
Last time we explored the methods at our disposal for replenishing the foremost trace elements from which Scleractinia build their skeletons. Several other scarcer and important ions become rapidly depleted in recirculating systems while others prevail despite a lack of supplementation. This extensive discipline is largely beyond the scope of this series; however, the author promises a fact-packed journey highlighted with some intriguing examples.
Granular ferric oxide (GFO) proficiently moderates dissolved inorganic forms of silicon (Si) and phosphorus (P) while it exerts a less significant impact on other ions (Riddle 2012).
The first microorganisms that newcomers encounter form golden-brown veneers on the surfaces of new setups for approximately a month to a month and a half of strong illumination. Several of these photosynthetic chromists of the infrakingdom Heterokonta are responsible for amnesic shellfish poisoning which manifests after people eat domoic acid-contaminated bivalves (Perl et al. 1990; Teitelbaum et al. 1990; Hasle 2002; Lu et al. 2012; Smith et al. 2017) where toxigenesis may be linked to chlorophyll a, temperature, and the bioavailability of iron (Fe; Trainer et al. 2009). Diatoms are a minor a yet significant nuisance in aquaria while their silicic acid (H4SiO4)-fueled blooms are referred to as “new tank syndrome” (Desikachary & Dweltz 1961; Maher et al. 2018) which likely originates from natural marine substrates.
Such coral sands and gravels have become obsolete of late and suppliers are selling terrestrially oolitic limestone as equivalents, which is amorphous and fossilized and as such, is not microscopically porous. These properties may be immaterial to the modern reef enthusiast inasmuch as they can purchase nitrogen-cycling sump bricks. Nevertheless, terrestrial limestone aggregate may or may not be associated with significant silicic acid.

Several “reefers” favor a scattering of substrate because LED luminaires lack meaningful backscatter and anything that enhances underside illumination is worthwhile. Side welling is just as important for branching colonies because they self-shade with stationary illuminants where LEDs were, and most probably still are, deficient. Metal halide lamps and fixtures produce potent backscatter, and their intensity satisfies the most light-demanding of SP hermatypes yet their upkeep and operating costs are exorbitant.
Diatoms build their cell walls from hydrated silicon polymerized by a series of condensation reactions which liberate water, whereas intracellular silicic acid is maintained above saturation so the frustules of living diatoms do not dissolve (Holmes-Farley 2003). The remnants of diatomaceous silicon are extensively recycled in surface waters and on their way to the abyss (Van Cappellen et al. 2002; Loucaides et al. 2012) yet remarkably, and fortuitously, the frustules of deceased diatoms are insoluble at marine aquarium pH.

Fig 1. A micrograph of Nitzschia cf. acicularis var. acicularis (Kützing) W. Smith 1853. Image courtesy of Antonio Guillén©, Proyecto Agua, Flikr.com.
Pseudo-nitzschia species belong to the kingdom Chromista, the subkingdom Harosa, the phylum Ochrophyta, the subphylum Khakista, the class Bacillariophyceae, the subclass Bacillariophycidae, the superorder Bacillariophycanae, the order Bacillariales, and the family Bacillariaceae (WoRMS 2022).
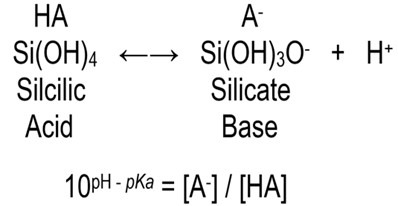
where square brackets represent concentration
pKa in seawater = 9.472
[A–] / [HA] @ 1/12 at pH 8.4
(Holmes-Farley 2003; Exley & Sjöberg 2013)
Silicic acid and its conjugate base silicate are in equal concentrations at pH 9.472 (100 = 1) which means the solution has reached optimal buffering.
Referred to as raphid diatoms and represented in phytoplankton, Pseudo-nitzschia species exploit a slit-like raphe that facilitates substrate adherence and gliding using polysaccharide-saturated microfibrils (Poulsen et al. 1999). Yet diatoms are exclusively associated with surfaces in reef aquaria (Lundholm & Moestrup 2002; Rines et al. 2002; Levialdi Ghiron et al. 2008; Lu et al. 2012) because captive waterborne “algal” blooms require around 1 mg l-1 (ppm) PO43- to subsist.

Fig 2. Food-grade diatomaceous earth (D.E.) is an animal and human dietary supplement comprising freshwater frustules.
Diatoms are so proficient at “scrubbing” environmental silicon that the bloom permanently subsides within two months. However, silicon is important to wild reef ecology and the captive demise of molluscs may be due to silicon dearth. They feed using a chitinous ribbon-like cutting/rasping appendage called a radula that requires iron (Fe) and silicon (Si) for its development and regeneration (Tzu-En & Chia-Wei 2007; Shaw et al. 2008; Faivre & Godec 2015). Soluble silicon may be important for developing larvae whereas dietary silicon is likely pertinent for snails; hence silicious microorganisms may be central to the mariculture of herbivorous molluscs (Holmes-Farley 2003).
Doctor Holmes-Farley’s dosing experiments utilized water glass which is an archaic solution of sodium silicate for preserving eggs. It is caustic and requires dissolving in a significant amount of reverse osmosis (R/O) water and drip feeding into a region of turbulent flow over many hours (Holmes-Farley 2003).
The author weighed out 1 gram of 50 percent waterglass and drew it into a 1 milliliter syringe and divided the volume by four to yield around 0.25 grams which raised the concentration of sodium silicate to ~0.28 mg l-1 (ppm) when dissolved in 500 milliliters of R/O water and administered dropwise to a 100 UK-gallon (120 US-gallon; 454-litre) system over several hours.
Diatomaceous blooms take around 10 days to attain momentum so be patient because overdosing is ecologically destabilizing.
Alternatively acidic calcium reactor effluent is ideal for dissolving substances that are refractory at seawater pH. The food grade human and animal health supplement, diatomaceous earth (D.E.) is the frustules of freshwater diatoms. Salifert® phosphate tests are supplied with a small red scoop, where one heaped scoop of D.E. for every 60 liters of system volume is solubilized each week in the effluent from a calcium reactor’s granular ferric oxide (GFO) column. See: Part V.
The above approach led to an upsurge of molluscan recruits that have kept pace with algal abundance now for several years.

Centralized block d transition elements of the periodic table have partially filled diffuse (d) or fundamental (f) electron subshells which excludes zinc due to its full 3d10 (Table 1.; Brockington et al. 1981). Iron (Fe; 3d6) is a valuable biochemical resource that all organisms require and amass (Bury et al. 2003) where animals acquire dietary Fe hence life can thrive throughout limited dissolved inorganic iron (DIFe). Fe is a conspicuous REDOX partner in several cellular processes while its variety of oxidation states makes it a versatile substrate and potent reducing agent for microbial exploitation. Remarkably the haem porphyrins (C34H32FeN4O4) of haemoglobin have centrally coordinated ferrous (Fe2+) or ferric (Fe3+) ions when they reversibly bind dioxygen (O2) in the erythrocytes of vertebrate blood (Fig 3.).
Microorganisms and some macroscopic photosynthetic life liberate low molecular weight iron-binding ligands called siderophores that are later scavenged and internalized which sustains an environmental reserve available to a mere few (Butler & Theisen 2010; Pérez-Pascual et al. 2017; Sanchez et al. 2018; Basu et al. 2019).
Most oceanic Fe is bound or chelated (Hopkinson & Morel 2009) which sustains DIFe well below saturation and expedites solubility (Sanchez et al. 2018) while marine “algae” are likely to uptake Fe via reduction from bound ferric to free ferrous (Hopkinson & Morel 2009).
Significant iron-rich atmospheric dust enters the oceans annually where some is captured and utilized by surface “algal” blooms like those of the cyanobacterial genus Trichodesmium (Zehr & Kudela 2011; Gradoville et al. 2017). Diazotrophs like cyanobacteria use the enzyme nitrogenase which engages an iron, molybdenum (Mo), and sulphur (S) complex (FeMo-co; Raugei 2019) to cleave and assimilate atmospheric or dissolved dinitrogen (N2) as ammonium (NH4+) which tends to leak environmentally (Lobo & Zinder 1988). Hence cyanobacteria sustain dissolved inorganic nitrogen (DIN) in the nutrient-poor ecosystems common to pristine wild reefs, yet they contribute to nitrate (NO3–) enrichment in aquaria.
DIFe limitation inhibits all microbes including pathogens and vital nitrifying biofilter consortia (Holmes-Farley 2002; Feldman et al. 2011; Keuter 2017) which is so efficacious that iron sequestration is a vital immune maneuver utilized in countless animals like humans and fish (Butler 1998) where “blonde” faeces are the harbinger of disease.
Fe is required for molluscan radulae (Tzu-En & Chia-Wei 2007; Shaw et al. 2008; Faivre & Godec 2015) while it and its neighboring transition element manganese (Mn; 3d5) are prerequisites for chlorophyll biosynthesis and indispensable co-factors for at least one member of the antioxidant enzyme family, superoxide dismutase (SOD) without which photosynthesis cannot occur (Rodriguez-Lanetty et al. 2005; Balling et al. 2008).
Zooxanthellae’s stress tolerance may be Fe and Mn dependent, where bleaching-promoting ionic dearth may render them defenseless (Balling et al. 2008).
The reef-cooling, upwelling-energizing trade winds are abating in the Pacific which diminishes SODs and expedites bleaching insofar as intermittent pulses of chilled water and nutrients like Fe and Mn remain on the seafloor (NOAA, cited in Wallace 2014). Notwithstanding, SODs disarm reactive oxygen species (ROS) which are useful photosynthetic intermediates that are unlikely to leak from zooxanthellae and “trigger” severance from their host (Nielsen et al. 2018).
Mn2+ associate with potentially harmful lanthanum ions (La3+) in the tissues of fresh- and salt-water organisms (Sardet et al. 1979; Herrmann et al. 2016) while elevated Mn2+ exert long-term deleterious impacts on aquatic ecosystems (Holmes-Farley 2003a; Aslett 2024).
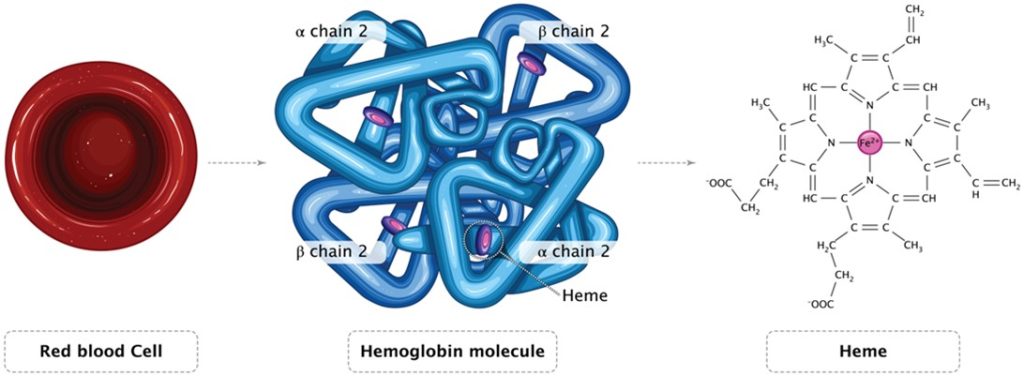
Fig 3. An illustration of an erythrocyte, the mammalian haemoglobin tetramer, and unbound ferrous haem which binds O2.
Anaerobic sulphate-reducing microorganisms preferentially reduce Fe3+ and Mn2+ over nitrate (NO3–) and sulphate (SO42-) which may stall euxinic denitrification, whereas the insoluble or colloidal sedimentary oxides of Fe and Mn are “swallowed” by marine substrate which supplies oxidation potential to anoxic zones (Nealson & Saffarini 1994).
Captive Goniopora stokesi wasting syndrome may be caused by an ionic deficiency which appears forestalled when seawater percolates through Hawaiian pumice (Sprung 2002).
Enriched Fe may inhibit skeletal accretion and exacerbate bleaching, whereas traces of Mn mitigate pigment loss and boost chlorophyll production and photosynthetic efficacy and rate (Biscéré et al. 2018).
Euphotic zone seawater comprises approximately 6 x 10-3 ppb (µg l-1) of iron most of which is ferric (Fe3+) due to the instantaneous oxidation of ephemeral ferrous (Fe2+), while a minority is represented by soluble ferric hydroxide (Fe(OH)3; Holmes-Farley 2002).
As with any potentially dangerous procedures, the reader prepares and uses these DIY supplements at their own risk, because the author, Reef Ranch Publishing Ltd, websites hosting this article, and their owners cannot be held responsible for any harm to pets, people, possessions, or properties.
Finfish diets are iron-rich because their requirements are challenging to appease (Bjørnevik & Maage 1993) while DIFe is rapidly depleted in recirculating reefs which may approach limitation every third day.
The lattice of crystalline water-soluble compounds retains water of crystallization, which impacts their mass and thus the amount required to concoct solutions of exacting ionic strength. Examples include copper II sulphate pentahydrate (CuSO4.5H2O) and magnesium sulphate heptahydrate (MgSO4.7H2O), whereas anhydrous (“dry”) chemicals lack water like CuSO4 albeit their hydration may be exothermic which evolves heat.
Doctor Holmes-Farley suggested dissolving 25 mg of food- or pharmaceutical-grade ferrous sulphate heptahydrate (FeSO4.7H2O) and 50.7 mg of trisodium citrate dihydrate (C6H5Na3O7.2H2O) in 250 ml of R/O water (Holmes-Farley 2002). Photodegradation-susceptible trisodium citrate chelates the ferrous ion (Fe2+) which precludes its oxidation but expedites its liberation in well-lit seawater (Holmes-Farley 2002; Valliere-Douglass et al. 2010).
Analytical balances weighing grams to five decimal places are upwards of 400GBP, while 100 or 1,000 times more concentrated stock solutions from which aliquots are taken to constitute the supplement are created when solubility allows. 10 g of FeSO4.7H2O and 21 g of C6H5Na3O7.2H2O are thoroughly dissolved in 100 ml of R/O water, from which 0.25 ml are added to 249.8 ml of R/O water to create the supplement.
Concentration lends stability and trisodium citrate retains significant antimicrobial properties (Nagaoka et al. 2010) while iron-rich solutions remain vulnerable to microbial attack. Besides, it is good practice to perform a procedure somewhat analogous to a botulinum cook which extends DIY preparation longevity and excludes the likely unbeneficial preservatives and chelating agents of proprietary supplements.
Source 300-ml polypropylene (PP) opaque bottles that withstand short bursts of 180oC (356oF) fitted with tilting disc-top or turret caps (Fig 4.).
- Fig 4. Tilting disc-top and turret caps. Both help forestall contamination but the former offers superior protection because the user handles the latter’s spout.
Botulinum cooks are used in the canning industry to kill Clostridium botulinum which is an anaerobic bacterium that produces a heat stable potent and deadly toxin, where foods are heated to 100oC. Six people died in the 1970s within a few hours of eating salmon from a perforated tin.
Stand the 300-ml bottles containing the 250-ml supplements with their tilting disc or turret caps fitted but open in a pan of cold R/O water to a level just beneath that in the bottles. Sealed bottles may explode so allow for expansion. Heat the pan until the water fully boils, withdraw the heat, cool to room temperature, and close the lids. Alternatively try submerging in a digital temperature-controlled kettle or support bottles using eggcrate fashioned frames. Full sterilization occurs in hob pressure cookers at 120oC for 15 minutes; however, some preparations may be affected by heat and you will require glass bottles but ensure their metal caps are loosely fitted.
Painstakingly label all bottles, keep them and compounds out of reach of children and pets, and store in a dark refrigerator from 4 to 6oC (39 to 43oF).
Seawater manganese ranges from ~8 x 10-2 to ~1 x 10-1 ppb (µg l-1; Sarzanini et al. 2001).
Manganese (II) chloride is an eye irritant and extremely toxic on contact, where nominal concentrations prove harmful to aquatic ecosystems. Wear PPE including clothing, gloves, goggles, and a fine particle facemask, and avoid ingestion, inhalation, and wash the chemical immediately from skin.
Thoroughly dissolve 20 g of manganese (II) chloride tetrahydrate (MnCl2.4H2O) and 57 g of trisodium citrate dihydrate (C6H5Na3O7.2H2O) in 100 ml of R/O water. Combine 0.25 ml of this stock solution with 249.8 ml of R/O water or the earlier iron preparation, botulinum cook, store in a dark fridge out of reach of children and animals, label the bottles as poison, and mark with a skull and crossed bones.
Administering 0.1-ml three times per week in a 250 US-gallon (208 UK-gallon; 944-litre) fully stocked reef abundant in ornamental algae, raises Fe2+ to a near-natural 2 x 10-3 ppb (µg l-1) assuming depletion every 48 hours (Holmes-Farley 2002) while Mn2+ is fortified by a conservative 5.9 x 10-3 mg l-1 (ppb; Sarzanini et al. 2001).
Table 1. The periodic table of elements.
There are 20 drops in each milliliter, and please adhere to dosing recommendations because we are supplementing ions that are not easy to quantify. Fe2+, Mn2+, and trisodium citrate appear to increase the incidence of rapid tissue necrosis (RTN) and are refractile. Boosting microbial populations and their metabolism will likely reduce microoxic calcium reactors and denitrifyers to anoxic; which may destabilize the mucosal microbiomes of corals which are their first line of defense.
The author retains a DIY Fe and Mn supplement that was botulinum cooked five years ago. The cap has been removed, and aliquots have been taken thrice weekly with a 1-ml syringe, while its straw coloration and lack of sediment remains redolent of an abundance of ferrous ions, insofar as spoiled preparations assume the appearance of a clear liquid with a rusty brown sediment (Holmes-Farley 2002).
Next we illuminate the role of the other rarer and readily expended trace elements of seawater and their replenishment as this journey unfolds.
To learn more, visit our website HERE
References
Aslett, C., G. (2024) The Complete Reef Aquarist, for hobbyists, the trade and academics – A Conservation Manual. Aslett, C., G. (ed.). Reef Ranch Publishing Ltd, Leeds, West Yorkshire, UK.
Balling, H., W., Janse, M. & Sondervan, P. (2008) Trace elements, functions, sinks and replenishment in reef aquaria. Advances in coral husbandry in public aquariums. Leewis, R., J. & Janse, M. (eds.). Burgers’ Zoo, Arnhem, The Netherlands. pp 143-156.
Basu, S., Gledhill, M., Beer, D., Prabhu Matondkar, S. & Shaked, Y. (2019) Colonies of marine cyanobacteria Trichodesmium interact with associated bacteria to acquire iron from dust. Communications Biology. 2(1), 1-8.
Biscéré, T., Ferrier-Pagès, C., Gilbert, A., Pichler, T. & Houlbrèque, F. (2018) Evidence for mitigation of coral bleaching by manganese. Sci Rep. 8, 16789.
Bjørnevik, M. & Maage, A. (1993) Effects of dietary iron supplementation on tissue iron concentration and haematology in Atlantic salmon (Salmo salar). Fiskeridirktratets Skrifter, Serie Ernæring. 6, 35-45.
Brockington, J., Stamper, P., J., Browning, D., R. & Skinner, A., C. (1981) Combined Chemistry. Brockington, J., Stamper, P., J., Browning, D., R. & Skinner, A., C. (eds.). Longman Group L:imited, Essex, UK. pp 617-618.
Bury, N., R., Walker, P., A. & Glover, C., N. (2003) Nutritive metal uptake in teleost fish. Journal of Experimental Biology. 205(1), 11-23.
Butler, A. & Theisen, R. (2010) Iron(III)–siderophore coordination chemistry: Reactivity of marine siderophores. Coordination Chemistry Reviews. 254(3), 288-296.
Butler, A. (1998) Acquisition and Utilization of Transition Metal Ions by Marine Organisms. Science. 281(5374), 207-209.
Desikachary, T., V. & Dweltz, N., E. (1961) The chemical composition of the diatom frustule. Proceedings of the Indian Academy of Sciences – Section B. 53, 157-165.
Exley, C. & Sjöberg, S. (2013) Silicon species in seawater. Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy. 117,.
Faivre, D. & Godec, T., U. (2015) From Bacteria to Mollusks: The Principles Underlying the Biomineralization of Iron Oxide Materials. Angew. Chem. Int. Ed. 54, 4728-4747.
Feldman, K., S., Place, A., A., Joshi, S. & White, G. (2011) Water: Baseline Values and Modulation by Carbon Dosing, Protein Skimming, and Granular Activated Carbon Filtration. AdvancedAquarist.com. https://www.advancedaquarist.com/2011/3/aafeature
Gradoville, M., Crump, B., Letelier, R., Church, M. & White, A. (2017) Corrigendum: Microbiome of Trichodesmium Colonies from the North Pacific Subtropical Gyre. Frontiers in microbiology, 8, 1780-1780.
Hasle, G., R. (2002) Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae. 1(2), 137-146.
Herrmann, H., Nolde, J., Berger, S. & Heise, S. (2016) Aquatic ecotoxicity of lanthanum – A review and an attempt to derive water and sediment quality criteria. Ecotoxicology and Environmental Safety. 124, 213-238.
Holmes-Farley, R. (2002) Chemistry and The Aquarium: Iron in A Reef Tank. AdvancedAquarist.com. https://www.advancedaquarist.com/2002/8/chemistry
Holmes-Farley, R. (2003) Silica in Reef Aquariums. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/1/aafeature1
Holmes-Farley, R. (2003a) Aquaria with low soluble metals. ReefKeeping.com. Volume 4. http://reefkeeping.com/issues/2003-04/rhf/feature/index.htm
Hopkinson, B. & Morel, F. (2009) The role of siderophores in iron acquisition by photosynthetic marine microorganisms. BioMetals. 22(4), 659-669.
Keuter, S. (2017) Longterm Monitoring of Nitrification and Nitrifying Communities during Biofilter Activation of Two Marine Recirculation Aquaculture Systems (RAS). International Journal of Aquaculture and Fishery Sciences. 10.17352/2455-8400.000029.
Levialdi Ghiron, J., Amato, A., Montresor, M. & Kooistra, W. (2008) Plastid inheritance in the planktonic raphid pennate diatom Pseudo-nitzschia delicatissima (Bacillariophyceae). Protist. 159(1), 91-98.
Lobo, A., L. & Zinder, S., H. (1988) Diazotrophy and Nitrogenase Activity in the Archaebacterium Methanosarcina barkeri 227. Applied and environmental microbiology. 54(7), 1656-1661.
Loucaides, S., Koning, E. & Van C., P. (2012) Effect of pressure on silica solubility of diatom frustules in the oceans: Results from long-term laboratory and field incubations. Marine Chemistry. 136-137, 1-6.
Lu, S., Li, Y., Lundholm, N., Ma, Y. & Ho, K., C. (2012) Diversity, taxonomy and biogeographical distribution of genus Pseudo-nitzschia (Bacillariophyceae) in Guangdong coastal waters, South China Sea. Nova Hedwigia. 95, 123-152.
Lundholm, N. & Moestrup, O. (2002) The marine diatom Pseudo-nitzschia galaxiae sp. nov. (Bacillariophyceae): morphology and phylogenetic relationships. Phycologia. 41(6), 594-605.
Maher, S., Kumeria, T., Aw, M., S. & Losic, D. (2018) Diatom Silica for Biomedical Applications: Recent Progress and Advances. Advanced healthcare materials. 7(19), e1800552.
Nagaoka, S., Murata, S., Kimura, K., Mori, T. & Hojo, K. (2010) Antimicrobial activity of sodium citrate against Streptococcus pneumoniae and several oral bacteria. Letters in applied microbiology. 51(5), 546-551.
Nealson, K., H. & Saffarini, D. (1994) Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annual review of microbiology. 48, 311-343.
Nielsen, D., A., Petrou, K. & Gates, R., D. (2018) Coral bleaching from a single cell perspective The ISME Journal. 12, 1558–1567.
Pérez-Pascual, D., Lunazzi, A., Magdelenat, G., Rouy, Z., Roulet, A., Lopez-Roques, C., Larocque, R., Barbeyron, T., Gobet, A., Michel, G., Bernardet, J. & Duchaud, E. (2017) The Complete Genome Sequence of the Fish Pathogen Tenacibaculum maritimum Provides Insights into Virulence Mechanisms. Frontiers in Microbiology. 8,.
Perl, T., Bédard, L., Kosatsky, T., Hockin, J., Todd, E. & Remis, R. (1990) An Outbreak of Toxic Encephalopathy Caused by Eating Mussels Contaminated with Domoic Acid. The New England Journal of Medicine. 322(25), 1775-1780.
Poulsen, N., Spector, I., Spurck, T., Schultz, T. & Wetherbee, R. (1999) Diatom gliding is the result of an actin-myosin motility system. Cell motility and the cytoskeleton. 44(1), 23-33.
Raugei, S. (2019) How Does Mother Nature Tackle the Tough Triple Bond Found in Nitrogen? Energy.gov. https://www.energy.gov/science/bes/articles/how-does-mother-nature-tackle-tough-triple-bond-found-nitrogen
Riddle, D. (2012) Aquarium Chemistry: Effects of GFO (Granular Ferric Oxide) on ‘Trace’ Metals Concentrations in Artificial Seawater. AdvancedAquarist.com. https://www.advancedaquarist.com/2012/2/chemistry
Rines, J., E., B., Donaghay, P., L., Dekshenieks, M., M., Sullivan, J., M. & Twardowski, M. (2002) Thin layers and camouflage: Hidden Pseudo-nitzschia spp. (Bacillariophyceae) populations in a fjord in the San Juan Islands, Washington, USA. Marine Ecology-progress Series. 225, 123-137.
Rodriguez-Lanetty, M., Scaramuzzi, C., Quinnell, R. & Larkum, A. (2005) Transport of symbiotic zooxanthellae in mesogleal canals of Zoanthus robustus? Coral Reefs. 24, 195-196.
Sanchez, N., Brown, E., Olsen, Y., Vadstein, O., Iriarte, J., Gonzalez, H. & Ardelan, M. (2018) Effect of Siderophore on Iron Availability in a Diatom and a Dinoflagellate Species: Contrasting Response in Associated Bacteria. Frontiers in Marine Science. 5,.
Sardet, C., Pisam, M. & Maetz, J. (1979) The surface epithelium of teleostean fish gills. Cellular and junctional adaptations of the chloride cell in relation to salt adaptation. The Journal of cell biology. 80(1), 96-117.
Sarzanini, C., Abollino, O. & Mentasti, E. (2001) Flow-injection preconcentration and electrothermal atomic absorption spectrometry determination of manganese in seawater. Analytica Chimica Acta. 435(2), 343-350.
Shaw, J., Macey, D. & Brooker, L. (2008) Radula synthesis by three species of iron mineralizing molluscs: Production rate and elemental demand. Journal of the Marine Biological Association of the United Kingdom. 88(3), 597-601.
Smith, J., Gellene, A., Hubbard, K., Bowers, H., Kudela, R., Hayashi, K. & Caron, D. (2017) Pseudo-nitzschia species composition varies concurrently with domoic acid concentrations during two different bloom events in the Southern California Bight. Journal of Plankton Research. 40(1), 29-45.
Sprung, J. (2002) Aquarium Invertebrates: Captive Husbandry of Goniopora, Spp. With Remarks About The Similar Genus Alveopora. AdvancedAquarist.com. https://www.advancedaquarist.com/2002/12/inverts
Teitelbaum, J., Zatorre, R., Carpenter, S., Gendron, D., Evans, A., Gjedde, A. & Cashman, N. (1990) Neurologic Sequelae of Domoic Acid Intoxication Due to the Ingestion of Contaminated Mussels. The New England Journal of Medicine. 322(25), 1781-1787.
Trainer, V., L., Wells, M., L., Cochlan, W., P., Trick, C., G., Bill, B., D., Baugh, K., A., Beall, B., F., Herndo, J. & Lundholm, M. (2009) An ecological study of a massive bloom of toxigenic Pseudo-nitzschia cuspidata off the Washington State coast. Limnol. Oceanogr. 54, 1461-1474.
Tzu-En, H. & Chia-Wei, L. (2007) Silica Biomineralization in the Radula of a Limpet Notoacmea schrenckii (Gastropoda: Acmaeidae). Zoological Studies. 46(4), 379-388.
Valliere-Douglass, J., F., Connell-Crowley, L., Jensen, R., Schnier, P., D., Trilisky, E., Leith, M., Follstad, B., D., Kerr, J., Lewis, N., Vunnum, S., Treuheit, M., J., Balland, A. & Wallace, A. (2010) Photochemical degradation of citrate buffers leads to covalent acetonation of recombinant protein therapeutics. Protein science: a publication of the Protein Society. 19(11), 2152-2163.
Van Cappellen, P., Dixit, S. & van Beusekom, J. (2002) Biogenic silica dissolution in the oceans: Reconciling experimental and field‐based dissolution rates. Global Biogeochemical Cycles. 16(4), 23-1-23-10.
Wallace, R. (2014) What Manganese and the Trade Winds Tell Researchers about the Coral Bleaching Epidemic of the Pacific. ScienceTimes.com. https://www.sciencetimes.com/articles/2172/20141224/what-manganese-and-the-trade-winds-tell-researchers-about-the-coral-bleaching-epidemic-of-the-pacific.htm
WoRMS (2022) Pseudo-nitzschia H. Peragallo in H. Peragallo & M. Peragallo, 1900. MarineSpecies.org. https://www.marinespecies.org/aphia.php?p=taxdetails&id=149151
Zehr, J. & Kudela, R. (2011) Nitrogen Cycle of the Open Ocean: From Genes to Ecosystems. Annual Review of Marine Science. 3, 197-225.






0 Comments