The importance of boron in marine aquaria is a subject that is not often discussed by hobbyists, despite the fact that many people dose it every day as part of their alkalinity supplements. In fact, most commentary on boron derives from manufacturers that are selling it in one fashion or another as a “buffering” agent. Unfortunately, these discussions nearly always lack any quantitative discussion of boron or the effects that it has (both positive and negative). This article describes in detail the forms and concentrations that boron takes in seawater, and shows how much pH buffering it actually provides, and why it does so. You may, in fact, be surprised to learn that boron actually contributes only a minor fraction of the buffering of normal seawater. At the end of the article, I also make suggestions on how to reduce the diurnal pH swing in reef tanks.
This article also discusses the sources and sinks for boron in reef tanks, and shows that while typical tanks may be slightly depleted in boron with respect to natural levels, most are not so significantly depleted that any correction is required. Nevertheless, how to test for and supplement boron is addressed.
Finally, this article also touches briefly on the limited knowledge of biological effects of boron in marine systems. Boron appears to be a necessary or desirable nutrient for certain organisms, but is also toxic to others at levels not far above natural levels (and below that present in one artificial salt mix).
Boron in Seawater
In natural seawater, boron is present at about 0.41 mM (4.4 ppm total boron) and takes two different chemical forms. The predominate form is boric acid, comprising about 70% of the total boron present. Boric acid, B(OH)3, consists of a central boron atom and three hydroxyl groups arranged in a triangle (Figure 1). The second form is borate, B(OH)4–. It consists of a central boron atom and four hydroxyl groups arranged in a tetrahedron (Figure 1). It carries a net negative charge, while boric acid is neutral. These two forms can interconvert in less than a second, so the two forms are in chemical equilibrium with each other.
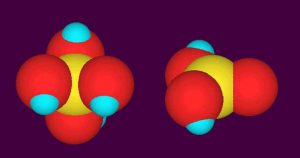
Figure 1. Space-filling molecular models of borate (left) and boric acid (right). The boron is yellow, oxygen is red, and hydrogen is blue.
Boric acid and borate have a variety of chemical and biological effects in normal seawater, though biological effects are rarely discussed in the marine aquarium hobby. The biological effects are, however, important in certain situations, and will be discussed later in this article. The most commonly known property of this system is its ability to buffer seawater against pH changes, and this aspect is discussed in detail in the next section.
Buffering of Normal Seawater
The exact percentage of boric acid and borate in any aqueous system is dependent on pH. The pKa of boric acid in seawater is about 8.55, depending on the temperature.1 That is, the pH where both forms are equally represented is about 8.55 in a normal tropical reef tank. At lower pH values, such as those in most reef tanks, there is more boric acid than borate.
The fact that the two forms are related by equation 1 explains why boric acid and borate together form a buffer system:
1) B(OH)3 + H2O → B(OH)4– + H+ (pKa ~8.55)
If the pH in a system containing both forms were to begin to rise for any reason, some of the B(OH)3 would be converted into B(OH)4–, releasing a proton, H+. The pH would then not rise as much as it otherwise would. Likewise, if the pH in that system were to begin to drop for any reason, some of the B(OH)4– would be converted into B(OH)3, taking up a proton. The pH would then not drop as much as it otherwise would.
This effect is exactly how a standard buffer works.
The other system that significantly buffers the pH in normal seawater is the bicarbonate/carbonate system:
2) HCO3– → CO32- + H+ (pKa ~ 8.92)
The relative buffering of these two systems (equations 1 and 2) depends on the amounts of each system present, and also on the pH. For any given buffer system, it turns out that the pH at which it gives optimal buffering corresponds to the pKa, where there are equal amounts of each of the two forms of the buffer present. Why exactly this is true is beyond this article, but it relates to the fact that for a given incremental change in pH, more of the buffer will change form at that pH than would change form at any higher or lower pH, and moreover, the farther that the pH is from the pKa, the smaller the effective capacity of that buffer system to resist pH changes.
Buffering capacity can be quantified using the buffer intensity, b, defined mathematically in a way that is easy to calculate, but that isn’t worth detailing here.2 The units of the buffering intensity can be expressed as meq/L or meq/L/pH unit (these are equivalent since pH is really a dimensionless parameter). Thinking about it as meq/L/pH unit makes it easier to understand that it is a measure of the amount of alkalinity (or acidity; either one measured as meq/L) that needs to be added to impact the pH up or down by one unit (though that is a substantial simplification).
In the case of normal seawater at pH 8.2, b = 0.19 meq/L/pH unit for the boric acid/borate system, and 0.63 meq/L/pH unit for the bicarbonate/carbonate system. These values are additive, and result in a total buffering of b = 0.82 meq/L/pH unit. Under these conditions, the boric acid/borate system provides about 23% of the total buffering, while the bicarbonate/carbonate system provides about 77%.
If the pH of normal seawater is raised to 8.5, the total buffering is b = 1.2 meq/L/pH unit, or about 40% greater than at pH 8.2 (because both systems are closer to the pKa). At this pH, the relative contribution of the two systems to the total capacity is only slightly different than at pH 8.2, with 20% from borate and 80% from carbonate.
If the pH of normal seawater is lowered to 7.8, the total buffering is b = 0.42 meq/L/pH unit, or about half that at pH 8.2 (because both systems are farther from the pKa). At this pH, the relative contribution of the two systems to the total capacity is also only slightly different than at pH 8.2, with 29% from borate and 71% from carbonate.
Buffering in Artificial Seawater
In artificial seawater the buffering can be very different than in natural seawater. In artificial seawater at pH 8.2 made using Seachem salt, where the boric acid/borate system is artificially very high (12X normal)3, the boric acid/borate system would provide a huge b = 2.3 meq/L/pH unit, which is a whopping 78% of the total buffering of b = 2.9 meq/L/pH unit . That amount of total buffering (3.5X the amount in normal seawater) will go a long way toward making the pH much more stable than in natural seawater, and is the reason that Seachem incorporates it into the salt mix. Whether this benefit is worth the concerns presented later in this article about unusually high boron concentrations is up to aquarists to decide for themselves.
Likewise, in artificial seawater with a normal amount of boron, but artificially high total alkalinity (say, 4 meq/L or 11 dKH, as is present in many reef tanks), the total buffering is about b = 1.2 meq/L/pH unit, or about 50% more than normal seawater. In this case, the relative contribution of the borate system to total buffer is less, or only about 16% of the total.
Would Depleted Boron Cause Big pH Swings?
Boron may be slowly depleted in some reef aquaria, and that possibility is discussed in detail below. If it is, would that impact the buffering in such a way that aquarists will experience substantially larger pH swings? Asked another way: Should aquarists worry about borate from a buffering perspective? Generally, no, in my opinion. In some situations, where pH stability is paramount, normal or excess borate is potentially preferred. In a normal reef tank, it is unlikely to be important at all.
Let’s look at the most extreme case: Would a tank with no boron experience excessive pH swings? First, let me qualify this entire discussion with the idea that there is little information available on the effect of pH swings on tank inhabitants. For example, there is no evidence that a tank with a diurnal pH swing of 0.10 or 0.01 pH units is any better (or worse) than one with a swing of 0.2 or 0.3 units. Larger swings may well swing you out of the overall optimal range (which I typically describe as pH 7.8 to 8.5), but the swing itself is not the concern there. If it turned out that pH swings of some particular magnitude were found to be problematic, that might change the discussion that follows. Note also that the ocean is not pegged to a particular pH, and lagoons, in particular, can shift by several tenths of a pH unit daily.1
So, a tank with a total alkalinity of 3 meq/L (typical of reef tanks) and normal boron levels, has 20% of it buffering, b, provided by boric acid/borate. Will removing that 20% of the buffering make the diurnal pH swing larger? Yes, certainly. How much larger?
While those effects can be calculated precisely (though it is tedious), or measured exactly in an experiment, here is a reasonable simplification that holds for small pH swings:
- pH change = (acid or based added)/ b
So if one has a pH swing of 0.10 pH units, and b were decreased by 20%, then the swing would become 0.125 pH units. Likewise a swing of 0.20 pH units becomes a change of 0.25 pH units.
While different aquarists may react differently to the magnitude of this effect, remember that it is the effect caused by a TOTAL lack of boron in the tank. In a recent study4 of reef tank water, it was found that typical reef tanks were not far from natural boron levels, and even the very lowest tank examined had half of the value, so would have about half of the swing increase shown above.
For comparison, a reef tank (pH 8.2) with no boric acid/borate at all, and a total alkalinity of 4 meq/L (11 dKH) has b = 1.0 meq/L/pH unit, or about 25% more total buffering than normal seawater.
These various comparisons should help aquarists understand the relative contribution of borate to buffering, and to understand that slight depletion (or excess) of boron will go unnoticed from a pH variation perspective. Even the total absence of borate might not be detected in the magnitude of the daily pH swing present in most reef tanks.
Depletion of Boron in Reef Tanks
Most artificial salt mixes3 have boron in approximately natural levels (0.41 mM; 4.4 ppm boron), with the exceptions of the brands Seachem (4.9 mM) and Coralife (1.26 mM), though a recent test4 showed boron in Instant Ocean a bit lower (0.31 mM) than the previous test (0.44 mM). 3 Starting with natural levels, if the amounts of boron entering the tank do not balance that being taken out, the boron levels may either rise or fall over time. In the 1 ½-year old reef tank of tank of Steve Nichols, for example, he used Instant Ocean salt mix, used no other specific boron additives and few water changes, and his tank was recently measured to have a boron concentration of 0.28 mM (3.0 ppm). That result is on the order of 40% lower than natural seawater, but only slightly lower (12%) than Instant Ocean salt mix in the same test4. In that study4, the average tank was depleted by only about 10% compared to natural seawater (although there were outliers at 2X natural levels and also at half the natural level).
Boron Sinks: Calcification
One primary sink for boron in reef tanks is expected to be co-precipitation with calcium carbonate. Typically, boron is found to be incorporated at about 50-100 ppm into corals, but the actual values vary considerably.5,6 Boron in coral skeletons has recently been a hot topic of study by chemical oceanographers. Specifically, the incorporation of different boron isotopes into coral skeletons has been hypothesized to be an indicator of the pH of the seawater, and hence a way to measure ancient seawater pH.
The reason for the differential isotope incorporation is that boric acid is slightly (2%) enriched in 11B relative to borate, which is enriched in 10B. The reason for this enrichment is that boric acid containing 10B is very slightly more acidic than boric acid containing 11B, and 10B is consequently more in the borate form at any given pH. The actual enrichment will depend somewhat on pH, since the two acids have different pKa values. Consequently, since it turns out boron is preferably incorporated into CaCO3 from the borate form, then looking at the isotope ratio in coral and foraminifera skeletons can lead one back to the pH that was present when the CaCO3 was deposited. While the isotope issue is fascinating, and open to debate, further discussion is beyond this article. Nevertheless, it has resulted in a great deal of study of boron in corals and other ancient sources of CaCO3, so the concentrations are well studied.
The amount of boron that is expected to be co-precipitated with calcium carbonate in a reef tank is, of course, a function of how much calcium carbonate is deposited. At the high end of calcification rates in reef aquaria,7 about 10 kg CaCO3/year per 100 gallons (a daily consumption of 1.4 meq/L of alkalinity), we can calculate that 50 ppm incorporation of boron results in a depletion of boron by 500 mg in a year, or 0.12 mM, about 30% of the natural boron level.
Similarly, at the middle to low end of calcification rates in reef aquaria, about 2 kg CaCO3/year per 100 gallons (a daily consumption of 0.28 meq/L of alkalinity), we can calculate that 50 ppm incorporation of boron results in a depletion of boron by 100 mg in a year, or 0.02 mM, about 6% of the natural boron level.
Boron Sinks: Algae
Another sink for boron is biological uptake by algae. Algae are not nearly so extensively studied with respect to boron as are corals and other organisms that deposit CaCO3, but the amounts involved can be significant. One study of 26 marine algae showed boron incorporated at 50-300 ppm of the dry weight.8
Calculations similar to those for calcium carbonate deposition (above) apply to the growth and harvesting of macroalgae. In many tanks, the deposition of calcium carbonate is likely to be a larger contributor to boron export than is the harvesting of macroalgae. However, algal removal may be the biggest boron export mechanism for tanks with lower calcification, but heavy macroalgae harvesting (like my own, where macroalgae harvesting may outpace calcium deposition on a weight basis).
Sources of Boron
Sources of boron, other than the salt mix used in marine aquaria, can be any of the following:
- Foods
- The dissolution of CaCO3/CO2 reactor media that contains substantial boron
- Other calcium and alkalinity supplements with incidental boron
- Alkalinity supplements that contain borate as an intentional additive
- Other “Special” Supplements (e.g., Combisan or Coral Vital that contain boron)9
- Foods. In a recent study of foods9,10 supplied to reef aquaria, it was shown that most contain very little in the way of boron. None had quantifiable levels, meaning they were all less than 2.5 ppm boron. If you added 5 grams of a food with 2.5 ppm boron to a 100 gallon tank every day, you would be adding a total of 4.5 mg of boron in a year, or 0.001 mM per year, which is less than 1% of the natural level of boron.Since algae can contain substantial boron,8 feeding macroalgae might be a significant source. If you fed 5 grams of dry macroalgae per day to a 100 gallon tank (a fairly hefty amount), then at 200 ppm boron in the macroalgae, that 5 grams contributes 1 mg of boron, or 0.24 mM to the tank per day. In a year, that would add 0.09 mM, which is about 22% of the natural boron level.
- Dissolution of CaCO3/CO2 media. This method seems like it should be a good source of boron, assuming that people are using crushed coral. For some reason, however, the commercial materials around are very low in boron, much lower than reported values for boron in coral skeletons. In two studies,11,12 it was found that boron ranged from less than 0.25 ppm (Koralith and also quarried limestone) to 0.7 ppm (Nature’s Ocean brand, Atlantic crushed coral) to 1.7 ppm (Super Calc Gold).
All of these media end up being small contributions to tank boron, even in cases with fairly high calcification. For example, a tank with a yearly calcification rate of 10 kg CaCO3 per 100 gallons of tank water (a daily addition of 1.4 meq/L of alkalinity) results in delivery of only 17 mg total in that 100 gallons, or only 0.004 mM (0.045 ppm) boron using the Super Calc Gold. That amount comprises only 1% of the natural level of boron. Of course, a media with higher boron concentrations, like coral skeletons matching those reported in the literature, could result in a net addition of fifty times that level, which then becomes quite significant.
Boron in Alkalinity Supplements
Some brands of alkalinity supplements or “buffers” are claimed to contain added boron (such as Marine Aquarium Buffer, Pro●Buffer dKH, and Superbuffer dKH made by Kent), while others do not (such as Reef Builder, Reef Buffer, and Reef Carbonate made by Seachem). Unfortunately, those that contain boron do not indicate how much, either on the bottle, or when the manufacturer is directly asked. The only statement made is that it is an “important contribution.” Consequently, it is not apparent, without lab testing, whether the amounts of boron present are large enough to be useful. It is also not apparent whether the amounts present might be high enough to invoke some of the undesirable effects of boron that are described below.
Two-Part Calcium and Alkalinity Additives: The Effect on Boron
Another series of products that should contain boron are the two-part calcium and alkalinity supplements that claim to leave only a natural seawater ionic residue after removal of calcium carbonate. That is, they contain all ions, including boron, in natural seawater ratios with the exception of calcium, carbonate, and bicarbonate (as is the case in B-ionic according to Bob Stark of ESV). Assuming that such supplements are properly formulated, then they should not cause an increase in boron above natural levels, though they may help “maintain” it if it is otherwise being depleted (just as a water change would).
Biological Aspects of Boron in Marine Aquaria
For such a ubiquitous chemical, there is actually very little known about the biological effects of boron. In one recent review,13 it is stated:
Vascular plants, diatoms, and some species of marine algal flagellates have abs. requirements for B, although the primary role remains unknown.
Further, some types of macroalgae (e.g., Gracilaria tikvahiae) apparently grow more rapidly in the presence of natural levels of boron than it its complete absence.14 In one case, it is known that blue-green alga Nostoc linckia uses boron to form borophycin, a potent cytotoxic compound that it may use for defense. 15
It has also been hypothesized by some in the hobby, but not demonstrated, that borate may be important for calcification. Just as with the issues around strontium, I’m not aware of any study that has tested the growth of a calcifying coral in the absence of borate, so the lack of any data may simply reflect the lack of anyone actually running a test. Nevertheless, there is no evidence that calcification is altered if boron is allowed to become depleted (or raised, for that matter).
While the data are very sparse, these studies suggest that it is desirable to maintain natural levels of boron in reef tanks.
Elevated Boron: Toxicity
At boron levels above that present in natural seawater, as is supplied in some artificial salt mixes and as may develop from overuse of boron supplements, boron begins to exert undesirable toxicity on a number of organisms. The studies on marine organisms are not wide ranging, so one must be careful in how to interpret levels above natural seawater since tests have not been run on most of the organisms that we keep.
In general, marine organisms (invertebrates and fish) are seemingly more prone to experience toxicity from boron than are freshwater species. The marine isopod Limnoria lignorum has a 24-hour LC50 (that is, the concentration at which 50% die in 24 hours) of only 2.6 mM (28 ppm boron).16 That is only about 6 times the concentration in natural seawater (and is BELOW the concentration in Seachem salt mix!). Similarly, the dab, Limanda limanda (a North Sea Fish), has a 96-hour LC50 of 6.8 mM (74 ppm boron).16
A lot of additional biological effects can be found on the web sites of the Canadian Environmental Protection Division Ministry of Water, Lands and Air Protection17 and the United Nations International Program on Chemical Safety. 18
Elevated Boron: Confounding Interpretation of Alkalinity Tests
One additional complication that comes from substantially elevated borate is the confounding of the interpretation of alkalinity tests. 19 When reef aquarists are concerned about alkalinity, they are almost invariably concerned with the alkalinity that comes from bicarbonate and carbonate, and it is largely used as a surrogate measure of bicarbonate, which is necessary for calcification.20 Nearly all hobby test kits measure alkalinity with a single titration that provides total alkalinity,19 which is the sum of bicarbonate, carbonate, and borate alkalinity. When the levels of boron are similar to natural levels, then the contribution of borate to that test is minimal, and is generally safely ignored in guidelines for alkalinity (for example, keeping a reef tank at
2.5-4 meq/L total alkalinity).
However, if the boron level is substantially above natural levels, as it is in the Seachem salt mix with 12x normal levels, borate can actually begin to dominate such tests, 19 and makes knowing the real bicarbonate and carbonate alkalinity much more difficult. Seachem sells a special borate alkalinity test kit to try to disentangle these effects, but that is only really necessary with tank water that contains greatly elevated boron levels.
Testing and Supplementing Boron
If you are interested in measuring boron in your tank, Salifert makes a boron test kit, as well as a supplement for adding boron. I’d advise maintaining a level close natural levels (0.41 mM; 4.4 ppm boron), rather than any elevated level with the goal of trying to attain better pH stability, but that is just my personal preference and you may feel differently about pH stability.
If you have a test kit, and want to supplement boron, you can also just use borax (from the grocery store; borax is 21.5% boron by weight) dissolved in water. One teaspoon will weigh about 4 grams,21 so 1 teaspoon in a 100 gallon tank will raise the boron level by 0.21 mM (2.3 ppm).
In general, I don’t think that most reef tanks are likely to suffer much if you don’t measure boron and just let the level be determined by the ebb and flow of boron from your salt mix and the various sinks in the tank. For whatever it is worth, I’ve not measured the boron level in my tank.
Increasing pH Stability
If you are concerned about pH stability, here is a list of actions that can be taken to reduce the diurnal pH swing in a reef tank:
- Increase aeration. Since the diurnal pH swing largely derives from changes in CO2 in the tank, “Perfect” aeration would nearly eliminate any pH swing. Nevertheless, perfect aeration is rather hard to accomplish in reef tank, but better aeration can decrease a large pH swing.
- Use limewater and other high pH alkalinity supplements only between late night and early morning.
- Use CaCO3/CO2 reactors only between late morning and late evening.
- Connect a reverse daylight tank or lit refugium to the existing system.
- Increase the carbonate alkalinity.
- Increase the boron level.
Happy Reefing!
References for Further Reading
- Chemical Oceanography, Second Edition. Millero, Frank J.; Editor. USA. (1996), 496 pp. Publisher: (CRC, Boca Raton, Fla.)
- Aquatic Chemistry Concepts. Pankow, J. F. (1991), 712 pp. Publisher: Lewis Publishers, Inc. The buffering intensity factor, b, was calculated according to equation 8.30 on page 150: b = 2.303Ca0a1 where C is the total concentration of the various forms of the buffer system in question (from reference 1 for normal seawater values) and a0 and a1 are the fractions present in each the two forms. a0 and a1 were calculated using seawater pKa values given in reference 1, using the equation 8.27 (p. 150): a0 = [H+]/([H+] K) and a1 = 1-a0.
- The Composition Of Several Synthetic Seawater Mixes by Marlin Atkinson and Craig Bingman: http://www.animalnetwork.com/fish2/aqfm/1999/mar/features/1/default.asp
- Its in the Water by Ron Shimek: http://reefkeeping.com/issues/2002-02/rs/feature/index.htm
- Boron isotopic compositions of corals: Seawater or diagenesis record? Gaillardet, Jerome; Allegre, Claude Jean. Paris, Fr. Earth and Planetary Science Letters (1995), 136(3-4), 665-76.
- Coprecipitation and isotopic fractionation of boron in modern biogenic carbonates. Vengosh, Avner; Kolodny, Yehoshua; Starinsky, Abraham; Chivas, Allan R.; McCulloch, Malcolm T. Res. Sch. Earth Sci., Aust. Natl. Univ., Canberra, Australia. Geochimica et Cosmochimica Acta (1991), 55(10), 2901-10.
- Calcification Rates in Several Tropical Coral Reef Aquaria by Craig Bingman: http://www.animalnetwork.com/fish2/aqfm/1998/mar/bio/default.asp
- Boron content of seawater and marine algae of the Finistere coast [France]. Maurice, J. Stn. Agron., INRA, Quimper, Fr. C. R. Seances Acad. Agric. Fr. (1983), 69(17), 1455-61.
- Necessary Nutrition, Foods and Supplements, A Preliminary Investigation by Ronald Shimek: http://www.animalnetwork.com/fish/data/foods.asp
- What we Put in the Water by Ronald Shimek: http://www.reefkeeping.com/issues/2002-04/rs/feature/index.htm
- Calcium Carbonate for CaCO3/CO2 Reactors: More Than Meets the Eye by Craig Bingman: http://www.animalnetwork.com/fish2/aqfm/1997/aug/bio/default.asp
- Alternative Calcium Reactor Substrates by Greg Hiller: http://www.animalnetwork.com/fish/library/articleview2.asp?Section=Aquarium+F rontiers+–+Biochemistry+of+Aquaria&RecordNo=1571
- Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. Hunt, Curtiss D. Grand Forks Human Nutrition Research Center, USDA-ARS, Grand Forks, ND, USA. Biological Trace Element Research (1998), 66(1-3), 205-225.
- Inorganic nutrition of marine macroalgae in culture.McLachlan, J. Atl. Res. Lab., Natl. Res. Counc. Canada, Halifax, NS, Can. Editor(s): Srivastava, Lalit Mohan. Synth. Degrad. Processes Mar. Macrophytes, Proc. Conf. (1982), Meeting Date 1980, 71-98.
- Structure and biosynthesis of borophycin, a new boeseken complex of boric acid from a marine strain of the blue-green alga Nostoc linckia.Hemscheidt, Thomas; Puglisi, Melany P.; Larsen, Linda K.; Patterson, Gregory M. L.; Moore, Richard E.; Rios, Jorge L.; Clardy, Jon. Department of Chemistry, University of Hawaii, Honolulu, HI, USA. Journal of Organic Chemistry (1994), 59(12), 3467-71.
- A comparative analysis of the toxicity of boron compounds to freshwater and saltwater species. Hovatter, Patricia S.; Ross, Robert H. Health and Safety Research Division, Oak Ridge National Laboratory, Oak Ridge, TN, USA. ASTM Special Technical Publication (1995), STP 1218(Environmental Toxicology and Risk Assessment: 3rd Vol.), 288-302.
- Ambient Water Quality Guidelines for Boron: http://wlapwww.gov.bc.ca/wat/wq/BCguidelines/boron/Photo
- United Nations International Program On Chemical Safety: http://www.inchem.org/documents/ehc/ehc/ehc204.htm
- What is Alkalinity? By Randy Holmes-Farley: http://www.advancedaquarist.com/2002/2/chemistry
- The Chemical and Biochemical Mechanisms of Calcification by Randy Holmes-Farley: http://www.advancedaquarist.com/2002/4/chemistry
- 20 Mule Team to the Rescue…Again by Craig Bingman: http://www.animalnetwork.com/fish2/aqfm/2000/feb/bio/default.asp



0 Comments