
The previous editorial discussed some unwelcome organisms that “stowaway” on new specimens while herein we explore parasites that consume SPS colonies, which can arrive on or within anything from another system or non-gamma irradiated foods. Exploit the recommended dilution of CoralRX Pro in aged seawater for new corals, where the final stage of any acclimation drip-dries specimens for a second or two. Cover the eyes of fish and do not expose sponges to air but never mix a supplier’s water with your own. See Part X.
Vegetable-eating copepods manifest as tiny white specks on the glass of established aquaria, albeit remarkably, two-thirds of all copepods are external (ecto-) or internal (endo-)parasites of fish and invertebrates where over 400 species impact more than 350 Cnidaria (Humes 1994). Another study concluded most are planktonic while ~50 percent are symbionts (Boxshall 2005). Widespread in temperate and colder seas, most inhabit the tropics (Rigby et al. 1999; Tucker et al. 2000; Johnson et al. 2004; Riddle 2010a; Fajer-Avila et al. 2011; Ismail et al. 2013; Osman et al. 2014; Nagasawa 2015; Soler-Jiménez et al. 2019).
Copepods are multicellular animals of the phylum Arthropoda, the subphylum Crustacea, the superclass Multicrustacea, and the class Copepoda comprising ~11,500 species. Members of the orders Harpacticoida, Siphonostomatoida, Cyclopoida, and Monstrilloida range from partially to exclusively parasitic (Boxshall 2005).
Podoplea superorder affiliates are cyclopiform where their anterior wider prosome transitions into their thinner urosome immediately before the fifth segmented somite which carries their final appendages (pedigers), while the urosome commences immediately after in non-parasitic Gymnoplea (Ho & Kim 2001). All are gonochoric where larger females carry egg sacks adhered to their genital somite formed from the fusion of two subdivisions of their tail (ramus; Fig 1.; Fig 2.; Boxshall 2005).
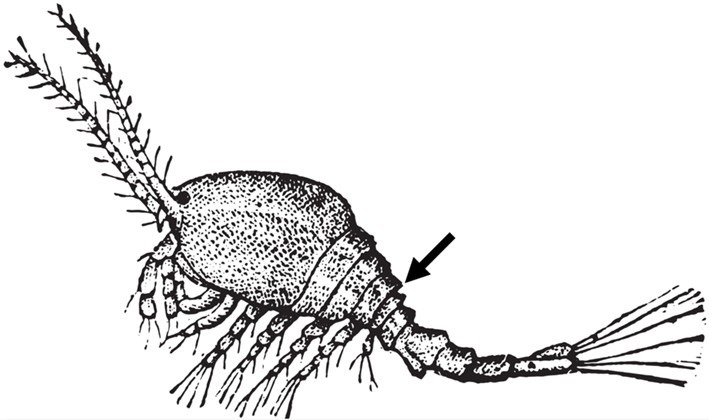
Fig 1. All parasitic copepods adhere to podoplean (cyclopiform) architecture, but imprecisely so, with the amorphous bag of female species of Sarcotaces that parasitize fish (Aitken 1942; Osman et al. 2014).
Nearly 40 genera infest corals such as Humesiella, Orstomella, Stockella, and Zazaranus; however, the genera Alteuthellopsis, Xarifia, Tegastes, and Parategastes infest SPS corals where the latter two are known as “red” or “black bugs” (Riddle 2010).
The extensive variety that parasitize fish are beyond the scope of this series yet their multiplicity is illuminated in Aslett 2024. Amphipod-like, laterally compressed, Tegastes species are gold with a single red eye with claw-like mandibles (Shimek 2002; Riddle 2010) where females carry red-punctuated uniocular embryos (Riddle 2010). Acropora species are susceptible to Tegastes acroporanus (Podoplea: Tegastidae; Fig 4.; Fig 5.; Christie & Raines 2016) where females often appear absent and thus infestations must be non-proliferative (Shimek 2002). Stylophora pistillata (Hexacorallia: Pocilloporidae) become home to Xarifia obesa (Podoplea: Xarifiidae; Cheng & Dai 2009) while “black bugs” may target Montipora (Riddle 2010). Species of Parategastes and Tegastes are nested in the infraclass Neocopepoda, the order Harpacticoida, and the family Tegastidae (WoRMS 2020c).

Fig 2. A micrograph of female cyclopiform copepods carrying developing larvae attached to their genital somites.
- Fig 3. Illustrations of Tegastidae drawn from the accurate biological representations within Riddle 2010a and World of Copepods, Walter & Boxshall 2020.

Fig 4. The genus Tegastes infesting Acropora. Image courtesy of Gregory HO ©, Ximina’s Photography. www.ximinasphotography.com
Alteuthellopsis corallina are western Indo-Pacific endoparasites of Acropora exigua (taxon inquirendum; Hexacorallia: Acroporidae), Astreopora species, Montipora verrilli, Pocillopora damicornis, Goniastrea retiformis (Merulinidae), Merulina ampliata, Platygyra daedalea, and other species of Platygyra (Humes 1981). The lineages of Alteuthellopsis and Parategastes/Tegastes diverge merely at the rank of family and subfamily (Peltidiinae; WoRMS 2020d).

Fig 5. “Red bugs” (Tegastes species) infesting Acropora (Fig 4.). Image courtesy of Gregory HO ©, Ximina’s Photography. www.ximinasphotography.com
The afflicted will likely exhibit limited polyp extension and diminished coloration (Shimek 2002; Riddle 2010a) while the exoskeletal microbiome of several comprise pathogenic bacteria of the genus Vibrio. They are typically <0.5 mm (<0.02”) where several amass coral pigments and are thus challenging to discern (Riddle 2010a).
Species of Tegastes are palatable to network pipefish (Corythoichthys flavofasciatus; Teleostei: Syngnathidae; Fig 6.; Delbeek & Sprung 2005) and most likely acropora crabs. See Part X. Elevated temperatures and enriched nutrients exacerbate pathology because they weaken corals (Ben-Haim & Rosenberg 2002; Ben-Haim et al. 2003) where most coral ailments cannot manifest at a steady 23.5oC (Aslett 2005; Riddle 2010).
Vigorous flow minimizes coral boundary layers which optimizes carbonate uptake, skeletal deposition, and photosynthesis (Riddle 2016; Munn 2019) whereas powerful currents dislodge, which thwarts colonization (Riddle 2010).
Dilutions of CoralRX Pro and ReVive comprise non-toxic natural plant extracts that irritate and expedite parasite dissociation through the sloughing of surplus mucus (Riddle 2010; Danireef 2017). The mucosal microbiome is the colonies first line of defense and these immersion therapies are undoubtedly stressful, yet they are preferable to a systemic treatment of a veterinary medicine that by necessity, must eradicate a vast cross-section of the ecosystem and annihilate diversity. Briefly purge corals in ReVive- or CoralRX Pro-free seawater before system introduction.
As with any potentially dangerous procedure, the reader uses these chemotherapeutics at their own risk, because the author, Reef Ranch Publishing Ltd, websites hosting this article, and their owners cannot be held responsible for any harm to pets, people, possessions, or properties.
Novartis AG’s milbemycin oxime is the active ingredient of Interceptor® which is licensed in the UK for dogs with heartworm or mange, whereas Interceptor® Plus contains both praziquantel and milbemycin oxime. Praziquantel is a useful wormer that helps eradicate the “eye parasite” Neobenedenia melleni, but it is non-copepodocidal. Both these drugs are controlled in the UK and as such, must be supplied by a veterinary surgeon if they are not used under license for the intended animal. Please adhere to national legislation. However, if milbemycin oxime is not controlled nationally, then hobbyists are free to source the chemical from elsewhere.
Milbemycin oxime will kill all members of the phylum Arthropoda including amphipods, barnacles, copepods, crabs, lobsters, and shrimp and will thus destabilize ecology and initiate reef malfunction, inasmuch as fish will require eutrophy-instigating supplementary nutrition, so this drug could permanently disrupt SPS mariculture. Do not use casually or as a prophylactic, and only administer if a systemwide infestation has been robustly substantiated microscopically. Move all ornamental Arthropods to quarantine, yet they may serve as re-inoculating vectors (Delbeek & Sprung 2005a).
Like praziquantel, milbemycin oxime requires dissolving in a small quantity of high-purity ethanol (drinkable alcohol) to make it water soluble, while more concentrated stock solutions or graduated syringes may assist accurate dosing in the absence of a costly analytical balance. See Part VI & VII. Ethanol feeds microaerophilic bacteria in suboxic zones which may create anaerobia, hence calcium reactors and denitrifyers may generate deadly toxins. Ensure the throughput of such reactors remains a rapid dribble. If water becomes suddenly grey, exploit GAC remembering to wash in copious amounts of R/O water beforehand and avoid flow-downregulating wavemaker cycles for up to 24 hours post-administration.
Remove granular activated carbon (GAC) and turn off UV sterilization and protein skimmer air but maintain systemwide circulation. Raise systemic milbemycin oxime to 0.015 mg l-1 (ppm) for six hours during which the water should remain clear. Whereupon reinstate protein skimmer air and UV sterilization and force water through fresh GAC. Deceased copepods may adhere to corals for up to 24 hours post-treatment after which a 25 percent water change was advised by the authors. The life cycles of Tegastes species remain enigmatic (Delbeek & Sprung 2005a) and thus readminister the therapeutic on day seven and 14 (Riddle 2010).

Fig 6. Network pipefish (Corythoichthys flavofasciatus; Teleostei: Syngnathidae) ostensibly consume parasitic species of Tegastes (Podoplea: Tegastidae).


Fig 7. An illustration of a cnidarian cnidocyte comprising a stinging nematocyst.
Nematocysts are capsular components of cnidocytes which comprise a stinging barbed tube-like tip and an eversible thread. Cnidarians including jellyfish and corals use nematocysts to inject venom where displacement of the cnidocil explosively everts the thread and expels the barbed tip (Fig 7.).
The “Acropora-eating” flatworms (AEFW; flukes; turbellarians) Prosthiostomum acroporae were recognized parasites of captive Acropora which rapidly multiplied in recirculating systems to denude their skeletons, yet they remained an enigma in the wild until they were spotted off Lizard Island on GBR in 2011 (Rawlinson & Stella 2012; Barton et al. 2019).
AFEW appear immune to Acroporidae’s nematocyst venom (Rawlinson & Stella 2012) while their gut- and parenchyma-bound host zooxanthellae, cnidocytes, and fluorescent proteins (FPs) amass to confer their eminent camouflage (Rawlinson et al. 2011; Rawlinson & Stella 2012; Hume et al. 2014). Temperature impacts adult length which ostensibly exceeds 4 millimeters (mm; 0.157”; Barton et al. 2019).
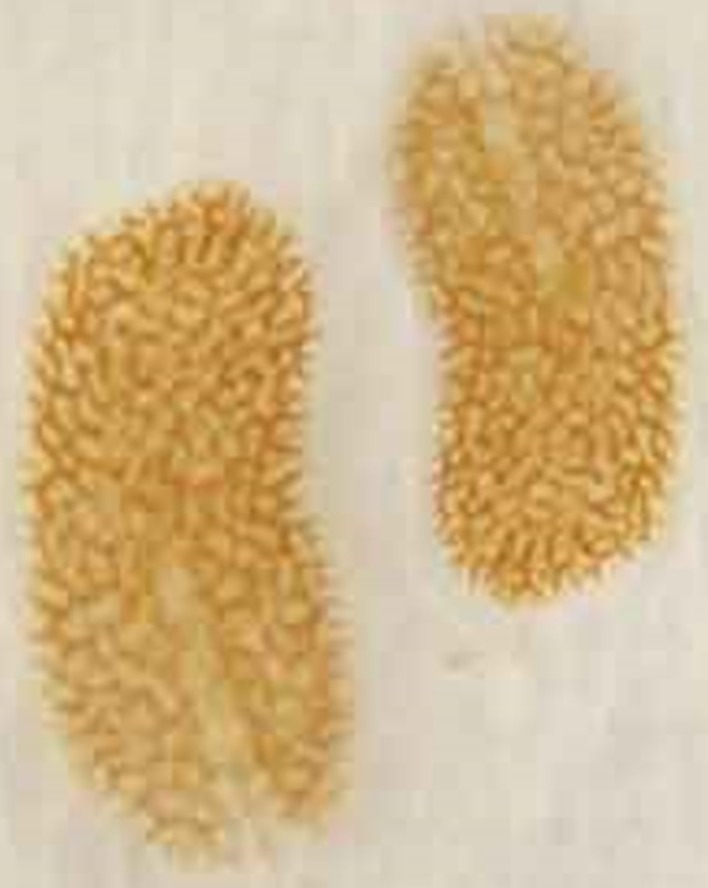
Fig 8. “Acropora-eating” flatworms (AEFW; Prosthiostomum acroporae; Polycladida: Prosthiostomidae). Image courtesy of Beau Henschen, Coral RX, Phillip Root ©.

Fig 9. Illustrations of an adult (left) and juvenile of the “Acropora-eating” flatworm (AEFW) Prosthiostomum acroporae (Polycladida: Prosthiostomidae) which are greater and less than 4 mm in length, where labels include both adult and juvenile organelles at merely approximate locations: [AV] accessory vesicle; [BR] brain; [CE] cerebral eye; [EC] egg chamber; [FG] female gonopore; [M] mouth; [ME] marginal eye; [MG] male gonopore; [MGL] Mehlis gland or shell gland; [MGP] Mehlis gland pouch; [OV] ovary; [PH] pharynx; [PS] penis stylet; [SV] seminal vesicle; [UT] uterus. Drawings from the accurate anatomical representations within Rawlinson et al. 2011 and Rawlinson & Stella 2012.

Fig 10. The life cycle of Prosthiostomum acroporae. [A] adult worms feed on coral and generate circular scars whilst they lay their eggs on bare skeleton. [B] egg masses are chemotherapeutic resistant, where embryonation and hatching occur in approximately 11 days at 27oC (~81oF) while two larvae emerge from each egg. [C/D] swimming lobes and ciliated tufts are present or absent, yet all competently swim and crawl. Hatchlings remain on the parent colony or seek a new host where they initially mature into males, and later oviparous hermaphrodites (Rawlinson & Stella 2012; Barton et al. 2019). Drawings from the accurate representations in Rawlinson et al. 2011, Rawlinson & Stella 2012, and Barton et al. 2019. Image of parasitized species of Acropora with genuine feeding scars courtesy of Beau Henschen, Coral RX, Phillip Root ©.
Comparative 28 svedberg (28S) ribosomal deoxyribonucleic acid (rDNA) sequence analyses and morphology recommended Amakusaplana should be nested within Prosthiostomum (Rawlinson et al. 2011; Litvaitis et al. 2019; Tyler et al. 2020; WoRMS 2020a; WoRMS 2020b) and an unclassified AEFW was discovered that was 10 times smaller than P. acroporae (Wang et al. 2019).
The morphology and occurrence of their sperm glands, seminal vesicles, Mehlis glands, and anterior indentation combined with ocular number and 16S ribosomal ribonucleic acid (rRNA) sequence analyses discerned P. acroporae from P. ohshimae, whereas the ventral suckers of settling larvae facilitate substrate recruitment in all but Prosthiostomum (Rawlinson et al. 2011).
Prosthiostomum are ranked in the phylum Platyhelminthes, the subphylum Rhabditophora, the order Polycladida, the suborder Cotylea, and the family Prosthiostomidae (Worms 2020b) and thus scientists often refer to members of this taxon as cotylean polyclad worms (Barton et al. 2019).
Egg deposition to hermaphrodite adult takes 64 and 38 days at 21oC (~70oF) and 27oC (~81oF), while elevated temperatures enhance egg viability and hatchling survivorship. Flukes proliferate wildly and overwhelm captive Acropora between 27oC and 30oC (86oF) while they subsist nine days without a host (Fig 10.; Barton et al. 2019).
Chemotherapeutic-impervious embryonated ova are deposited in clusters on denuded skeleton (Barton et al. 2019) where the lobes and ciliary bands of hatchlings augment their planktonic phase (Rawlinson et al. 2011; Rawlinson 2014). Two juveniles emerge from each egg with or without such buoyancy-assisting features because all can proficiently swim and crawl (Fig 10.; Barton et al. 2019).
Prosthiostomum montiporae of “Monitpora-eating” flatworm (MEFW) parasitize corals of the genus Montipora in poor conditions (Jokiel & Townsley 1974) because they are commensals that become opportunistic pathogens (Rawlinson et al. 2011). P. montiporae are slender compared with P. acroporae which may reach 12 mm (0.47”) but are usually 4 to 8 mm (0.16 to 0.3”) while their photophobia constrains them to shaded regions (Jokiel & Townsley 1974). Nevertheless, the diminished upwellings of LEDs may expedite infestations of plating colonies.
Cnidarian cells are found in the gut of several polyclads, yet apart from AE- and ME-FWs, merely Stylochoplana inquilina (Polycladida: Stylochoplanidae) is a recognised predator of the anemone Calliactis armillatus (Hexacorallia: Hormathiidae; Poulter 1975; Rawlinson et al. 2011).
Six-line wrasse (Pseudocheilinus hexataenia) bioremediate 100 percent of worms but eggs remain unharmed, yet unconventional peppermint shrimp (Lysmata vittate) ingested 60 and 80 percent of eggs and worms (Barton et al. 2019a). Chemotherapeutics must synchronize with worm life cycles because their encapsulated embryos are resistant, where embryonation and larval settlement occur on day 13 at 28oC (82.4oF). Hence treatments are repeated on the 13th and 38th day.

Fig 11. Six-line wrasse (Pseudocheilinus hexataenia) ostensibly eat AE- and ME-FWs but ignore their eggs.
The controlled veterinary medicine levamisole hydrochloride (HCl) is efficacious (Barton et al. 2019; Barton et al. 2021) which is licensed for merely cattle and sheep in the UK. It must therefore be supplied by a veterinary surgeon for aquarium use. Please adhere to national legislation, whereas aquarists may source the compound from elsewhere in countries where its use remains unrestricted. 99.8 percent purity and above powders are most suitable, where levamisole HCl and praziquantel work synergistically to clear 90 percent of platyhelminths (Barton 2021). Many of which will be key players that benefit captive reef ecology. PraziPro is a praziquantel-comprising fluke remedy, and Salifert® Flatworm eXit is effective and obviates quarantine (Ehlers 2017) whereas Coral RX Pro detaches worms (Henschen 2018b). See Part X.
To be clear, the author does not endorse levamisole HCl yet has included a therapeutic protocol for completeness, while HCl stands for hydrochloric acid which may adversely impact pH. The drug is dosed at 4.4 mg l-1 in reefs where it circulates for 4 to 5 hours without GAC, protein skimmer air, or an energized UV sterilizer, while supplementary aeration is provided by streams of 1-cm (0.4”) air bubbles (Delbeek & Sprung 2005; James404 2011).
4.4 mg l-1 is the absolute upper threshold (James404 2011) whereas alternative literature suggests a concentration of 4 mg l-1 (Delbeek & Sprung 2005). Painstakingly ascertain system volumes, calculate dosages, and weigh accurately using stock solutions and serial dilutions when necessary. Notwithstanding, aquarists have imparted anecdotes of fish, coral, and invertebrate demise (Bug 2009).
25 mg l-1 of levamisole HCl in aged seawater may prove helpful as a 10-minute prophylactic dip (Wijgerde et al. 2013) while flukes are dislodged by vigorous flow so direct currents with a turkey baster.

Fig 12. Aquarium Solutions PraziPro® is a proprietary medication containing praziquantel.

Fig 13. The reef exhibit at Eilat’s Coral World Underwater Observatory in Israel.
Horseshoe crabs, mites, scorpions, and sea spiders are animal arthropods of the subphylum Chelicerata, which have a jointed exoskeleton with a ventral central nervous system (CNS). Their blood is duck-egg blue copper-based haemocyanin like that of crustaceans, molluscs, octopuses, and squid (Stromberg 2014). Haemocyanins are sizeable oxygen-transporting metalloproteins that exploit six residues of the amino acid histidine where their imidazole ring sidechains electrostatically coordinate with two cuprous (Cu+) or cupric ions (Cu2+) when either deoxygenated or oxygenated (Conant et al. 1933; Shimek 2003; Rannulu & Rodgers 2005). Which happens to be one reason why copper-based remedies are so lethal to invertebrates.
Sea spiders lack antennae but have claw-like chelicerae which are analogous to chelifores (Fig 14.; Waloszek & Dunlop 2002; Brenneis et al. 2008; Shimek 2015). Their surface-area optimized exterior facilitates gas exchange while their six side vesseled, single arteried, primitive tubular heart disseminates oxyhaemocyanin (Shimek 2003; Shimek 2015). They require liquified prey or those comprising tiny particles because their tubular three-lipped muscular proboscides penetrate and macerate cells with their ridgelike armature through which they drink (Shimek 2003). Proboscis lumens integrate a pharynx, a hairy debris filter, and a particulate grinder called the reusenapparat (Fahrenbach & Arango 2007; Dietz et al. 2018). The oesophagus progresses into a rudimentary gut where nutrients are absorbed in their upper legs (Fahrenbach & Arango 2007). Some species remain as larvae for a year and live up to nine (Shimek 2015).

Fig 14. “SPS coral-eating” sea spider anatomy: [A] opisthosoma (abdomen); [CH] chelicera; [E] eggs brooded by an ovigerous leg; [P] palp; [PR] proboscis; [P] prosoma (head/thorax).
According to the fossil record, they emerged around five hundred million years ago during the Cambrian (Waloszek & Dunlop 2002) while their phylogeny remains tenuous inasmuch as Ammotheidae and Callipallenidae require reclassification. Austrodecidae, Colossendeidae, and Pycnogonidae are the lowest ranking clades (Arango & Wheeler 2007) while all are order Pantopoda and class Pycnogonida affiliates (WoRMS 2020e).

Fig 15. A “SPS coral-eating” sea spider consistent with the observations of Melev’s Reef 2019.
Their head and thorax-analogous prosoma is frequently abridged to an unsegmented tube which may bear four pairs of legs yet some have five or six pairs. Their long pedipalps are sensory which assist motility while their unappendaged abdomen-like opisthosomas may comprise vestigial respiratory organs (Shimek 2003).

Fig 16. A brooding male sea spider.
Frequently well camouflaged, their locomotion and sexual reproduction are unhurried (Shimek 2003; Shimek 2015). They are gonochoric where spermatozoa and eggs are produced from the base of male and female limbs. Eggs are gathered, fertilised, and brooded by adapted male appendages called ovigers (Fig 14.; Fig 16.; Bain & Govedich 2004; Shimek 2015; Brenneis et al. 2017). Embryos develop into parasitic suckling larvae with two limbs and six appendages (Shimek 2008; Shimek 2015) where each molt has chelifores, ovigers, and palps (Dietz et al. 2018).
Protonymphon larvae shed several times on one kind of host which they may switch when they reach adulthood (Shimek 2003). Chorion-associated attaching larvae are brooded by males until free-swimming where 12 or more molts may be required before sexual maturity (Bain 2003; Shimek 2015).
Sea spiders are detached using tweezers (Henschen 2018a); however, most exhibit a lack of host fidelity and as such, leave a wake of destruction. Infested colonies are best discarded
(Shimek 2003) and submerge new specimens in the prescribed dilution of Coral RX Pro in aged seawater (Henschen 2018a) and ruthlessly sustain oligotrophy and moderate temperature.
Photon flux, spectral cohesion, component wavelengths and their application, are decisive in SPS mariculture where next we commence to reveal the practical application of light within reef displays.

To learn more, visit the Reef Ranch website
References
Aitken, A. (1942) An Undescribed Stage of Sarcotaces. Nature. 150, 180-181.
Arango, C. & Wheeler, W., C. (2007) Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics. 23, 255-293.
Arnaud, F. & Bamber, R., N. (1987) The biology of Pycnogonida. Advances in marine Biology. 24, 1-96.
Aslett, C., G. (2005) What the Scientists Know, Coral Disease: Cause or Coincidence. Marine World Magazine. 23, 38-41.
Aslett, C., G. (2024) The Complete Reef Aquarist, for hobbyists, the trade and academics – A Conservation Manual. Reef Ranch Publoshing Ltd, Leeds, West Yrokshire, UK. In press. https:///www.reefranch.co.uk/ pp 363-368.
Bain, B. (1991) Some observations on biology and feeding behavior in two southern California pycnogonids. Bijdragen tot de dierkunde. 61(1), 63-64.
Bain, B., A. & Govedich, F., R. (2004) Courtship and mating behavior in the Pycnogonida (Chelicerata: Class Pycnogonida): a summary. Invertebrate reproduction & development. 46(1), 63-79.
Bain, B., A. (2003) Larval types and a summary of postembryonic development within the pycnogonids. Invertebrate reproduction & development. 43(3), 193-222.
Barton, J., A., Hutson, K., S., Bourne, D., G., Humphrey, C., Dybala, C. & Rawlinson, K., A. (2019) The Life Cycle of the Acropora Coral-Eating Flatworm (AEFW), Prosthiostomum acroporae; The Influence of Temperature and Management Guidelines. Frontiers in Marine Science. 6, 524.
Barton, J., A., Neil, R., C., Humphrey, C., Bourne, D., G. & Hutson, K., S. (2021) Efficacy of chemical treatments for Acropora-eating flatworm infestations. Aquaculture. 532,.
Barton, J., Humphrey, C., Bourne, D., G. & Hutson, K. (2019a) Biological controls to manage Acropora-eating flatworms in coral aquaculture. Aquaculture Environment Interactions. 12,.
Ben-Haim, Y. & Rosenberg, E. (2002) A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Marine Biology. 141, 47-55.
Ben-Haim, Y., Thompson, F., L., Thompson, C., C., Cnockaert, M., C., Hoste, B., Swings, J. & Rosenberg, E. (2003) Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. International Journal of Systematic and Evolutionary Microbiology. 53(1), 309-315.
Boxshall, G., A. & Justine, J., L. (2005) A new genus of parasitic copepod (Siphonostomatoida: Caligidae) from a razorback scabbardfish, Assurger anzac Alexander (Trichiuridae) of New Caledonia. Folia Parasitologica. 52, 349-358.
Brenneis, G., Bogomolova, E., V., Arango, C., P. & Krapp, F. (2017) From egg to “no-body”: an overview and revision of developmental pathways in the ancient arthropod lineage Pycnogonida. Front Zool. 14, 6.
Brenneis, G., Ungerer, P. & Scholtz, G. (2008) The chelifores of sea spiders (Arthropoda, Pycnogonida) are the appendages of the deutocerebral segment. Evolution and Development. 10(6),.
| Aslett 2024 8 |
Bug, A., R. (2009) Evaluation of Chemical Eradication Methods of Acoels (Acoelomorpha) From Marine Aquaria. AdvancedAquarist.com. https://reefs.com/magazine/evaluation-of-chemical-eradication-methods-of-acoels-acoelomorpha-from-marine-aquaria/
Child, C., A. (1998) Nymphon torulum, new species and other Pycnogonida associated with the coral Oculina varicosa on the east coast of Florida. Bulletin of Marine Science. 63, 595-604.
Christie, B., L. & Raines, J., A. (2016) Effect of an Otic Milbemycin Oxime Formulation on Tegastes acroporanus Infesting Corals. Journal of aquatic animal health. 28(4), 235-239.
Conant, J., B., Chow, B., F. & Schoenbach, E., B. (1933) The Oxidation of Hemocyanin. J. Biol. Chem. 101, 463-473.
Danireef (2017) Revive Coral Cleaner – it prevents stress and eliminates parasites. Reefs.com. https://reefs.com/2017/12/23/revive-coral-cleaner-prevents-stress-eliminates-parasites/
Delbeek, J., C. & Sprung, J. (2005) The Reef Aquarium: Science, Art, and Technology. Two Little Fishes Inc., d.b.a. Ricordea Publishing, 1007 Park Centre Blvd., Miami Gardens, Florida 33169, USA. p 452.
Delbeek, J., C. & Sprung, J. (2005a) The Reef Aquarium: Science, Art, and Technology. Two Little Fishes Inc., d.b.a. Ricordea Publishing, 1007 Park Centre Blvd., Miami Gardens, Florida 33169, USA. (eds.). pp 650-652.
Dietz, L., Dömel, J., S., Leese, F., Lehmann, T. & Mlezer, R., R. (2018) Feeding ecology in sea spiders (Arthropoda: Pycnogonida): what do we know? Front Zool. 15, 7.
Ehlers, A. (2017) What Are Acropora Eating Flatworms and How to Treat Them. Reef Builders. https://reefbuilders.com/2017/01/09/what-are-acropora-eating-flatworms-and-how-to-treat-them/
Fahrenbach, W., H. & Arango, C., P. (2007) Microscopic anatomy of pycnogonida: II. Digestive system. III. Excretory system. J. Morphol. 268, 917-935.
Fajer-Avila, E., Guzman-Beltran, L., Zarate-Rodriguez, W., Zaragoza, O. & Almazan-Rueda, P. (2011) Pathology caused by adult Pseudochondracanthus diceraus (Copepoda: Chondracanthidae), a parasite of bullseye puffer fish Sphoeroides annulatus. Revista de Biologia Marina y Oceanografia. 46, 293-302.
Henschen, B. (2018a) Zoanthid Eating Spiders. CoralRX.com. https://coralrx.com/2018/12/26/zoanthid-eating-spiders/
Henschen, B. (2018b) Advanced Aquarist Evaluation of Coral Rx. https://coralrx.com/2018/12/27/advanced-aquarist-feature-article-evaluation-of-chemical-eradication-methods-of-acoels-acoelomorpha-from-marine-aquaria/
Ho, J. & Kim, I. (2001) New species of Hatschekia Poche, 1902 (Copepoda: Hatschekiidae) parasitic on marine fishes of Kuwait. Syst Parasitol. 49, 73-79.
Hume, B., D’Angelo, C., Cunnington, A., Smith, E. & Wiedenmann, J. (2014) The corallivorous flatworm Amakusaplana acroporae: an invasive species threat to coral reefs? Coral Reefs. 33(1), 267-272.
Humes, A., G. (1981) Harpacticoid Copepods Associated With Cnidaria in the Indo-west Pacific. Journal of Crustacean Biology. 1(2), 227-240,
Humes, A., G. (1985) Cnidarians and copepods: a success story. Transactions of the American Microscopical Society. 104(4), 313-320.
Humes, A., G. (1994) How many copepods? Hydrobiologia. 292, 1-7.
Ismail, N., Ohtsuka, S., Maran, B., A., Tasumi, S., Zaleha, K. & Yamashita, H. (2013) Complete life cycle of a pennellid Peniculus minuticaudae Shiino, 1956 (Copepoda: Siphonostomatoida) infecting cultured threadsail filefish, Stephanolepis cirrhifer. Parasite (Paris, France). 20, 42.
James404 (2011) Levamisole In-Tank Treatment for AEFW. ReefCentral.com. http://www.reefcentral.com/forums/showthread.php?t=2027706
Johnson, S., Treasurer, J., Bravo, S., Nagasawa, K. & Kabata, Z. (2004) A review of the impact of Parasitic Copepods on Marine Aquaculture. Zoological Studies. 43,.
Jokiel, P., L. & Townsley, S., J. (1974) Biology of Polyclad Prosthiostomum (Prosthiostomum) sp, a New Coral Parasite from Hawaii. Pacific Science. 28, 361-373.
Litvaitis, M., K., Bolaños, D., M. & Quiroga, S., Y. (2019) Systematic congruence in Polycladida (Platyhelminthes, Rhabditophora): are DNA and morphology telling the same story? Zoological Journal of the Linnean Society. 20, 1-27.
Melev’s Reef (2018) Benign Flatworm. https://www.melevsreef.com/critter/benign-flatworm
Melev’s Reef (2019) Pycnogonid spider. https://www.melevsreef.com/critter/pycnogonid-spider
Munn, C., B. (2019) Marine Microbiology: Ecology & Applications, Third Edition. Munn, C., B. (ed.). CRC Press, Taylor & Francis Group, London. pp 273-326.
Nagasawa, K. (2015) Parasitic copepods of marine fish cultured in Japan: a review, Journal of Natural History. 49(45-48), 2891-2903.
Osman, H., Mohamed, M. & El-Refaey, A. (2014) Studies on Sarcotaces Sp. (Copepoda, Philichthyidae) Infestation (Black Bag Disease) among Some Marine Fish Species of Arabian Gulf, Saudi Arabia. World Applied Sciences Journal. 32, 1780-1788.
Poulter, J., L. (1975) Hawaiian polyclad flatworms: Prosthiostomids. Pac Sci. 29(4), 317-339.
Rannulu, N., S. & Rodgers, T., M. (2005) Solvation of copper ions by imidazole: Structures and sequential binding energies of Cu1(imidazole)x, x = 1–4. Competition between ion solvation and hydrogen bonding. Phys . Chem. Chem. Phys . 7 , 1 0 1 4 – 1 0 2 5.
Rawlinson, K., A. & Stella, J., S. (2012) Discovery of the Corallivorous Polyclad Flatworm, Amakusaplana acroporae, on the Great Barrier Reef, Australia – the First Report from the Wild. PLoS ONE. 7(8),.
Rawlinson, K., A. (2014) The diversity, development and evolution of polyclad flatworm larvae. EvoDevo. 5, 9.
Rawlinson, K., Gillis, A., Billings, R. & Borneman, E. (2011) Taxonomy and life history of the Acropora-eating flatworm Amakusaplana acroporae nov. sp. (Polycladida: Prosthiostomidae). Coral Reefs. 30, 693-705.
Riddle, D. (2010) Aquarium Corals: Stony Coral Parasites: Red and Black Bugs: Identification Guide, Preventive Measures, and a Review of Treatment Protocols. Reefs.com. https://reefs.com/magazine/aquarium-corals-stony-coral-parasites-red-and-black-bugs-identification-guide-preventive-measures-and-a-review-of-treatment-protocols/
Riddle, D. (2010a) Aquarium Corals: Stony Coral Parasites: Part One: Copepods, Not Just Red Bugs. https://reefs.com/magazine/aquarium-corals-stony-coral-parasites-part-one-copepods-not-just-red-bugs/
Riddle, D. (2016) Feature Article: Montipora digitata: A Stony Coral for All Hobbyists. The Marine Aquarium Council of North America. BulkReefSupply.com. https://youtu.be/fBktpJ3umAs
Rigby, M., L., O., C., Cribb, T., Euzet, L., Elisabeth, F., Galzin, R., Holmes, J., C. & Morand, S. (1999) Checklist of the parasites of coral reef fishes from French Polynesia, with considerations on their potential role in these fish communities. Cybium. 23,.
Shimek, R. (2002) A Spineless Column: Bitty bugs – Copepods in the reef aquarium. http://www.reefkeeping.com/issues/2002-10/rs/index.php
Shimek, R. (2008) Ron Shimek: Flatworms http://www.ronshimek.com/flatworms.html
Shimek, R., L. (2003) A Spineless Column. Along Came A Spider, Reefkeeping.com. http://reefkeeping.com/issues/2003-01/rs/index.php
Shimek, R., L. (2015) Oddities 03 – Pycnogonids, Underwater Tuffet Sitters? https://www.reef2rainforest.com/2015/11/12/oddities-03-pycnogonids-underwater-tuffet-sitters/
Shimek, R., L. (2015) Oddities 03 – Pycnogonids, Underwater Tuffet Sitters? https://www.reef2rainforest.com/2015/11/12/oddities-03-pycnogonids-underwater-tuffet-sitters/
Soler-Jiménez, L., C., Morales-Serna, F., N., Aguirre-Macedo, M., L., McLaughlin, J., P., Jaramillo, A., G., Shaw, J., C., James, A., K., Hechinger, R., F., Kuris, A., M., Lafferty, K., D. & Vidal-Martínez, V., M. (2019) Parasitic copepods (Crustacea, Hexanauplia) on fishes from the lagoon flats of Palmyra Atoll, Central Pacific. ZooKeys. 833, 85-106.
Stromberg, J. (2014) Why some animals have blue, green, or purple blood. Vox.com. https://www.vox.com/xpress/2014/10/31/7133779/blood-blue-green-purple
Tucker, C., Sommerville, C. & Wootten, R. (2000) The effect of temperature and salinity on the settlement and survival of copepodids of Lepeophtheirus salmonis (Krøyer, 1837) on Atlantic salmon, Salmo salar L. Journal of Fish Diseases. 23(5),.
Tyler, S., Artois, T., Schilling, S., Hooge, M. & Bush, L., F. (2020) World List of turbellarian worms: Acoelomorpha, Catenulida, Rhabditophora. Prosthiostomum Quatrefages, 1845. Tyler, S., Artois, T., Schilling, S., Hooge, M. & Bush, L., F. (eds.). World Register of Marine Species (WoRMS). http://www.marinespecies.org/aphia.php?p=taxdetails&id=142239
Waloszek, D. & Dunlop, J., A. (2002) A Larval Sea Spider (Arthropoda: Pycnogonida) From The Upper Cambrian `Orsten’ of Sweden, and The Phylogenetic Position of Pycnogonids. Palaeontology. 45(3), 421-446.
Walter, T., C. & Boxshall, G. (2020) World of Copepods database. http://www.marinespecies.org/copepoda https://doi.org.10.14284/356
Wang, Q., Yan, L. & Zheng, X. (2019) Morphological and histological characterization of a new Acropora-eating flatworm: A potential threat to captive acroporid corals. Aquaculture. 512. 734384.
Wijgerde, T. (2002) Epizoic flatworms impair coral feeding: evidence for parasitism. https://reefs.com/magazine/epizoic-flatworms-impair-coral-feeding-evidence-for-parasitism/
Wijgerde, T., Schots, P., Van Onselen, E., Janse, M., Karruppannan, E., Verreth, J. & Osinga, R. (2013) Epizoic acoelomorph flatworms impair zooplankton feeding by the scleractinian coral Galaxea fascicularis. Biology open. 2(1), 10-17.
Wijgerde, T., Spijkers, P., Verreth, J. & Osinga, R. (2011) Epizoic acoelomorph flatworms compete with their coral host for zooplankton. Coral Reefs. 30(3), 665.
WoRMS (2020a) World Register of Marine Species. Amakusaplana Kato, 1938. http://www.marinespecies.org/aphia.php?p=taxdetails&id=480067
WoRMS (2020b) World Register of Marine Species. Prosthiostomum Quatrefages, 1845. http://www.marinespecies.org/aphia.php?p=taxdetails&id=142239
WoRMS (2020c) World Register of Marine Species. Parategastes Sars G.O., 1904. http://www.marinespecies.org/aphia.php?p=taxdetails&id=115445
WoRMS (2020d) World Register of Marine Species. Alteuthellopsis Lang, 1948. http://www.marinespecies.org/aphia.php?p=taxdetails&id=150019
WoRMS (2020e) World Register of Marine Species. Ammotheidae Dohrn, 1881. https://www.marinespecies.org/aphia.php?p=taxdetails&id=1562





0 Kommentare