Mushroom Corals: Order Corallimorpharia
There are only about fifty described species of corallimorpharians, but, despite this limited diversity, they are well-known amongst aquarists. The genera commonly exported are fairly homogenous in resembling anemones that have nearly lost their tentacles, but there are some very anemone-like exceptions—like the “orange ball anemone” Pseudocorynactis. In truth, this group is actually far more closely related to the stony corals, which at first glance seem quite different. How then do we differentiate these three groups?
An important distinction must be made concerning the placement of the tentacles in anemones and corallimorphs. In many anemones, the tentacles are located only around the margin of the oral disk, whereas in corallimorphs these tentacles extend towards the mouth to fill most of the disk. This is not universally true, as some anemones have secondarily evolved a tentacle-filled oral disk. In fact, nearly all of the common aquarium anemone species violate this rule—Stichodactyla, Radianthus, Entamacaea, Cryptodendrum, Heterodactyla. This even caused confusion amongst taxonomists, who at one time considered Ricordea as belonging amongst these anemones. The differences that exist involve subtle nuances of the nematocysts and body musculature, but a good rule of thumb is that corallimorphs lack the ability to rapidly move themselves in the manner of an anemone.
The anatomy of a corallimorph is essentially identical to the stony corals, save for the lack of a secreted skeleton. But, as discussed earlier, stony corals were not always stony, and this begs the question: Are corallimorphs actually a distinct evolutionary lineage, or are these merely stony corals that lost their skeleton? This latter scenario is known as the “naked coral” hypothesis.
Definitive answers to this mystery have not been forthcoming, but recent studies analyzing the complete mitochondrial genomes of numerous genera have allowed researchers to make some headway. The results so far have provided strong evidence that corallimorphs are indeed a monophyletic group sister to the stony corals, though until studies of nuclear DNA are completed there is still some room for uncertainty. The species at the base of the corallimorph tree is Corallimorphus profundus, a large, anemone-like species known from the deep ocean floors and shallow waters of Antarctica. Its mitochondrial genome is ordered in much the same manner as in scleractinians, but the genetic code contained in it shows it to belong with the Corallimorpharia.
Comparing Corallimorphus to the basalmost groups of stony corals allows us to envision what the common ancestor of these groups might have resembled and how it might have lived. Presumably, this ur-coral was an azooxanthellate suspension feeder living in the ocean depths, and it probably would have looked nearly identical to an anemone in the same manner that Corallimorphus does today. Only later would this primitive coral develop a fragile aragonitic skeleton and become recognizable as a true Stony Coral.
Stony Corals: Order Scleractinia
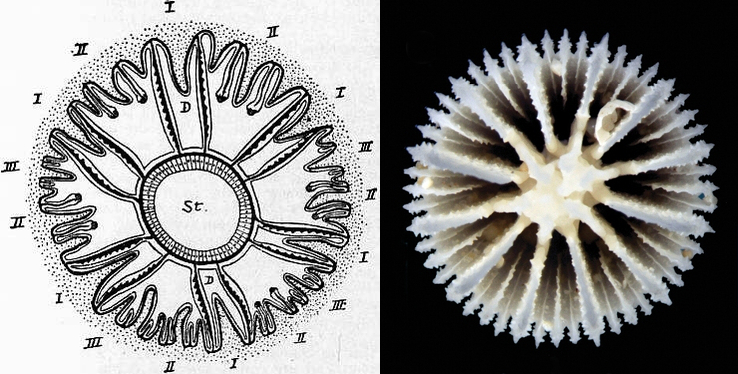
Illustration of mesenteries and septa (dotted). Septal growth occurs in discrete cycles. I first, II second, III third, and so on. Compare to skeleton on right, Cryptotrochus. Credit: 1911 Encyclopaedia Britannica, and Cairns & Kitahara 2012
The roughly 1500 species of stony corals make this one of the most successful branches of cnidarian life. The group is easily identified by its hexacorallian body plan and aragonitic skeleton, though, as indicated above, the exclusion of the corallimorphs is mostly a case of semantics. These groups are essentially identical, save for the skeleton.
The fossil record of scleractinians begins in earnest back during the Middle Triassic. This follows the mass extinction event of the Permian/Triassic boundary, a moment when competing groups like tabulate and rugosan corals disappeared into extinction. This clearly drove a sudden diversification amongst the scleractinians, as most major lineages appear almost instantly in the fossil records. So now we must ask, “Whither the pre-Triassic Scleractinia?” Surely this group didn’t appear de novo.
There are two possible explanations for this. The most obvious is that the pre-scleractinians (which for all intents and purposes would be identical to a corallimorpharian) simply left no fossil record. This is perhaps to be expected for a soft-bodied group, as fossilization is always a haphazard process. The other explanation is that pre-Triassic fossils do exist, but they have not been recognized as such. And this does indeed seem to be the case.
Only in the last twenty years have early scleractinian-like fossils been discovered. Classified under the name scleractiniamorphs, these corals were initially dismissed as failed experiments in anthozoan evolution that died out long before the “true” stony corals. This made some sense, given that the earliest of these corals, Kilbuchophyllia, was some 455 million year old, placing it more than a hundred million years before the mass appearance of the mid-Triassic scleractinians. But as genetic evidence mounted that the stony corals likely preceded their fossil record by many millions of years, it became more evident that these fossils were in fact the earliest representatives of the scleractinians.
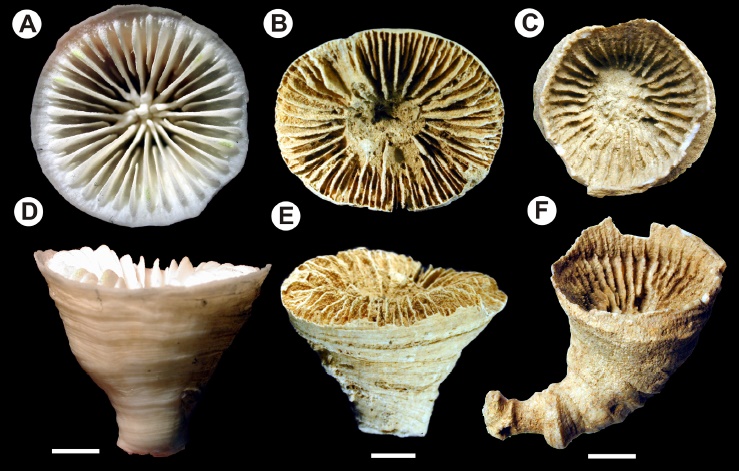
The extant Gardineria (A,D) versus 230MYA Margarophyllia (B,E) & 380MYA Ptychophyllum (C,E). Note the similar smooth, epithecal walls. Credit: Stolarski et al 2011
One corroborating piece of evidence is the structure of the outer wall of the coral. In nearly all extant scleractinians, the outer wall is formed by calcification similar to the rest of the corallite, with this structure being called the “theca”. In many groups, a secondary calcified layer (the “epitheca”) is added atop the theca, with this epitheca differing fundamentally in its composition from the rest of the corallite. This can be seen most easily in free-living groups like Rhizotrochus or Trachyphyllia, where the outer surfaces of skeleton are covered in a thin, annulated shellac of calcium carbonate. What the fossil record indicates is that the earliest corals lacked a theca, and instead only had this epithecal layer circumscribing the coral. This is never the case in modern groups, with one exception…
Gardineriidae is an unfamiliar group of corals to aquarists, due to its restricted habitat in the depths of the ocean. But, like the earliest fossils stony corals, this group possesses only the epithecal layer around its polyps. Another primitive group, the Micrabaciidae—characterized by its diaphanous skeleton, branched septa, and fleshy polyps, is also comparable to earlier fossils. This is congruous with the major phylogenetic changes made in light of genetic study. Extant stony corals are now broken into three major groups. There is a basal group, formed by the gardineriids and micrabaciids. There is a “complex” group, formed by species like Acropora that have fragile, porous skeletons. And there is a “robust” group, comprised of more solidly built lineages, like brain corals, as well as plate coral and many others.
The classification of these last two groups continues to be in a constant state of flux, with new taxonomies being proposed on a regular basis. Slowly, we are replacing outdated hypothesis based on subjective morphological characters with quantifiable analytics of molecular sequences. It might be many more years before we fully understand the big picture of stony coral evolution, but certainly we are closer to that point than at any time in the 250+ years since scientists began their study of them. One can’t help but wonder what future scientific inquiry will discover, for there are undoubtedly more evolutionary surprises yet to be revealed.
Summarizing Coral Evolution
It’s clear from studying the molecular clock of anthozoans that the calcareous coral taxa must have first evolved long before their appearance in the fossil record might suggest. When you consider that these groups diverged from soft bodied ancestors like the actinarian sea anemones, it makes sense that the earliest representatives would have been likewise soft-bodied. The ecological and environmental pressures which drove anthozoans to create skeletons has happened on at least three occasions, and quite probably more than that. In fact, there’s no way to know if the scleractinian skeleton evolved a single time, or was arrived at independently in the gardineriid/micrabaciid lineages and the “complex”/”robust” lineage.
To be sure, Precambrian reefs would have been a poor imitation of those we are familiar with today. Their diversity was low; the species were small and innocuous; and their taxa would be unfamiliar. The Cambrian was likewise depauperate in its reef diversity, with calcifying sponges and archaeocyaths dominating the fauna. It was only in the Ordovician that we see the first large, successful groups of calcareous corals, the Tabulata and Rugosa, as well as the possible proto-scleractinian Killbuchophyllia. Diversity in the former groups would escalate through this period, ultimately reaching its peak during the Devonian which followed. It’s interesting to note that this period, dominated by some of the warmest temperatures and highest CO2 concentrations in earth’s history, was known for having the most expansive coral reefs known from the fossil record. While scientists are right to worry about the existential threat modern stony corals face from a warming climate, it seems naïve to imagine this group is incapable of eventually adapting to such an environment.
From their peak in the Middle Devonian, species diversity would slowly dwindle. The end of the Devonian is associated with a general cooling of the planet, with large-scale glaciation prevalent. This strongly depleted the ability of corals to form large reefs, and the eventual extinction of the stromatoporoid sponges—vital to the agglutination of reef structures—would lead to an extended period of diminished coral diversity. Carboniferous reefs would see a resurgence in reef growth, with colonial rugosans increasing in prominence, albeit at a lesser abundance than their Devonian peak. This is a period when other groups, including calcareous algae, would become important to reef ecosystems. Towards the end of this geologic period, the major supercontinents of Lauratia and Gondwana began merging together, limiting the warm waters that existed between them. This resulted in more cooling, a general reduction in reef formation, and the further decline of Tabulata and Rugosa.
The Permian which followed would see another period of planetary warming, with a change to the ocean’s chemistry which began favoring the formation of aragonite over calcite. There was one final burst of speciation for the rugose and tabulate corals, with some reefs in what is now Southeast Asia being dominated by these taxa. But eventually, over a period of 5-8 million years, these groups began disappearing from the fossil record. This marks the beginning of one of the most cataclysmic extinction events in earth’s history, during which some 90% of the marine life on earth disappeared in the span of just a few thousand years. While the cause of this shift in earth’s biota is poorly understood, the result was that calcium carbonate deposition essentially ceased entirely for many millions of years. One theory posits that massive volcanic eruptions in Siberia resulted in a sudden increase in atmospheric CO2/methane concentrations and massive global warming, which in turn reduced the upwelling of oxygen-rich polar waters, ultimately leading to widespread anoxic conditions in what has been dubbed a “runaway greenhouse”. This would obviously be detrimental to coral survival, in the same way that an aquarium without circulation doesn’t stay alive very long. An end Permian reef tank would have been a depressing sight indeed. There are obvious parallels between this era of earth’s history and the climatic changes we appear to be shifting perilously towards.
Following the Permian extinction, we now move into the Mesozoic. The first several million years saw minimal reef formation, with those communities dominated by non-coral taxa. It was only when the anoxic conditions diminished in the Middle Triassic that the earliest definitive scleractinians began to appear. The approximately 19 genera of this period (none growing more than a few centimeters across) were minor players in the formation of reefs. We do know from their biochemical remains that they were already zooxanthellate, but it would be some 30 million more years before stony corals assumed their status as major hermatypic producers. This marked the first true resurgence of coral reefs since their heyday during the Middle Devonian some 150 million years earlier. Groups common to aquarists, like acroporids, would finally become recognizable.
Towards the end of the Triassic, corals would again diminish in number, with only eleven genera known to have survived into the Jurassic. The causes are murky, but are probably related to changing climatic conditions associated with increased volcanism and continental movements. Coral reefs would rebound again through the Jurassic, with some groups of this period (e.g. Caryophyllia) thriving up to today. Ultimately, another major extinction event at the end of the Cretaceous—the same one that brought about the end of the dinosaurs—reduced their number by perhaps 80%.
The next 65 million year of coral evolution shows a similar pattern of speciation and extinction, though to a lesser extent than the major events of the past. Sharp increases in temperature and C02 show a characteristic depression in the diversity of coral taxa. One of these changes, marking the boundary between the Paleocene and Eocene, is thought to have taken place over the geologically brief period of a few thousand years. Contrast this with the scientific consensus of current climate change predictions, which projects a similar amount of warming over a period of just a century or two and it becomes clear the risk corals are heading towards. The fossil record provides evidence of just how resilient stony corals can be. Throughout their history they have endured environmental upheavals that have eliminated countless other taxa, and still they persisted, recovered and thrived. What the future hold for corals, only time will tell, but it seems naïve to think they won’t find some way to adapt to another changing world.
References
Bayer, F. M., Muzik, K. M. 1976. “A New Solitary Octocoral,gen. Et Sp. Nov. (Coelenterata: Protoalcyonaria) from New Zealand.” Journal of the Royal Society of New Zealand 6.4: 499-515.
Clarkson, E. N. K. 2009. Invertebrate Palaeontology and Evolution. 4th ed. London: Blackwell Science
Duerden, J. E. 1905. “The Morphology of the Madreporaria, VI The Fossula in Rugose Corals.” Biological Bulletin. 9 (1): 27-52
Ezaki, Y. 1998. “Paleozoic Scleratinia: Progenitors or Extinct Experiments?” Paleobiology 24(2): 227-234.
Goldberg, W. M. 2013. The Biology of Reefs and Reef Organisms. Chicago: University of Chicago Press.
Goldberg, Walter M., Theodore L. Hopkins, Susan M. Holl, Jacob Schaefer, Karl J. Kramer, Thomas D. Morgan, and Kiho Kim. “Chemical Composition of the Sclerotized Black Coral Skeleton (Coelenterata: Antipatharia): A Comparison of Two Species.” Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 107.4 (1994): 633-43.
Gudo, M. 2002. “Soft Body Reconstructions of Palaeozoic Corals: Implications for the System of Anthozoa (Coelenterata).” Lethaia. 35.4: 328-44.
Han, J., Kubota, S., Uchida, H-o., Stanley, G. D. Jr, Yao, X., et al. 2010. “Tiny Sea Anemone from the Lower Cambrian of China.” PLoS ONE 5(10): e13276. doi:10.1371/journal.pone.0013276
Kitahara, M. V., Lin, M.-F., Forêt, S., Huttley, G., Miller, D. J., Chen, C. A. 2014. “The “Naked Coral” Hypothesis Revisited – Evidence for and Against Scleractinian Monophyly.” PLoS ONE 9(4): e94774. doi:10.1371/journal.pone.0094774
Liu, P., S. Xiao, C. Yin, C. Zhou, L. Gao, and F. Tang. 2008. “Systematic description and phylogenetic affinity of tubular microfossils from the Ediacaran Doushantuo Formation at Weng’an, South China.” Paleontology. 51:339–366
Lin, M. F., Kitahara, M. V., Luo, H., Tracey, D., Geller, J., Fukami, H., Miller, D. J., and Chen, C. A. 2014. “Mitochondrial genome rearrangements in the scleractinia/corallimorpharia complex: implications for coral phylogeny.” Genome Biol. Evol., 6(5):1086–95.
Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, Grigorenko AP, Dailey C, Berezikov E, Buckley KM, Ptitsyn A, Reshetov D, Mukherjee K, Moroz TP, Bobkova Y, Yu F, Kapitonov VV, Jurka J, Bobkov YV, Swore JJ, Girardo DO, Fodor A, Gusev F, Sanford R, Bruders R, Kittler E, Mills CE, Rast JP, Derelle R, Solovyev VV, et al. 2014. “The ctenophore genome and the evolutionary origins of neural systems.”’ Nature 510(7503):109-114.
Riemann-Zürneck K., Iken K. 2003. “Corallimorphus profundus in shallow Antarctic habitats: bionomics, histology, and systematics (Cnidaria: Hexacorallia).” Zool. Verh. Leiden. 345:367-386.
Reimer, J.D., Lin, M., Fujii, T., Lane, D.J.W., Hoeksema, B.W. 2012. “The Phylogenetic Position of the Solitary Zoanthid Genus Sphenopis (Cnidaria: Hexacorllia).” Contributions to Zoology 81(1): 43-54
Rodríguez, E., Barbeitos, M., Brugler, M.R., Crowley, L., Gusmão, L., Häussermann, V., Grajales, A. & Daly, M. 2014. “Hidden among sea anemones: The first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals.” PLoS ONE, 9(5): e96998. http://dx.doi.org/10.1371/journal.pone.0096998
Stolarski, J., M.V. Kitahara, D.J. Miller, S.D. Cairns, M. Mazur, and A. Meibom. 2011. “The Ancient Evolutionary Origins of Scleractinia Revealed by Azooxanthellate Corals.” BMC Evolutionary Biology 11.1: 316.
Van Iten, H., Marques, A.C., Leme, J.M., Pacheco, M.L.A.F., Simoes, M.G., 2014. “Origin and early diversification of the phylum cnidaria verrill: Major developments in the analysis of the taxon’s Proterozoic–Cambrian history.” Palaeontology. 57: 677–690.











0 Comments