary | Secondary | Tertiary |
|---|---|---|---|
| 1+ | <10 | None | None |
| 2+ | >10 | <5 | None |
| 3+ | >10 | >5 | <5 |
| 4+ | >10 | >10 | >5 |
Subculture Results
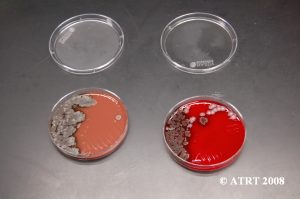
The subculture testing definitively showed three distinct colony types of Bacillus species. Even with the naked eye, distinct morphologies were apparent-mucoid, wet and rough, and pink colony types. Microscopic analyses clearly showed gram positive spore-forming rods with differing morphologies.
Conclusion
The results of this test confirm the manufacturer’s claim that the Sanolife product (INVE Technologies) contains three strains of the bacteria Bacillus. With the recent tests showing positive effects of these bacteria when added to both broodstock and to larval animals (INVE 2008), further development of probiotics may prove useful. Further experimentation with flora and fauna for aquaculture is certainly warranted following these results, especially given the potential outcomes.
References and Suggested Readings
- Blundell, A., Durrant, R., (2009), “Probiotic Analysis Part I”, Advanced Aquarist Online Magazine. USA.
- Ciprandi, G., et al (1986), “In vitro effects of Bacillus subtilis on the Immune Response”, Chemioterapia. Issue 5, pgs 404-407.
- Green, D., et al (1999), “Characterization of Two Bacillus Probiotics”, Applied and Environmental Microbiology. September 1999, American Society for Microbiology, Vol. 65, No. 9, pgs 4288-4291.
- INVE Technologies, Package Insert from ProBiotic Sanolife Mic-R.
- Irianta, A., Austin, B., (2002), “Probiotics in Aquaculture”, Journal of Fish Diseases, Blackwell Publishing. USA.
- Gomez-Gil, B., Roque, A., Turnbull, J., (2000), “The Use and Selection of Probiotic Bacteria for Use in the Culture of Larval Aquatic Organisms”, Aquaculture V. 191 I. 1-3. USA.
- Maza, G., (1983), “Genetic studies on the transfer of antibiotic resistance genes in Bacillus subtilis strains”, Chemioterapia. Issue 2, pgs 64-72.
- Olafsen, J., (2001), “Interactions Between Fish Larvae and Bacteria in Marine Aquaculture”, Aquaculture V. 200 I. 1-2. USA.
- Skjermo, J., Vadstein, O., (1999), “Techniques for Microbial Control in the Intensive Rearing of Marine Larvae”, Aquaculture V. 177 I. 1-4. USA
- Verschuere, L., Rombaut, G., Sorgeloos, P., Verstraete, W., (2000), “Probiotic Bacteria as Biological Control Agents in Aquaculture”, Microbial and Molecular Biology Reviews Vol. 64 No. 4. USA.





0 Comments