For many reefkeepers, correcting undesirable calcium 1 and alkalinity 2 values can be among the most vexing of the chemical problems encountered in maintaining a reef tank. Most reefkeepers know that if these parameters are not maintained appropriately, corals and other organisms may have difficulty 3 in depositing calcium carbonate skeletons. Understanding how to solve such problems, however, proves more elusive. Unfortunately, it is often not as simple as adding more of whatever is depleted.
Here, for example, is a real question that typifies the problem:
I’m having problems raising my calcium levels above 200 ppm. I have been using kalk for about two weeks for all top off water, about 3/4 gal a day. The level has never gone above 250 ppm and drops back to under 200 ppm. I bought some Turbo Calcium and tried it as the product label recommended but am having no real success. I have never used Turbo Calcium before and was wondering how much of it I could dose safe. I only have 2 mushrooms and 2 damsels. Any advice?
Unfortunately, calcium and alkalinity are linked 4,5 in many ways in reef tanks, and these links can lead to serious problems if they are not fully understood. If, for example, you add too much of a calcium supplement, you will drive down alkalinity as you get precipitation of calcium carbonate in the tank. Likewise, adding too much of an alkalinity supplement can result in reduction of calcium. Consequently, trying to correct one problem can cause another. Moreover, if you try to correct a calcium or alkalinity “problem” with the wrong type of additive, you might accomplish nothing more than creating limestone in your tank.
This article will clarify the different types of calcium and alkalinity problems encountered in typical reef tanks, and will describe in detail how to “solve” each of them. In reading through the article, you may feel that I am making it unduly complicated. Remember, however, that this article describes how to solve many different problems, while any given tank can only have one of them, so only a small section will apply.
Unlike most of my articles, this one is not written to provide a deep understanding of the science behind calcium and alkalinity in reef tanks. Those topics have been dealt with in several of my prior articles. This one reads more like a recipe. Nevertheless, this is the procedure that I go through (in my mind) when giving advice about correcting calcium and alkalinity additions, and there really is no shortcut that will ensure success (and plenty that ensure failure).
At the end of the article, I’ll also emphasize the best way to avoid these problems: using balanced calcium and alkalinity additions. I believe that using such additives would eliminate the majority of problems that people have with calcium and alkalinity, and I very strongly recommend their use.
One caution: many people get faulty readings from aquarium test kits. Some of these problems are the fault of the kit, and some the fault of the user. Regardless, if an aquarist were to “correct” a problem that was really only due to a faulty measurement, then the tank may go from fine to disaster. So please, before making any big corrections to water chemistry, confirm the reading with a different kit, preferably a different brand. This caution should especially apply if the measurement does not seem to make sense based on what you have previously added to the tank.
Recommended Ranges
Before getting into problems and solutions, let’s first define what constitutes a problem and what does not. Based on published studies3 involving the calcification of corals and other organisms, I recommend the following:
Alkalinity2 (due to bicarbonate and carbonate but not borate, so those using Seachem salt must raise this value substantially to accommodate the borate being counted in standard alkalinity tests)
2.5 – 4 meq/L or 7 – 11 dKH or 125 – 200 ppm CaCO3 equivalents
Calcium:
380 – 450 ppm calcium ion or 950 – 1125 ppm CaCO3 equivalents
If you are anywhere within these ranges for both parameters, you do not need to perform any correction on your tank chemistry, though you may choose to do so for other reasons. In this sense it makes no difference what the relationship is between the two values. If alkalinity is 4 meq/L, it is not inherently any “better” for calcium to be at 380 ppm or 450 ppm. Also, these ranges are somewhat arbitrary, especially at the high end. In fact, the primary reason for having a high end at all is that it is often difficult to keep one of these parameters above the minimum end of the range if the other is over the top end. So if one of these parameters is slightly above the high end, and the other is OK, that is not a problem worth worrying about.
One of the reasons that you may find compelling to adjust values even when within the recommended range (or outside but close to it) relates to test kit errors. All measurements of calcium and alkalinity have some uncertainty associated with them. Even if the kit is a reliable one, you may still want to strive to be in the center of the range to make it less likely that you are actually outside of it and only appear to be inside of it due to uncertainties in the measurement. This issue is especially important at the low end of the ranges, and not so important at the high ends.
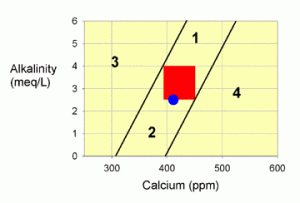
Figure 1. A graph showing possible values for calcium and alkalinity in marine aquaria. The red zone is the recommended target, and the blue dot represents values in natural seawater. Each numbered zone outside of the target area has a specific set of directions to get back to the target.
Calcium and Alkalinity Problems
If your tank falls outside of these ranges for either or both of these measurements, then how you need to go about correcting them does indeed depend on the relationship between the two. It is this aspect of calcium and alkalinity maintenance that causes problems for many aquarists.
Figure 1 shows a graph of calcium and alkalinity for marine tanks. The red zone in the middle represents the desired range for both parameters. The blue dot represents the values present in natural seawater. We will use this figure to determine a course of action for each of the four numbered zones outside of the red target area. So the first step is to see where your tank falls on the graph, and then follow the directions given for that zone.
Corrections for Zone 1
Zone 1 is the easiest problem to correct. Unfortunately, it is also very uncommon. In this case, both calcium and alkalinity are on the high side of normal. Moreover, if you leave the tank alone, the problem will likely correct itself, and you will end up in the red target zone (though you may also pass through it into zone 2 if you wait too long).
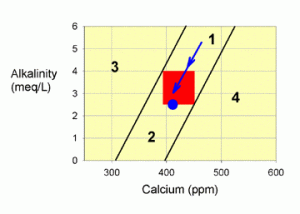
Figure 2. A graph showing how to correct values within zone 1 by allowing calcium carbonate to be deposited in the tank (the blue arrow).
What this zone implies is that both calcium and alkalinity are elevated, and that by removing calcium carbonate from the water, either through biotic deposition into coral skeletons or coralline algae, or through abiotic precipitation, as on heaters, the levels of each will drop in an appropriate ratio. More specifically, the tank parameters will move along a line parallel to the two lines bordering this zone, and directly into the red target zone (the blue arrow in Figure 2). If you are smack in the middle between these two lines, as in Figure 2, then you will continue to move in the middle of these two lines down into the target zone.
This movement can continue right out the bottom end of the target zone (into zone 2), of course, so once you reach the target zone, you’ll have to reinitiate normal calcium and alkalinity additions.
Corrections for Zone 2
Zone 2 is also an easy problem to correct, and is very common. In this case, both calcium and alkalinity are on the low side of normal. If you take any tank in the red target zone, and let corals and coralline algae grow (i.e., calcify), then you will move into zone 2. Just as above (Figure 2), you will move downward in a fashion such that you parallel the lines bordering the zone. That fact is why it is so common to have this problem: anyone not adding enough calcium and alkalinity to balance demand in the tank will likely enter this zone.
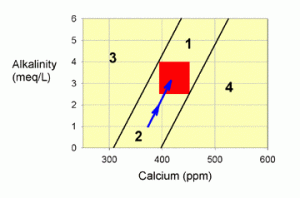
Figure 3. A graph showing how to correct values within zone 2 by supplementing with a balanced calcium and alkalinity additive system (the blue arrow).
To correct this situation back to the red target zone, one wants to add calcium and alkalinity in a balanced fashion. Fortunately, there are many ways to achieve this balance, such as with limewater (kalkwasser), calcium carbonate/carbon dioxide reactors, and the many balanced two-part calcium and alkalinity supplements (such as B-ionic, C-Balance, etc.). The more that you add of these, the further upward your correction will go (the blue arrow in Figure 3). Of course, adding too much will push you into zone 1, but if that happens you can just sit back and watch it drop back to the target zone. I’ll discuss balanced additives more at the end of the article.
Of course, you can add calcium and alkalinity that are not “balanced” (that is, not tied to one another in any specific ratio). For example, adding calcium chloride and sodium bicarbonate will work fine, but you must fine-tune exactly how much of each you add. Consequently, more careful monitoring of calcium and alkalinity levels is necessary if you go this route.
Remember that manufacturer recommendations are based on maintaining a tank, not in making substantial corrections. To make such corrections, you may need to add much more than is recommended. If you are adding a balanced additive (and the pH is not getting out of the range of about 7.9 to 8.5) then the worst that is likely to happen from overdosing (other than truly huge overdosing) is wasting some money and causing some extra precipitation of calcium carbonate on your heater and other parts of the tank. These balanced additives are discussed in more detail at the end of the article.
On the other hand, if you are using independent calcium and alkalinity additives, you must be very careful to not create an imbalance by adding too much of one relative to the other. Directions for deciding how much of these types of additives can be found below, but you should rely on frequent testing more than recommended amounts, to determine how much of these types of additives to put into the tank.
Finally, if you are adding large amount of calcium and alkalinity supplements, but just cannot maintain the desired values, you might want to measure the magnesium level in the water. Magnesium plays an important role in preventing the abiotic precipitation of calcium carbonate1, and if it is substantially depleted, you may be experiencing excessive amounts of calcium and alkalinity loss to this route. Magnesium gets the blame far more frequently, in my opinion, than it is likely responsible, but since it is easy to check with a test kit and easy to supplement if necessary, there’s no reason to not see if it is a problem. I’d advise aiming for a natural seawater level of about 1300 ppm.
Corrections for Zone 3
Zone 3 problems are a little harder to correct, and are fairly common. It is, in fact, the problem in the real question posed at the beginning of this article (it doesn’t say so there, but the alkalinity was 3.2 meq/L). This problem is typically caused by overdosing alkalinity RELATIVE to calcium, but does not necessarily imply that calcium is either too high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.
One more word about this zone before getting to solutions: Many tanks end up here because aquarists are trying to correct pH problems by adding “buffer.” In my opinion, one should not try to correct any pH problem by simply adding an alkalinity supplement. If you are low on alkalinity, it is a fine course of action to raise the alkalinity. But if alkalinity is OK, or even high, adding an alkalinity supplement to alter the pH may simply create a worse problem. Better solutions to pH problems are discussed in this recent article6.
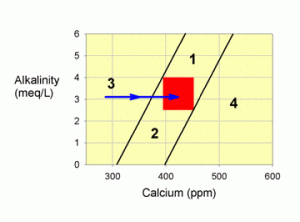
Figure 4. A graph showing how to correct values within zone 3 by adding a calcium additive, such as calcium chloride (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the right hand edge of zone 3), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
I would advise correcting this problem by adding a calcium chloride supplement until you have moved into the target zone (or zones 1 or 2 that you can then treat as described above) as shown in Figure 4. Almost any brand of calcium chloride will do (Kent Turbo Calcium, Kent Liquid Calcium, ESV, etc.). Certain other calcium supplements may also be OK (such as just the calcium component of the two-part calcium and alkalinity additive systems), but you do not want to add any alkalinity. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the edge of the zone, while you want to move over to it, and cross it.
If calcium is less than 400 ppm, I’d suggest using this handy online calculator7 to determine how much dry calcium chloride is necessary to move back to the target zone. Note that it is a minimum estimate because it does not know how much alkalinity you have, so it cannot know if you are only raising calcium directly (which it calculates) or are also precipitating calcium carbonate (when alkalinity is high this will probably happen, but is typically not a problem other than that it uses up some of what you add).
If the calcium is above 400 ppm in this zone (unlikely, but it does happen), then you can safely either do nothing until it drops and you need to add more calcium, and treat it as suggested in the previous paragraph, or you can add some calcium immediately, move into zone 1, and then just let it drop on its own.
Corrections for Zone 4
Zone 4 problems are also a little harder to correct. It is typically caused by overdosing calcium RELATIVE to alkalinity, but does not necessarily imply that alkalinity is either to high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.
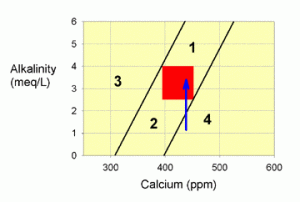
Figure 5. A graph showing how to correct values within zone 4 by adding an alkalinity additive, such as baking soda (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the left hand edge of zone 4), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
If alkalinity were less than 4 meq/L (11 dKH; the most common situation in zone 4; shown in Figure 5), I would advise correcting this problem by adding an alkalinity supplement until you have moved into the target zone (or zone 1). For systems with a pH of 8.2 or above, baking soda (sodium bicarbonate) is a good choice. For systems with a pH below 8.2, washing soda (sodium carbonate) is a good choice (though use some baking soda too if the correction is a large one and the pH gets too high; that is, above pH 8.5 or so).
In gauging how much to add, here are some rough guidelines:
- Baking Soda
- To raise 50 gallons of tank water by 1 meq/L will require about 16 grams of baking soda (sodium bicarbonate; sodium hydrogencarbonate). Since a level teaspoon of baking soda weighs just under 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.4 meq/L (~1 dKH).
- Washing Soda
- To raise 50 gallons of tank water by 1 meq/L will require 10 grams of washing soda (sodium carbonate). Since a level teaspoon of washing soda weighs just over 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.6 meq/L (~1.7 dKH).
One special note about washing soda: Apparently some Canadian brands of washing soda contain surfactants. VIP brand, in particular, contains them and a reef keeper using them on my advice turned his tank into a bubble bath. On questioning, the manufacturer did indicate that a surfactant is present. The same reef keeper says the local Arm & Hammer brand in Canada smells strongly of perfume. I’d avoid perfumed brands, if possible. My Arm & Hammer Super Washing Soda purchased in the US apparently contains no significant surfactants, and is not perfumed. Nevertheless, anyone using washing soda for the first time ought to put some in water and stir it around to see if soapy bubbles form. If so, I’d suggest finding another brand.
Many commercial alkalinity supplements will also be fine for this purpose, as long as no significant calcium is added. In general, I don’t prefer those that contain substantial borate. The alkalinity component of the two-part calcium and alkalinity additive systems would be OK. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the left edge of the zone, while you want to move over to it, and cross it.
If alkalinity is more than 4 meq/L (11 dKH; an uncommon situation), then you can safely either do nothing until it drops and you need to add more alkalinity, and treat it as suggested in the previous two paragraphs, or you can add some alkalinity immediately, move into zone 1, and then just let it drop on its own.
Using Balanced Additives
Just as in many other fields of human endeavor, the easiest way to solve reef tank chemistry problems is to prevent them from happening in the first place. That may sound trite, but there are ways to maintain reef tanks that require substantially fewer measurements of calcium and alkalinity than are required with other supplementation schemes. In fact, I haven’t measured either in my tank for over a year, and yet I expect that the levels are fine, just as they have always been whenever I have measured them. So while I am not saying that you should never measure things, I am saying that using appropriate methods may greatly cut down on the likelihood of having chemical problems, and thereby on the required frequency of measurement.
Let’s first look at how calcium and alkalinity are consumed in reef tanks.
Calcium is largely consumed by formation of calcium carbonate. This happens biologically3 in corals, coralline algae, mollusks, and a variety of other organisms. It can also happen abiotically1, such as by precipitation on heaters and pump impellers. In a reef tank with rapidly calcifying organisms, this effect will so predominate any other calcium export mechanism, that no others need be considered for this purpose.
Alkalinity2 for our purposes here is comprised of bicarbonate and carbonate. The vast majority of alkalinity depletion in most tanks also comes about by the precipitation of calcium carbonate, as described above. In this process, as alkalinity is depleted by 1 meq/L, calcium will be depleted by 20 ppm. There are some other processes that can lead to alkalinity depletion, including partial cycling of nitrogen (from organic compounds to nitrate and no further) and the incorporation of magnesium into calcium carbonate, but these are generally much less important than calcification.
Consequently, alkalinity depletion in most tanks (especially in short time frames) is tightly coupled to calcium depletion, and if one supplements calcium and alkalinity in proportions equal to those that they are being removed, then it is MUCH less likely that calcium and alkalinity will become imbalanced4 and thereby trickier to correct. That is, the only problems that you will encounter are those in zones 1 and 2 (not enough or too much of these additives). Using a balanced scheme, you should not ever end up in zones 3 and 4, where you have substantial imbalances between calcium and alkalinity.
Additionally, overdosing of balanced additives typically seems to simply result in an increase in the amount of calcium carbonate that is being precipitated in the tank, and does not, in general, lead to substantial increases in dissolved calcium and alkalinity. This fortunate circumstance comes about largely because of the supersaturation of calcium carbonate1 in reef tanks.
There are many ways to add calcium and alkalinity to reef tanks in ways that are balanced with output. These include limewater (kalkwasser)8 regardless of how it is dosed, calcium carbonate/carbon dioxide reactors, the two-part calcium and alkalinity additives (e.g., B-ionic, C-balance, Kent Tech CB, etc.), and a small selection of one-part additives, such as calcium acetate. I strongly recommend that you adopt on of these in your tank maintenance routine, and then never add any other calcium or alkalinity sources unless you are certain of what you need.
If you choose to add limewater, but find that using saturated limewater to replace all evaporated water does not quite keep up with calcification demand9, you can stretch the capacity of limewater by adding vinegar to the solution to enhance the solubility of the calcium hydroxide10.
Summary
I hope this article proves useful is understanding and dealing with the different types of calcium and alkalinity problems encountered by reef keepers. Once you get calcium and alkalinity under control, you are far along the road to having your tank chemistry optimal for its inhabitants. Once you accomplish that, then you can spend your time either enjoying your tank, or reading additional reef chemistry articles.
References
- Calcium in Reef Tanks, http://www.advancedaquarist.com/2002/3/chemistry
- What is Alkalinity?, http://www.advancedaquarist.com/issues/2002/2/chemistry
- The Chemical and Biochemical Mechanisms of Calcification, http://www.advancedaquarist.com/issues/2002/4/chemistry
- The relationship Between Calcium and Alkalinity, http://reefkeeping.com/issues/2002-04/rhf/feature/index.htm
- More About Calcium and Alkalinity, http://www.animalnetwork.com/fish2/aqfm/1998/july/bio/default.asp
- Solutions to pH Problems, http://www.advancedaquarist.com/issues/2002/6/chemistry
- Online Calcium Chloride Calculator, http://www.andy-hipkiss.co.uk/cacalc.htm
- Limits To Limewater…Revisited, http://www.animalnetwork.com/fish2/aqfm/1999/aug/bio/default.asp
- Calcification Rates in Several Tropical Coral Reef Aquaria, http://www.animalnetwork.com/fish2/aqfm/1998/mar/bio/default.asp
- Expanding the Limits of Limewater: Adding Organic Carbon Sources, http://www.animalnetwork.com/fish2/aqfm/1999/oct/bio/default.asp
- Limewater, Acetic Acid and Sand Clumping, http://www.animalnetwork.com/fish/library/articleview2.asp?Section=&RecordNo=181



0 Comments