This article represents a sequel to my previous paper Behavior and Breeding of Peppermint Shrimp (see Advanced Aquarist Online Magazine, April, 2004) and is a continuation of my work in rearing the delicate larval stages of ornamental crustaceans. It is a work unfinished since as of this writing I have yet to complete the larval stages to metamorphosis of the Fire Shrimp, the species I am working with this year. With the completion of an advanced rearing system I have faith that I will be successful. It is another chapter, a collection of notes updating my advances. Indeed, there will be more to come.
Discussion
The joy of the marine aquarium hobby is that it is ripe for new frontiers in captive breeding possibilities and one can go as far as one likes even to make it a career and give life purpose. One of the greatest visionaries of modern marine aquatics, Martin Moe, showed us this path as the reefkeeping dawn emerged. Research institutions are breaking barriers in mariculture such that it literally is as simple as choosing any aquatic animal you want and chances are there are ways to rear them to adulthood. Choose a valuable animal to captive breed and you could single handedly save an entire species from over harvesting! The commercial constraints that still limit making most marine life tank raised is the investiture of man hours and dubious payback especially when it is still much cheaper to wild collect them. That indeed is a finite resource which is dwindling yearly and once gone will be a wake up call to consumers! Thus progress in mariculture is often limited to universities, research institutions, and individuals.
The Fire Shrimp, Lysmata debelius, is a prime example. My love affair with this animal began years ago when my aquatic abilities were poor as was that of the folks that brought them here. The inability to keep any alive at that time dismayed me and I was biased against ever keeping one again. Boy, how times have changed! My breeding pairs greet me daily with trusting forays into my hands and I am rearing hundreds of their larvae. My recent success breeding their close relative, the Peppermint Shrimp ( Lysmata wurdemanni ), in addition to learning of the success of some labs completing the life cycle of L. debelius and their advanced techniques convinced me to devote all my energy to this species.
As hermaphrodites, any two shrimp can form a pair. In broodstock pairing attempts both adults were moved to a totally separate environment which minimized territoriality issues. L. debelius is very aggressive towards conspecifics, tolerating only its mate in all but the largest of aquaria. The same 55 gallon tank that previously housed my adult peppermints became the broodstock home of four adult fire shrimp (two breeding pairs) separated by a tank divider with a large netting across the top (mesh large enough to allow larvae to pass through). The same larval collection device as described in my previous article is equally effective in isolating the zoeae (larvae) of fire shrimp and is currently employed for this species. The hatching of eggs, molting, and copulation cycle is identical to that of L.wurdemanni, yielding weekly batches of zoeae from each pair.
Conventional Rearing Tank
After my last batch of L. wurdemanni completed metamorphosis I used the same larval collection and rearing methodologies for the debelius zoeae. From literary sources I knew that the traditional bare tank and airstone setup would be inferior for the full development of the considerably more delicate zoeal stages of fire shrimp. Nevertheless I wanted to familiarize myself with them and make observations as I proceeded with construction of an advanced larval rearing tank known as a “planktonkreisel.” One improvement with the conventional rearing tank was the addition of a dedicated biological/chemical filtration system with a trickle tower, protein skimmer, and an activated carbon tower. Water was trickled in and recirculated back to the filter through a fine mesh fabric panel to retain larvae and their food.

Harvested copepods from peppermint shrimp growout trays are supplemental live prey for fire shrimp larvae.
May 24, 2004: First hatch obtained. Having read recent findings demonstrating that newly hatched debelius zoeae consumed significant amounts of Tetraselmis, a cryo-preserved phytoplankton was added directly into the larval collector the night of the hatch.
May 25: Second hatch obtained. At this time the larvae were in their first zoeal stage, approximately 2 mm. long, with pink coloration and lacking eyestalks. New hatch Artemia nauplii was added to the larval tank daily.
May 26: Larvae molted to their second zoeal stage, gaining eyestalks in the same manner as L. wurdemanni. No dead larvae were noticed. At this point daily feeds added were Artemia nauplii, phytoplankton, Cyclop-eeze, pulverized flakes, and later instar Artemia metanaupliiar stages present from prior addition. Rotifers were not included as they were unnecessary in L. wurdemanni culture, however, this subject is addressed again below.
May 27: Healthy larvae swim in a hovering formation, in a head-stand orientation in the lull zone of the upper area of the tank.
May 28: Larvae molted to what is apparently the third zoeal stage as they have more pronounced antennules and endopods.
May 30: Larvae seem to be swimming in a tumbling manner, unable to maintain control of their normal head-stand orientation. This is possibly indicative of weakened energy levels.
May 31: The strange appendages which characterize mature Lysmata larvae, frail, long, paddle-like “spear legs” (as Kirkendoll called them) were first seen. These are the pereiopods (parapodia) whose purpose is debated but is undoubtedly a major reason for the mortalities of zoeae due to injuries they sustain with aeration trauma.
Larval swimming was improved; more control of head-stand position. There was no observed interest in pulverized flakes at this point quite unlike L. wurdemanni which were observed eating flakes on their first day after hatch. Naturally that tendency gives peppermints a significant energy source and survival advantage. Also a remarkable variety of Artemia stages (to juvenile adult) were present in the larval tank likely due to phytoplankton dosing and water quality with biological and chemical filtration.
June 2: Larvae appear to have reached a fifth zoeal stage, being larger with pereiopods more elongate and apparently being used (as is thought) for steering through the water.
Fire shrimp larval coloration is vividly beautiful with hues of pink and gold in some variation. Moreover an even bluish tint of their antennules is noted. These zoeae were undoubtedly a tough batch as only 4 or 5 dead were noticed thus far. Additional hatches of larvae were periodically added to the rearing tank as they were obtained during this time.
Still there was no sighting of larvae eating flakes or Cyclop-eeze. Pulverized flakes were no longer offered in an effort to lower pollution levels.
Feeds now offered were Cyclop-eeze in the auto feeder, phytoplankton, and Artemia nauplii only. Some zoeae were observed consuming Artemia.
June 4: Still no observation of larvae eating Cyclop-eeze although the red coloration of many individuals would strongly suggest it. Some injured larvae were witnessed missing one or both pereiopods and a few more sightings of dead larvae were seen raising serious concerns about their survival. Conversely however, another large percentage of larvae seemed vibrant and active as always, leading me to believe a divergence of strong and weak may be occuring.
Since twelve days had passed and as of yet no zoea had been positively observed to eat flakes or Cyclop-eeze, the option to add another live zooplankton prey item, copepods, would be undertaken. Although I have been skeptical of aquarium copepods as an effective larval food (unlike Artemia), their abundant natural populations in the peppermint growout trays makes them a readily available alternative live food item. It seemed foolish to not take advantage of this resource as long as Artemia would still be offered daily. I believe this copepod may be a Calanoid Acartia species (the most popularly cultured) as they swim freely in swarms rather than the Harpacticoid specks crawling across the glass, but I am not sure. Pelagic copepods are undoubtedly a major part of natural larval crustacean diets.
June 5: There is an unmistakable trend in larval losses that very likely is tied in with aeration trauma. Many larvae are struggling with one injured pereiopod, having lost one or both. There really is no solution with this species’ later zoeal stages and aeration as reducing the air output may be gentler but the heavier biomass of mature larvae cause them to sink in the corners of the tank. There they tail-scoot around, swimming hopelessly to get back up in the water flow until they are exhausted and die. With stronger air output they stay suspended but are blown up into the bubbles so fast their appendages are twisted up or torn off. The 5th pereiopods of these later zoeal stages exhibit a frail, more delicate morphology subject to injury, stress, and subsequent microbial infection in an organically heavy environment.
July: The strongest of these held on to life a few more weeks but would never metamorphose as all their energy was expended on trying to swim in an inferior set of water flow conditions. I had expected this and the desire to see them in the optimized conditions of the planktonkreisel compelled me to complete its construction. Some of these remaining larvae were transferred to the kreisel for testing trials to observe how they would respond, thus their existence had had a meaningful purpose. A modest number of that strong original hatch actually lasted six weeks which said a lot for their determination to survive.
The “Plankton Carousel”
Soon after my first success with raising peppermint shrimp I became acquainted with the esoteric culturing techniques of meroplanktonic crustacea and the concept of the planktonkreisel. This type of advanced zooplankton design quickly became a new obsession and I was determined to create one. Primarily they are referenced in the keeping of medusae (jellies) and in public aquarium displays have been seen by many people.
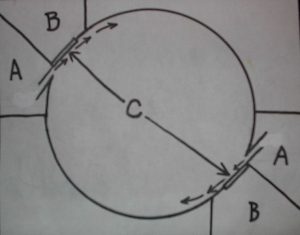
Planktonkreisel diagram with dual inflow (A), outflow (B), chambers and fabric screens (C). Zooplankton lives in tank’s round interior.
Getting detailed information on these systems is not easy. Many of the papers are only accessible through certain channels and the information is highly specialized. It would appear that there are two different types of “kreisels”, a cylindrico-conical upweller refined from no less than a century of lobster hatchery development and Greve’s 1968 Helgoland design, a tank with a rounded interior for keeping medusae from which the name kreisel (carousel) originates. The circular motion of this system is much like that of a Ferris Wheel. Gentle diffuse inflows maintain delicate planktonic forms in continuous supension and fine mesh fabric panels protect them and their food from passage back to the pump.
Palmtag and Holt (2001) described protocols for rearing L. debelius larvae in screened cylindrical downwellers and Calado, Narciso, Morais, Rhyne, and Lin (2002) described remarkable successes rearing a diverse number of decapod crab and shrimp larvae in cylindrico-conical upwellers. Nevertheless a simplified version of Greve’s Helgoland style kreisel became the chosen rearing system for this study in a 20 gallon extra-high tank partly due to the ease of modification by glueing in rounded bottom corners. This type of tank is also known as a “tumbler” which I call a “tumbler kreisel” or “downweller kreisel”. An extremely gentle, diffuse inflow of water is deposited via a spraybar at one top corner creating a downwelling circular movement. A slanted removeable fabric screen is located in the other top corner for water outflow with a bubble curtain positioned at its base preventing larvae from getting stuck against it. Since the bubble curtain is so close to the top with only a low output it doesn’t cause damage to larvae as would a deeper air release.
Throughout August detailed refinements were made on the kreisel particularly concerning the spraybar, its flowrate, and the fabric screen panels which had to slide perfectly into a groove. By September all the kinks had been worked out and it was ready to receive larvae.
Sept., 2004: A decision was made to put the larvae on a reverse photoperiod for a couple of reasons. Since I worked during the day, having the larvae’s daylight on throughout the night enabled me to be around for closer monitoring and I wasn’t pressured to start my Artemia hatches as much as when trying to get to work in the morning. Also the most important reason was that the sooner larvae get food the better their survival would be into their first week. Hatches typically occur around 1:00 AM and the quick subsequent transfer into the kreisel by 2:00 with new hatch Artemia nauplii available provided immediate nutrition and minimized potential energy loss swimming in the collector overnight.
This was a great project for an insomniac like myself!
The planktonkreisel allows for better viewing of larvae. The previous tank’s aeration marred visibility with so many tiny bubbles in the tank. Some further observations made at this time reflected the energy status of early stage zoeae.
Phototactic responses of L. debelius zoeae are seemingly not as pronounced as with L. wurdemanni. Phototaxis is displayed in stronger, successfully feeding zoeae.
Zoeae with red pigmentation exhibit higher survival than discolored ones. A definite trend I observed was that many larvae had a pink-red coloration and those were unquestionably the stronger individuals. Other larvae with a washed out, grey, or white coloration apparently had received a lesser percentage of nutrition from the adult during embryonic development. Usually those would become white corpses by the next day. Twice daily feedings of broodstock pairs with the highest quality feeds, rich in protein, vitamins, and omega 3 fatty acids is critical for larval survival as nutrition is passed to embryonic larvae before hatch.

Current Lysmata rearing system. the left tank will be used for cleaner shrimp larvae. One of the interchangeable screens is visible in front of the skimmer/biofilter.
Lysmata zoeae are poor swimmers and depend upon the buoying currents of the open sea for suspension in nature. They swim by paddling their legs up and down and the rate of speed of these movements is an indicater of their energy status and as to whether they were successful in Artemia predation up to that time. This is an important factor especially on their second day of life as to whether they will make it to their next zoeal stage when they gain eyestalks.
Debelius larvae take two days to advance to their second stage (clearly discerneable from appearance of eyestalks) unlike L. wurdemanni which usually molt to that stage after their first day.
Currently a regimen has developed with the new rearing system which begins at 9:00 PM when I’m home from work. Two fluorescent lights click on over the kreisel tank beginning the larvae’s photoperiod that lasts throughout the night. The flow through the spraybar is stopped and the water level is lowered in the tank below the screen’s position. Then the screen which has a larger mesh (allowing older Artemia instar II metanauplii to flush out over the day) is replaced with a finer mesh screen than retains the newly added Artemia instar I nauplii. The water level is brought back up and spraybar inflow is resumed.
A new batch of Artemia cysts are pre-hydrated in freshwater for about 30 minutes and then aerated in 1.017 density saltwater for the next 24 hours. The Artemia culture from the previous evening is harvested into a coffee filter and then added to the kreisel for that day’s (reverse daytime) feeding. Water flow to the biological/chemical filtration is suspended throughout the photoperiod so as not to diminish phytoplankton levels which are also added. When larvae reach their later zoeal stages, Cyclop-eeze in the auto feeder and live copepods are also added in addition to the Artemia and phytoplankton.
In the morning the circulation to the biological/chemical filtration is resumed. The flow through the spraybar is again suspended, the water level lowered, and the finer mesh screen replaced with the other larger mesh screen allowing uneaten Artemia to flush from the tank. Water level and flows are resumed and the lights are clicked off with the entire tank dark all day long while I’m at work.
Rotifers and Lysmata Larviculture
If one were to ask the pertinent question, “Are rotifers necessary to successfully rear Lysmata larvae?” I would say “no”. However, from hours of observation of newly hatched zoeae, I would have to add that feeding sizeable quantities of rotifers during the first few days along with the ample Artemia nauplii ration will give greater survival of zoeae through the first week. In fact, typical protocols for rearing many decapod larvae call for Brachionus plicatilis being fed the first day exclusively and switching to Artemia the next. My belief is that ample quantities of both Brachionus and Artemia should be offered during the first week of Lysmata larval life for optimum survival. Ingestion of unicellular microalgae by newly hatched zoeae has been demonstrated and co-culturing phytoplankton along with zooplankton in the rearing tank should be standard. Very likely the addition of a third live prey, late stage, pre-metamorphose pelecypod veligers (approximately 250 microns) would
even more fully optimize results during the first zoeal stage. Traditional methodology calls for the successive stages of enriched Artemia metanauplii to subadult and adult be available (along with pureed seafoods) to the zoeae as they gain size and maturity. The swimming movements of tiny live foods appear necessary for stimulating successful predation responses in early stage Lysmata debelius and L. amboinensis zoeae.
The difference that can enable a Lysmata zoea to get by the first couple of critical days of life on new hatch brine shrimp alone hinges on the fact that they have the ability to grab onto and chew a particle unlike many fish larvae, notably clownfish, which can only swallow whole a small soft food particle, namely, rotifers. Artemia nauplii are simply too big and spikey for new fish larvae. Moreover even when the larval fish is big enough for Artemia it is important that there be no cyst shells present to choke on. Cyst shells, though undesireable, don’t pose a problem for Lysmata zoeae aside from pollution and clogging of screens. Most labs would logically decapsulate them. There is absolutely no doubt that L. wurdemanni can be successfully reared in large numbers on Artemia alone by virtue of the unique fact that they actively grab at larger objects as stated above inculding pulverized flake. Not so for L. debelius or L. amboinensis (the Cleaner Shrimp) which are very passive
indeed. In her book How to Raise and Train Your Peppermint Shrimp, April Kirkendoll summed it up best in the chapter on rearing L. amboinensis (and my observations would be very similar for L. debelius) stating, “_You can raise the cleaner shrimp larvae on newly hatched brine shrimp but you will lose significant numbers of them during the first week. Only the largest and most willing to grab brine shrimp will survive. You will get more to survive if you raise rotifers to feed them for at least the first one or two stages”._ So there it is. Only the strongest fire shrimp larvae that can successfully grab Artemia nauplii on the first day will make it into their first week.
Outlook
Perfecting this system has been an all-summer-long project which has been extraordinarily frustrating at times yet exhilirating overall. To recreate the naturally gentle circular motion of pelagic zooplankton under the waves and to await the eminent appearance of the successive stages of fire shrimp larvae makes this an exciting, pivotal time in my work. Now it remains to be seen if the superior suspension characteristics of the planktonkreisel yields the success it would seem to promise.
Another likely challenge will be supplying settlement cues to induce metamorphosis in Lysmata debelius. That seems to be a wide open question, but the later feeding of adult Artemia, larger enriched frozen food items (which the kreisel keeps effectively suspended) and the placement of textile strips for postlarval settlement will be good strategies to try.
When I saw pictures of tiny postlarval fire shrimp from the English labs I knew that this Holy Grail of aquaculture achievement is possible. I have faith that I am nearing the destination of my pilgrimage.
References
- R. Calado, L. Narciso, S. Morais, A. L. Rhyne, and J. Lin, 2002. A Rearing System for the Culture of Ornamental Decapod Crustacean Larvae.
- A. Kirkendoll, 2001._How to Raise and Train Your Peppermint Shrimp._
- F. Nicosia and K. Lavalli, 1999. Homarid Lobster Hatcheries: Their History and Role in Research, Management, and Aquaculture.
- F. Hoff and T. Snell,1987. Plankton Culture Manual.
- M. Moe, 1989. The Marine Aquarium Reference, Systems and Invertebrates.
- J. Lin, D. Zhang, and A. Rhyne, 2002. Broodstock and Larval Nutrition of Marine Ornamental Shrimp.
- M. R. Palmtag and G. J. Holt, 2001. Captive Rearing of the Fire Shrimp.
- F. Simoes, F. Ribeiro, and D. A. Jones, 2002. Feeding Early Larval Stages of Fire Shrimp, Lysmata debelius (Caridea, Hippolytidae).
- W. Greve, 1968. The “Planktonkreisel”, a New Device for Culturing Zooplankton.





0 Comments