We often hear that we cannot artificially replicate the amount of light that falls upon a natural reef. This assumption is likely based upon visions of shallow reefs bathed by an unblinking noontime sun, where both sky and ocean are crystal clear. This mental snapshot is supported by beautiful photographs of various paradises in travel brochures, and is something we want to capture and display in our homes. But which is the best way to achieve this? Can we as the masters of small water-filled glass boxes really hope to achieve the intensity of natural sunlight, and more importantly, do we really want to? How does light quality affect zooxanthellae, and ultimately the symbiosis between the coral animal host and its symbionts? What role can the photoperiod potentially play? Can coral farmers decrease grow-out times by using prolonged illumination periods?
This article will look at the evidence gleamed from various peer-reviewed journal articles as well as data gathered here in Hawaii over the last few years. We’ll begin our discussion with that of something not often considered when lighting reef aquaria – light dosage.
Light Dosage, or Daily Light Integral (DLI)
As hobbyists, we generally think of lighting in the terms of two separate components: Intensity and Photoperiod. In reality, these two are interlinked and can be used to calculate a third component: The Daily Light Integral (DLI). One of the best analogies I’ve heard about the value of DLI is as follows: An instantaneous measurement of PAR is analogous to the number of raindrops falling upon a given area in a given time. We should be more interested in the total amount of PAR falling upon a given area per day, which is similar to inches of rainfall per day.
DLI is important since it allows us to mathematically determine the total amount of radiation falling on a particular object (such as a coral), which then can be manipulated to arrive at other datum (such as an average amount of light).
The formula for DLI is simple. It is Photosynthetically Active Radiation (PAR, reported as micromole per square meter per second, or molm²sec) times the photoperiod in seconds.
For example, let’s determine the DLI for a coral receiving 250 molm²sec for 12 hours:
250 molm2sec * (12 hours * 60 minutes per hour * 60 seconds per minute, for a total of 43,200 seconds) = 10,800,000 molm² in 12 hours. We can divide the result by 1,000,000 to arrive at usable shorthand of Mol per Day, which is 10.8 Mol per Day.
Another, slightly more complicated, example:
- Actinic lamps delivering 100 molm2sec on at 6 am, off at 8 pm (14 hours). A metal halide lamp delivering an additional 300 molm2sec is on from 11 am to 6 pm (7 hours).
- Actinic lamp DLI = 100 molm2sec * (14 * 60 * 60) = 5.04 Mol per Day.
- Metal halide DLI = 300 molm2sec * (7 * 60 * 60) = 7.56 Mol per Day, for a total DLI of 12.6 Mol per Day.
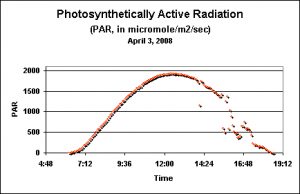
Figure 1. ”Terrestrial’ PAR values as recorded by a data logger just prior to the seasonal Pocillopora meandrina spawnings in Hawaii. This day is shown because of its relative lack of afternoon cloud cover.
These DLIs are useless without a reference point. What is the DLI of sunlight falling upon a location with real coral reefs (such as the Big Island of Hawaii)? Unless you wish to sit in the baking sun taking PAR measurements every couple of minutes all day long, the task seems impossible (or at least most tedious and extremely boring). Fortunately, there are some very good data loggers available. These instruments can be easily programmed to make PAR measurements at various time intervals. All you’ve got to do is program the device, put it in place and retrieve the data once done. The examples below were made by a WatchDog™ data logger manufactured by Spectrum Technologies. Figure1 shows the PAR values taken above water at Kealakekua on the Big Island of Hawaii just days before the seasonal spawning events of the stony coral Pocillopora meandrina. This is a good example of the amount of PAR falling on the ground during a relatively cloudless day (something of a rarity here in Kona, since clouds build around the Hualalai volcano during the heating of mid-morning and usually spread out over the ocean in the afternoon). The data logger was programmed to take a light measurement every five minutes, for a total of 150 measurements during the day lasting about 12.5 hours. Spectrum Technologies’ Basic 8™ software relieves us of the tedium of calculating the DLI. A few keystrokes are all it takes to make the calculation, which in this case is 48 Mol per Day.
Underwater Light Measurements and DLIs
As we all know, water rapidly absorbs light and alters its intensity and spectral quality. Fortunately, PAR sensors are relatively immune to the effects of shifting spectral qualities and can accurately report light intensities seen at various depths. So, what is a good estimate of a DLI found in only a few feet of water? To answer this question, the PAR sensors (easily waterproofed with silicone cement) obtained from Spectrum Technologies were attached to a WatchDog™ data logger and either housed on a floating laboratory or secured onshore.
Spring DLI Measurements

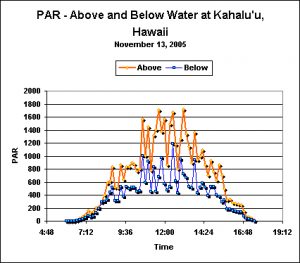
Figure 2. ‘Air’ and ‘Water’ PAR measurements. Note that a ‘lensing’ action by passing waves can make sunlight more intense at depth than at the water surface. The sharp drop in light intensity at noon is due to cloud cover. See text for details.
These measurements were made one day prior to the stony coral Pocillopora meandrina spawnings at Kahalu’u Beach Park, Hawaii (Big Island). The morning was exceptionally clear, with some cloud cover present at noon and a gradually clearing during the afternoon (see Figure 2). Water depth ranged from less than 1 foot during the morning low tide to about 3 to 3.5 feet at high tide in the afternoon. Rising surf necessitated recovery of the floating laboratory and sensors at approximately 4:30 pm (sunset was at 6:45 pm); therefore the originally calculated DLI estimations were a bit low. However, I am comfortable with the estimations made to ‘fill in the blanks’ which increased both water and air DLIs by approximately 1 Mol photons.
It should be noted that recovery of the equipment was a dangerous proposition, due to violent wave action even in sheltered, shallow water. See here for additional details on conditions seen during the seasonal coral spawning the next day:
Once data were downloaded and analyzed, these were the results:
- ‘Air’ DLI: 41 Mol photons/Day
- ‘Reef’ DLI: 30 Mol photons/Day
Late Fall (November) DLI Measurements
Roughly the same procedure described above was used in obtaining light data in November 2005. The site was a semi-protected tide pool at Kahalu’u’s Outrigger Resort in Keauhou, Hawaii and is located about 1,000 feet south of the Kahalu’u Beach Park (described above). See Figure 3.
Analyses of data revealed the following DLIs:
- ‘Air’ DLI = 30 Mol photons/Day
- ‘Reef’ DLI = 16 Mol photons/Day
Effects of Excessive Photoperiod and Spectral Quality

Figure 4. A Euphyllia glabrescens was used in the experiments. Photo by the author, taken at Jenn Myerscough’s Imagine Ocean in Canton, Georgia.
Successful symbiosis between the coral host and its zooxanthellae depends upon the maintenance of a stable population of symbionts. A number of factors can influence the number of zooxanthellae contained within the coral, including their nutritional states possibly resulting in nutrient limitations, temperature modulations (high or low) resulting in expulsion of symbionts, and so on. It is critical that outside factors influencing dramatic shifts in zooxanthellae populations are minimized. Recent research has revealed that an excessively long photoperiod and spectral qualities can temporarily disrupt the reproduction cycle of zooxanthellae. Wang et al. (2008) have described effects of both of these factors in a highly detailed examination of zooxanthellae isolated from a ‘Torch’ coral (Euphyllia glabrescens; See Figure 4). Information presented below is based almost entirely upon these researchers’ results. Before beginning our examination, we should be familiar with the reproductive phases of plants in general and zooxanthellae in particular.
The Plant Cell Cycle
Zooxanthellae reproduce in an established order involving replication of genetic material and cellular division (See Figure 5). This cycle is subdivided into 4 distinct phases, including:
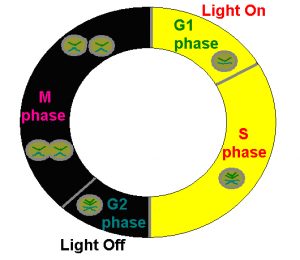
Figure 5. The Plant Cell Cycle is applicable to the genus Symbiodinium (zooxanthellae) and represents observed cycles discussed in the text. After Wang et al., 2008.
- G1 Phase: The freshly divided zooxanthellae are in a growth phase and the rate of protein synthesis is very high. The zooxanthellae are at their highest photo-efficiency.
- S Phase: The synthesis phase, where chromosomes are replicated in anticipation of cell division.
- G2 Phase: A ‘resting’ phase where feedback to the zooxanthellae determines ‘go’ or ‘no-go’ on cellular division.
- M Phase: The phase where cellular division and hence reproduction (Mitosis) occur.
The cycle is now complete and the newly divided cells enter the G1 Phase. The process begins anew. Interestingly, in order to maintain zooxanthellae population densities, some corals and anemones expel zooxanthellae as they enter the M Phase.
Wang et al. established timing of zooxanthellae reproduction phases when maintained at a photoperiod of 12 hours of light (40 -100 molm²sec) and 12 hours of darkness. See Table 1.
| Hour | Reproductive Phase |
|---|---|
| 5 | Growth (G1) |
| 11 | Growth (G1), Synthesis (S), Reproduction (G2 and M Phases) |
| 17 | Growth (G1) and Reproduction (G2, M Phase) |
| 23 | Growth (G1) and Reproduction (G2, M Phase) |
| 29 | Growth (G1) |

Figure 6. Over 50% of zooxanthellae are reproducing (that is, in mitosis) at Hour 23 when maintained under ‘pure’ blue light for 12 hours. Lamps were off between Hours 12 and 24. After Wang et al., 2008.
Effects of Altered Light Spectral Qualities
We’re beginning to realize spectral quality plays important role in the health and growth of corals’ zooxanthellae and hence the coral host. More importantly, we are starting to understand why and how light quality affects zooxanthellae and host pigmentation.
Wang and his group of researchers exposed zooxanthellae isolated from the stony coral Euphyllia glabrescens to differently ‘colored’ light in order to examine the effects of spectral quality on the symbionts’ reproductive cycles. LEDs provided essentially pure ‘blue’, ‘red’ and ‘infrared’ light. (Note that these results are probably not just applicable to light generated by LEDs, but to any essentially monochromatic light). See Figures 6-9.
In a nutshell, ‘blue’ light and a mixture of ‘blue’ ‘red’ and ‘infrared’ wavelengths were about the same in promotion of normal zooxanthellae reproduction (although the ‘blue’ light seems to be slightly more effective).

Figure 7. Only ~20% of zooxanthellae cells are dividing at Hour 23 when maintained under ‘pure red’ light. After Wang et al., 2008.
Exposure to only ‘red’ light significantly inhibits the productive cycle (is this the reason for the slightly less efficiency of the ‘mixed’ light?). Infrared light apparently plays no part in regulation of zooxanthellae reproductive cycle, and the algal cells remain in the G1 Phase with no DNA synthesis or mitosis.
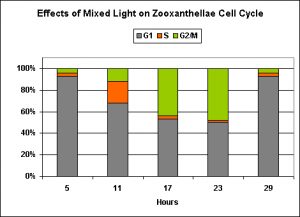
Figure 8. This ‘mixed’ lighting (consisting of blue, red and infrared light) is less efficient than ‘blue light’ at promoting mitosis in zooxanthellae. This is likely due to the presence of red light, since infrared has no effect on zooxanthellae reproduction (See Figure 9). After Wang et al., 2008.
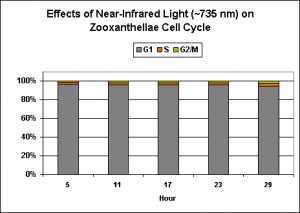
Figure 9. Near-infrared ‘light’ does not play a beneficial part in promoting zooxanthellae reproduction. After Wang et al., 2008.
Effects of Prolonged Illumination
If you read some of the reef-related internet sites, you’ve likely run across at least one thread questioning the effects of prolonged photoperiods on zooxanthellae. Again, Wang and his group have provided some answers (at least for some Clade B zooxanthellae).
In an experiment where continuous illumination was provided for 72 hours, zooxanthellae maintained a natural progression of reproductive phases for at least the first 11 hours. At Hour 17, unnatural populations of phases were noted, and the trend lasted for the duration of the experiment. Notably, zooxanthellae contained an abnormal number of chromosomes (designated as ‘3-4 Chromo’ in Figure 10) and failed to divide in an orderly fashion.
Conclusions
Is It Possible to Provide ‘Natural’ Amounts of Light?
Yes. It is possible to provide the same number of photons (light energy) to captive symbiotic invertebrates – even those found in extremely shallow water. For instance, corals fully-exposed to intense sunlight (such as demonstrated in Figure 2) can receive ~30 Mol photons per Day. Using this formula, we can arrive at an average light intensity.
- First, we convert 30 Mol photons to mol by multiplying by 1,000,000.
- Hence, 30 Mol * 1,000,000 = 30,000,000 mol.
- 30,000,000 divided by the length of the photoperiod (say, 12 hours) = 2,500,000 mol.
- 2,500,000 divided 60 minutes per hour = 41,667 mol.
- 41,667 mol divided by 60 seconds per minute = 694 molm²sec.
It might take some effort to illuminate an aquarium with this amount of light, but it is possible. But do we really want to provide this amount of light?
Is It Necessary to Provide Maximal Illumination?
No. Common sense and a quick look at reef aquaria proves that most photosynthetic invertebrates will thrive under conditions of relatively little light. There is no evidence that I am aware of that suggests corals’ zooxanthellae require supersaturating light intensities in order to maintain growth rates and/or provide proper nutriment to the coral animal. Most ‘common’ corals saturate (that is, photosynthesis is at a maximum rate) at light intensities ranging from 200 to 450 molm²sec.
Can I Increase the Photoperiod and Provide Less Light in Order to Maintain ‘Natural’ Light Dosage?
Existing evidence suggests that extended photoperiods, or worse, non-stop illumination should be avoided (this should not be construed to mean cycles mimicking daylight and weak moonlight are to be resisted).
Done mostly for my own amusement, Table 2shows the PAR values required to simulate Spring and Fall DLIs of shallow water corals in Hawaii. DLIs are listed at the far left. Scroll down from Hours of Illumination (at top) to determine the PAR values (in molm2sec) required to deliver either the Spring or Fall DLI. Italicized fields designate levels warning of over-illumination of the most light-tolerant corals (Pocillopora meandrina, Porites lobata, and various Acropora spp.). Bolded highlighting warns of an excessive photoperiod, possibly resulting in disruption of zooxanthellae reproductive cycles. Note that some low-light corals will bleach under these light intensities!
| Hours of Illumination | 30 Mol photons/Day | 16 Mol photons/Day |
|---|---|---|
| 1 | 8333 | 4444 |
| 2 | 4167 | 2222 |
| 3 | 2778 | 1481 |
| 4 | 2083 | 1111 |
| 5 | 1667 | 889 |
| 6 | 1388 | 741 |
| 7 | 1190 | 634 |
| 8 | 1041 | 556 |
| 9 | 926 | 494 |
| 10 | 833 | 444 |
| 11 | 758 | 404 |
| 12 | 694 | 370 |
| 13 | 641 | 342 |
| 14 | 595 | 317 |
| 15 | 556 | 296 |
| 16 | 521 | 278 |
| 17 | 490 | 261 |
Is It OK to Extend the Photoperiod Beyond ‘Natural’ Lengths?
Existing evidence suggests that photoperiods of at least 17 hours per day can cause disruptions, if only temporarily, of zooxanthellae belonging to Clade B isolated from the stony coral Euphyllia glabrescens. This might seem trivial, but consider that at least some corals regulate symbiont populations by expelling zooxanthellae cells entering the M phase (mitosis). In this situation, it could be possible that the coral host expels an excessive number of zooxanthellae. Unfortunately, the experiments of Wang et al. examining the effects of prolonged illumination were terminated at 72 hours. However, this evidence combined with the fact that constant or prolonged photoperiods are unnatural, it is probably best to avoid periods of illumination exceeding much more than 14 or 15 hours per day.
Isn’t A Large Dosage of Red Light Unnatural to Zooxanthellae?
Yes, in many cases. Since red light is rapidly absorbed by the water column, corals inhabiting depths of more than just a few meters do not receive a lot of red light. In aquaria, we desire some red light in order to observe the sometimes gaudy coloration of fishes and invertebrates. Obviously, many corals thrive in these conditions and it is difficult to state that small amounts of red light have any lasting, truly negative effects.
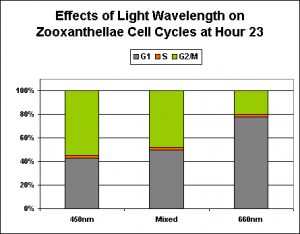
Figure 10. Effects of essentially monochromatic and mixed light on zooxanthellae reproduction at Hour 23 of a photoperiod consisting of 12 hours light and 12 hours darkness.
However, the evidence continues to mount that ‘strong’ red light can have detrimental effects, if even temporarily, on corals and other photosynthetic invertebrates. The effects of ‘pure’ blue, red, and infrared wavelengths have been examined individually and in combination. The results strongly suggest that ‘pure’ red light (at ~660 nm) can inhibit zooxanthellae reproduction rates.
Some photosynthetic invertebrates (such as Aiptasia anemones, and stony corals Pocillopora damicornis and Acropora formosa) regulate symbiont populations through measured expulsion of zooxanthellae when they enter the division stage, and is perhaps due to the inability of the host to contain them. However, not all corals preferentially expel zooxanthellae entering the mitotic stage. These corals are known to include Hawaiian Porites compressa, Montipora verrucosa (now M. capitata), and Fungia scutaria (Baghdasarian and Muscatine, 2000). To further complicate the matter, some corals only very slowly expel zooxanthellae and this slow expulsion rate is not likely to have an effect on symbiont populations (these corals include Xenia macrospiculata, Heteroxenia fuscescens, Millepora dichotoma and Stylophora pistillata from the Red Sea; Hoegh-Guldberg et al., 1987). It is possible that different zooxanthellae clades have different mechanisms of dealing with host physiology and/or other environmental factors.
See Figure 10 for a comparison of different colored light on the reproductive cycles of zooxanthellae.
This concludes our discussion of light intensity and spectral quality on zooxanthellae.
I’m excited about projects that are now in the planning stages. Perhaps most importantly is the Pocillopora meandrina mass-spawning in the first week of May 2009. Last year was a bumper crop for these corals, but no reports of spawnings have been made by numerous volunteer observers so far this year. I’m hoping to see a mass spawning soon, and the goal of my research this year is to get coral planula larvae to settle (something that has not occurred in any lab I’m aware of with any repeatability).
Questions? Comments? I’m best reached at [email protected].
References
- Baghdasarian, G. and L. Muscatine, 2000. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull., 199: 278-286.
- Smith, G. and L. Muscatine, 1999. Cell cycle of symbiotic dinoflagellates: variation in the G1 phase-duration with anemone nutritional status and macronutrient supply in the Aiptasia pulchella-Symbiodinium pulchrorum. Mar. Biol., 134: 405-418.
- Wang, L.-H., Y.-H. Liu, Y.-M. Ju, Y.-Y. Hsiao, L.-S. Fang and C.-S. Chen, 2008. Cell cycle propagation is driven by light-dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral Reefs, 27:823-835.



0 Comments