About two years ago a thread1 started up on the SPS (small-polyp scleractinian) Coral Keepers sub-forum on Reef Central. I read through the thread and suddenly felt rather ill. The thread described a species of small ‘bug’, almost like a ‘mite’ that infected genus Acropora corals and basically sucked the life out of them. I dashed down to one of my fragment grow out tanks and sure enough, there they were, just as described on the forum. I’d been wondering why some of the frags in my grow out tank were growing slowly, or not at all, and had poor color. I dashed upstairs to examine corals in my main SPS display system, no sign of them, ‘phew!!’ Dodged that bullet!
Where Do They Come From?
I undoubtedly got the parasites from someone I traded Acropora coral fragments with. I’d always loved the idea of acquiring the next new ‘hot’ colored Acropora that grew at light speed under normal output fluorescent bulbs. I’d been shipping and receiving Acropora from all over the US over the past few years. Clearly, I paid dearly for not practicing ‘safe trading.’ Of course the parasites must originally have come from the wild, but in the wild something must suppress their growth, or prey on them. When browsing pet shops I’ve noticed that it is very rare to find a wild collected Acropora with the parasites. Over the course of numerous scuba dives trips in the Pacific and Caribbean I also have yet to find an Acropora in the wild infested with these parasites.
What Do They Infect?
In my experience the parasites ONLY infect corals of the Acropora genus. I have had numerous other SPS corals in the same tank, frequently right next to infected Acropora colonies that never became infected themselves. Some of these included Pocillipora damicornis, Stylophora, multiple Montipora spp., Heliopora, and even the closely related Anacropora. My experience also has been that the parasites only affect certain Acropora species. Exact species identifications within the Acropora genus are of course very difficult to assign, but I’ve found that the commonly know ‘Green slimer’ Acropora (sometimes called A. youngei ) seems to be immune to the parasites. Could it be that the extra thick slime coat this Acropora secretes somehow protects it? Some Acropora species are only moderately affected and are likely carriers of the disease. Such colonies continue to grow well, and healthy, the owner thinks nothing of trading the fragments of that colony with other
reefkeepers. Once in a new tank, the parasites travel to Acropora species/variants more to their appetite and can eventually kill such colonies. My experience has been that if left untreated, only about 20% of Acropora species/variants are killed by the parasites, perhaps another 25% are stunted, or loose color, maybe another 25% are carriers, and the final 30% are immune and do not become colonized. Of course in times of other tank stresses like high temperatures, poor circulation, low calcium or alkalinity values, the additional ‘pressure’ that these parasites put on a colony might be enough to push other colonies ‘over the cliff.’
What Does An Infection Look Like?
The first indication that a hobbyist has that a colony is infected with the parasites is usually a loss of color. The colony will loose its colored tips (if it has them), and in general turn a very pale color. Upon close inspection, even with the naked eye, the tiny yellowish ‘bugs’ with a red spot on one end of their bodies become apparent. The parasites can actually move about on the colony quite quickly and easily, and this motion can often be detected with the naked eye. One of the best places to look for the parasites is on the shaded side of the colony, where the polyps may be more sparse, and the tissue smoother. I estimate the size of the parasites to be about 500 microns, or 1/2 of a millimeter.
Two videos of the organisms – taken by Ramon Primicias:
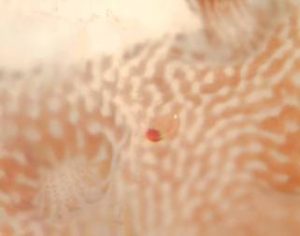
Figure 2 – Close up photo of the parasite on an Acropora – note size in relation to coral polyp – photo by author
What Are These Critters?
Good question, and one that is not easily answered. Dr. Ronald L. Shimek described the parasites as harpacticoid copepods probably of the genus Tegastes2, but the parasite is not easily distinguished even under the microscope from stenothoid amphipods which are also found on Acropora and are not parasites. He further commented that to his knowledge, no scientific research is being done on these organisms to determine their effect on corals.
I sent formalin-preserved specimens to coral researchers at the Waikiki Aquarium in Honolulu and they were baffled. They forwarded the specimens to The Bishop Museum in Waikiki. The invertebrate zoologist at the museum, Dr. Luscius G. Eldredge identified the organisms as micro-amphipods due to their “large colored eyes, short antennae, and laterally-compressed, bent abdomen.” Other experts in amphipods or copepods that I attempted to contact, either couldn’t be bothered, or were of little help.
Why should I care?
Well, in a nutshell, these parasites can kill some species/variants of Acropora. It may be a slow process, taking perhaps weeks or months, but watching that Acropora colony die that grew in five years from a one-inch fragment to a colony that eventually breaks the water’s surface is not what I call fun. Also, once you have acquired the parasites and word gets around, you may be the ‘Typhoid Mary’ of your local reefkeeping community, and certainly no one will want to be trading corals with you!
Okay, I’ve got them and I care, how do I get rid of them (the first step in recovery is admitting you have a problem)
The method of eradication, if it can be accomplished will depend upon the characteristics of the Acropora infected, the level of infestation, and the nature of the system that the affected colony(s) are in.
1. Mechanical Means
If the infestation is not too bad you can blast them off a colony with a powerhead. This works particularly well if you ‘sneak’ up on them; i.e., blast them suddenly with a powerhead before they have a chance to ‘hunker down.’ Then you must check very carefully with a magnifying glass (or preferably a dissecting microscope) to make sure you got them all. If you blast the colony repeatedly over several days, you might just rid the colony of all of the parasites. You might also have to pluck a few off with a tweezers. I cleared one branch of a particularly colorful Acropora with this method, but in hindsight, if I’d had a clean piece of the Acropora in an uninfected system I certainly wouldn’t have bothered. Of course this powerhead blasting technique should only be used in a tank or container where the now free-swimming parasites won’t infect other colonies. It should also be mentioned that the parasites will sometimes crawl into a polyp and subsequently crawl out,
apparently none the worse for the wear, so a single observation of a colony after a powerhead blasting should not be used as a 100% certainty of the successful removal of all of the parasites. If you have time, and enough tanks, the best test of removal would likely be placing a colony that you know is particularly susceptible to the parasites next to a colony you believe you have just ‘cleaned’, and wait for several weeks or maybe even months. If the susceptible colony is clean after two months you can be nearly certain that you have ‘cleaned’ the originally infected colony.
2. Biological Means
The fish I’ve tried for control include a six-line wrasse ( Pseudocheilinus hexataenia ), a blue Mandarin ( Synchiropus splendidus ), and an orange- spotted filefish ( Oxymonacanthus longirostris ). None of these fish had any impact on the populations that I could see. The orange-spotted filefish is known to feed on certain SPS corals, and I had hoped it would also consume the parasites as a side dish, but despite much time observing the fish in a tank with the parasites I never saw the fish consume them. Please no flames on the keeping of an orange-spotted filefish; while it is a very difficult fish to maintain long term, I have succeeded in the past with this fish. Pipefish of the genus Corythoichthys have also been recommended, but not tested, as a possible control of the Acropora parasites 3. This genus of pipefish ‘crawls’ along the surface of corals and rocks, pecking at various minute crustaceans and other organisms. While not the easiest fish to maintain, these pipefish4 can be successfully kept in a healthy and diverse reef system. Other aquarists have tried citron gobies (Gobiodon citrinis ) and neon gobies ( Gobiosoma oceanops ) without success.1
The only species of fish that some have reported success with is the yellow clown gobies ( Gobiodon okinawae ).1 I’ve kept this species of goby before and while they are beautiful and entertaining little fish, they have a somewhat annoying habit of clearing the tissue away from sections of branching hard coral to make a spot to lay their eggs. Fortunately, usually once they clear a spot they don’t do much more damage. Some hobbyists have reported that the gobies will clear the parasites from Acropora colonies that they nest in, but will not clear the parasites from other colonies in the tank. Unfortunately, the gobies are not a panacea, as other hobbyists have reported zero success with these gobies.
Some reefkeepers have proposed that the common symbiotic Xanthiid crabs that nearly always inhabit wild Acropora colonies and sometimes defend these colonies from certain threats might be of some value in the control of the Acropora parasite.1 However, others have also had infected Acropora colonies, complete, with a Xanthiid crab completely ignoring the parasites. I conjecture that the colonies that had the crabs and no parasites were coincidentally a species of Acropora that happened to not be susceptible to the parasites.
Obviously there must, in nature, be some organism or environmental factor that limits the numbers and destructiveness of these Acropora parasites. Ideally in the future on the reefkeeping forums such an organism would be well known and a simple, quick, and easily obtained critter could be recommended. One such example of an easily obtained pest control organism that comes to mind is the now well known peppermint shrimp’s ( Lysmata wurdermanni ) control ability for Aiptasia genus anemones that so commonly become problematic in the captive reef environment. If anyone is aware of such an organism for the Acropora parasite control I hope they make the information widely available.
3. Chemical Means
The one chemical means of control that has worked for me was the coral dip called Reef Dipâ manufactured by Seachem. I used it at the maximum recommended dosage for a 15 minute dip and it seemed to be a 100% kill with minimal effect on the Acropora colonies that I experimented with. I performed the dip twice on two consecutive days. However, I have had variable results with this dip. The dip, even when administered several times over days or weeks was not 100% effective for highly infected Acropora colonies. The parasites would often return, even when the treated colony was placed into a new tank that was free of Acropora. Some have reported more success using this dip at many times it’s recommended dosage level and duration of treatment.5 Lugol’s strong iodine solution can be used in a similar manner to the SeaChem dip. The SeaChem dip appears to be iodine based, but might have other components making it more effective than Lugol’s solution alone. Lugol’s solution can be used at 10 drops per liter for a 15 minute treatment in an isolated container. Such a dip is commonly used on newly acquired hard corals to prevent RTN (rapid tissue necrosis) outbreaks in SPS dominated tanks.
I have also experimented with Tetra Medica’s Oomedâ to remove the Acropora parasites. Oomed is a quinine based chemical preparation for treating fish diseases that is no longer available in the US. Some have found this chemical useful for controlling the red photosynthetic flatworm ( Convolutriloba retrogemma ) in reef systems. Observing an Acropora branch under a microscope I found that this chemical irritated the mites at low concentrations, and killed them at high concentrations, but these high concentrations might be too much for most Acropora. The mites clearly started to be much more active and moved about or swam off the coral more quickly when the Oomed was used. This high activity level might help facilitate mechanical removal. I’ve not tried to treat a whole tank with the Oomed, only individual colonies have been treated in external dips. I’ve also tried freshwater dips, but not found this to be a good solution as the parasites seem to
be able to ‘hide’ in the slime coat of the Acropora, and a dip of sufficient length to kill the parasites also kills the coral. Of course this ‘killing of the patient’ is the overriding problem with all of the mentioned treatments/eradication methods.
One additional chemical whole tank removal technique was related to me by a local reefkeeper.5 He found that while treating his tank with Red Slime Removerâ from Ultralife for control of a plague of green cyanobacteria a side benefit was that the Acropora parasites also seemed to disappear. In a later experience he came to believe that the mere chemical treatment with the Red Slime Removerâ was not sufficient, but significant quantities of red or green cyanobacteria also had to be in the tank for the chemical treatment to also kill the Acropora parasites.
4. The Waiting Game
This, in my experience, is a 100% effective method of eradicating the parasite from a reef system. If ALL Acropora genus corals are removed from a tank and the tank is left ‘_Acropora_ free’ for a week, preferably two weeks, any ‘clean’ Acropora colonies later added to the tank will not become infected. Unfortunately, for well-established tanks that have Acropora encrusted all over the rock structure it may be nearly impossible to do this. I performed this technique on two of my fragment grow out tanks and was successful in the eradication of the parasite both times. It was heart- wrenching throwing out mildly infected beautiful Acropora colonies from the tanks, but I saw no alternative after months of external experiments. I still count my stars that while I was unaware of the existence of the parasite in my fragment grow out tanks it did not infect my main hard coral display tank. I was always careful only to move new Acropora colonies or fragments into my main display tank, when I was fairly certain there was nothing ‘strange’ going on in my fragment tanks, and when I had not added any newly acquired corals to the fragment grow out tanks in quite some time.
In closing I would recommend that anyone with a reef tank with significant number of Acropora colonies should be particularly careful when introducing a newly acquired Acropora specimen. At a minimum, careful visual examination of the colony with a small magnifying glass, and a prophylactic chemical dip are in order. Aquarists with well-established Acropora display tanks should consider the above plus a lengthy quarantine and careful observation in a separate system.
References
- Red bugs on your SPS? Share your experiences. http://reefcentral.com/forums/showthread.php?s=4d406c12c379c7edad80665479e31a 7d&threadid=39956
- Bitty Bugs: Copepods in the Reef Aquarium, by Ronald L. Shimek. http://reefkeeping.com/issues/2002-10/rs/index.htm
- Personal communication, September, 2002, Bruce Carlson.
- There’s More to Pipes Than Just PVC: The Genus Doryrhamphus and Other Pipefish, by Henry C. Schultz, III. http://reefkeeping.com/issues/2003-04/hcs3/index.htm
- Personal communication, November, 2002, Jake Maki.




0 Comments