We are here for the second article in the Salifert test series, introducing the two remaining test kits in the triad: The Salifert KH and Mg (Carbonate Hardness / Alkalinity and Magnesium) tests.
As with calcium, it is essential to be able to measure carbonate hardness and magnesium values in the marine aquarium; only then can you properly supplement and have healthy, well-growing hard corals. In fact, periodic water changes are not meant to keep the triad stable!
Packaging and contents
Salifert tests for KH and Mg look very similar to the calcium test. A small cardboard box with everything you need inside to run 100-200 KH tests (again depending on your aquarium KH you will use more or less reagent) and 50 Mg tests.
The KH test comes with two liquid reagents, two syringes and the plastic test tube (as well as multilingual instructions, not in Italian but in Dutch, French, German, and English, at least in the German version). The Mg test has an almost identical supply but the reagents (logically) are for measuring magnesium.

Even for these tests, the components are “in bulk” in the box, and we have to gloss over the very economical aspect, which we find easy given the price.
Usage

To measure KH with the Salifert test, you need to take 4 ml of water from the aquarium with the syringe provided and put it into the test tube (we previously rinsed it with aquarium water). Next, 4 drops of KH-Ind reagent are added and mixed by shaking the test tube for 5 seconds. Then we aspirate 1 ml of KH reagent with the “precision” syringe to which we will have added the pink nozzle. Also for this test, it is important to note that when we aspirate the KH liquid we must bring the plunger of the syringe to the 1 ml mark and not the liquid itself. Also the readings of the values on this syringe should be done by looking at the base of the plunger, regardless of the liquid level (this also applies to magnesium).
Adding the liquid KH drop by drop and shaking the test tube a couple of seconds between drops you must wait for the color change from blue to pink (the opposite of calcium!). Once that happens, read the value on the syringe (i.e., where the base of the plunger is) and compare it with the table in the instructions to find out the KH value in German degrees. In this case, if we are in between two values the calculation is more difficult, since 0.02 ml of reagent corresponds to a change of 0.3-0.4 dKH (depending on the values). In this case we would certainly have preferred a scale that is easier to interpret. In fact, the table allows us to measure values between dKH 0 and 15.7 but we would have preferred to focus on “useful” values and a higher accuracy.
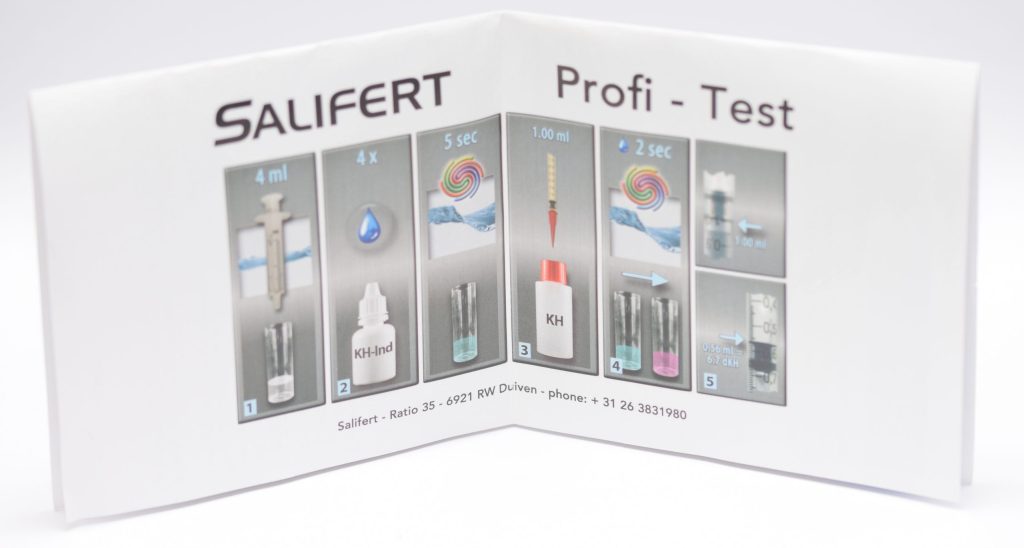
In my case, I measured a value of 0.48 ml, which corresponds to 8.0 dKH. Salifert indicates an alkalinity value of 7-8 dKH, which means my aquarium is in range.
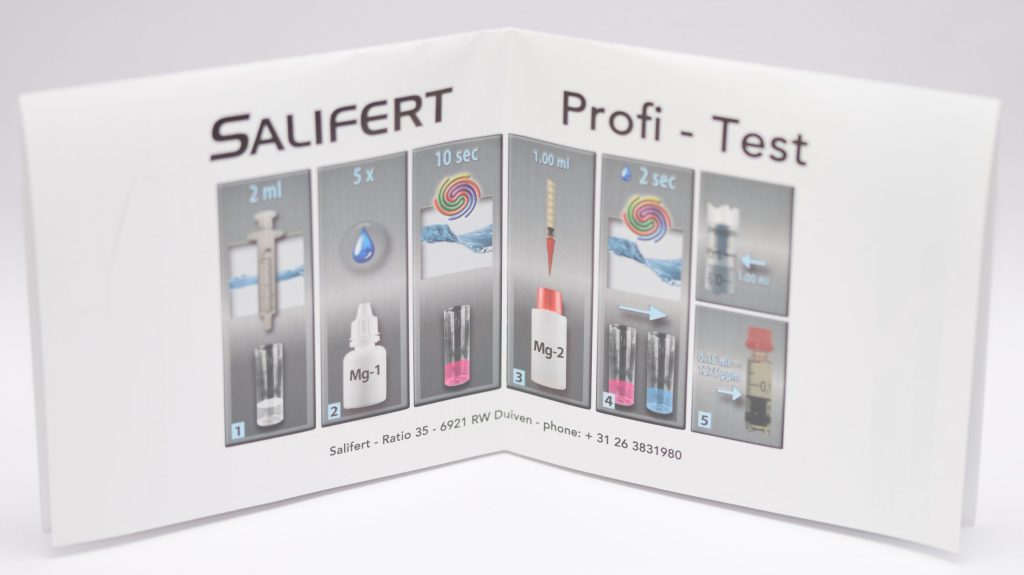
The magnesium measurement is similar, but with different amounts of water and reagents: In fact, only 2 ml of aquarium water and 5 drops of Mg-1 reagent are used, which must be shaken for 10 seconds before starting to add the second reagent drop by drop. The color change from pink to blue also occurs here. In this case 0.02 ml of reagent corresponds to 30 mg of magnesium, and values from 30 ppm to 1500 ppm can be measured.
To find out what values are recommended check our article: The ideal pollutant and triad values in the marine reef aquarium.
Quality
A test must have basically 3 characteristics to be valid. First, it must always show the same value when repeated, without fluctuating too much (accuracy); second, it must not depend on the person or conditions present when the test is taken (reliability); and, crucially, the value must show the correct value (correctness)!
Accuracy
For KH, the table provided allows values of 0.02ml to be read, but with the syringe we can identify changes as small as 0.01ml (with good light and good eyesight). The interpolation of the table is rather complicated, compared to calcium. So considering only the values on the table and choosing the one that comes closest to our result, the test has an accuracy of 0.3-0.4 dKH. The same applies to magnesium, where the distance between the values on the table is 30 ppm magnesium.

Reliability
To check reliability, I ran the test three times at close range (so that the parameters in the aquarium could not be changed). I did not have a third person run the test to check if the values came out the same, but I consider that in certain “doubtful” situations (i.e., in the middle of the color change) two different people may or may not add a drop of reagent, I myself was undecided, as if in some cases a “half drop” was needed, as in calcium. Therefore, I would say that the test result may vary by about 0.2 dKH in measuring carbonate hardness and 15 ppm in measuring magnesium, to be added to the previous value.
For this reason, I consider the results of a water sample made with the Salifert KH/Alk test to have a maximum fluctuation of 0.5-0.6 dKH. For magnesium, on the other hand, the oscillation is as high as 45 ppm. Unfortunately, we had a single Salifert KH and Mg test available, so we cannot say whether there is variation between different batches.
In the three measurements taken for KH I measured values on the syringe of 0.48, 0.47 and 0.49 ml, which correspond to 8.0, 8.15 (interpolating) and 7.85 (also interpolated). The oscillation was thus 0.3, as expected.
For magnesium, the measurements were 0.22, 0.17 and 0.22, corresponding to 1170, 1245 and again 1170, with an error of 75 ppm. This value is beyond the range I expected (and was measured trying to be as quick as possible) and again stands to show how one must take the time to perform the tests with absolute precision and concentration. In fact performing these tests takes a certain amount of time. I was undecided whether or not to include the 1245 value in the average to measure its correctness, but decided that since it was beyond tolerance, this test should be considered null.

Correctness
To verify the accuracy of the I Salifert KH and Mg tests, I sent a sample of my aquarium water to Triton. The KH value unfortunately is not indicated by the Triton test, so we will focus on magnesium. With the Salifert “successful” tests I got a value of 1170 ppm, the Triton test done with the same water shows a value of 1260 mg/l.
It should be noted that although it is often said that ppm equals mg/l this is incorrect, since ppm is milligrams per kg and not milligrams per liter. A liter of pure water actually weighs one kg (and so in fresh water this is an absolutely correct simplification). In seawater, salt leads to a density of 1.023 kg/l, so it is correct to make a transformation from ppm to mg/l before comparing the two values.
In practice: The 1170 mg/l indicated by the Sailfert test corresponds to 1197 mg/l (as the Salifert test package insert also tells us – In this I must say brava Salifert!). Compared to the actual value (Triton) of 1170 mg/l we are faced with an error of 2.3%, a very good value! Even with regard to magnesium dosing in the aquarium, an error of less than 30 mg/l is absolutely tolerable.
While I have not had a chance to compare KH values I believe they may be similar to calcium and magnesium (i.e. 2.5% – 5% error). This would be very good. On the other hand, the total accuracy of the test of 0.5-0.6 dKH seems to me to be too low to calculate the dosage and set up dosometric pumps. It will not be impossible but will require a few more days and several measurements. In this case other tests have higher accuracy.

In conclusion
The Salifert KH and Mg (carbonate hardness and magnesium) tests are similar to the calcium test: cheap but workable. The magnesium accuracy is sufficient and the test proves correct when compared to Triton. KH unfortunately has a slightly too wide scale to set our dosimeter pumps but can give us a good indication for any imbalances in the aquarium.
For this reason, I would recommend these tests, especially because of the excellent value for money. If you want an impeccable KH measurement, however, you’d better look elsewhere.
[Text by Simone D’Archino, translated by Aisja Baglioni]










0 Comments