This month, we’ll take a look at something most hobbyists would prefer to never see – coral parasites of genera Alteuthellopsis, Parategastes, and Tegastes (the latter two commonly called ‘red’ or ‘black bugs’).
- Order: Harpacticoida
- Family: Peltidiidae
- Genus: Alteuthellopsis
- Family: Tegastidae
- Genus: Parategastes, Tegastes
As a general rule, ‘bugs’ of Order Harpacticoida are smaller in size than Xarifiid copepods, with a length of about 0.5mm being the maximum size. They are generally considered to be endoparasites and are thought to reside mainly in coral polyps’ gastrovascualr cavity.
Order: Harpacticoida; Family: Peltidiidae; Genus: Alteuthellopsis
Alteuthellopsis corallina
- Hosts: Acropora exigua, Astreopora sp., Goniastrea retiformis, Merulina ampliata, Montipora verilli, Platygyra daedala, Platygyra sp., andPocillopora damicornis.
- Maximum Reported Size (female): 0.63mm
- Maximum Reported Size (male): 0.57mm
- Color: Slightly opaque gray, red eye, egg sacs dark brownish gray.
- Locality: Eniwetok Atoll, Marshall Islands, Lizard Island, GBR
- Reference: Humes, 1981
Order: Harpacticoida; Family: Tegastidae; Genus: Parategastes, Tegastes: Common Name: Red bugs, black bugs
‘Red bugs’ are the bane of Acropora keepers and it is popular belief among hobbyists that they are the only parasites of stony corals. The ‘evil’ Tegastes acroporanus has been officially described from but one Acropora species.
Copepods of the family Tegastidae (Crustacea, Copepoda, Harpacticoida) are characterized by a laterally compressed amphipod-like body, a modified male genital area, and nauplii possess a claw-like mandible (Lang 1948, Ivanenko et al. 2008). About 60 species belonging to 6 genera have been described, although those listed as parasitic to corals are small relative to the number of Xarifiid species known to infest corals. As a rule of thumb, Tegastidae copepods are much smaller than xarifiid copepods.

Figure 134. Tegastes cnidicus. Although not a known coral parasite (this species prefers hydrozoans), its shape is representative of its kind. Lateral view.
Except for the deep-sea species Smacigastes micheli (Ivanenko & Defaye 2004), all tegastid species have been found in shallow water habitats in association with algae, bryozoans and/or cnidarians. Not all are animal parasites. For instance, Tegastes nanus is found in association with the red alga Ceranium and brown algae genera Fucus and Laminaria. Tegastes falcatus feeds upon suctorian cilicates found on the bryozoan Flustra foliacea, while T. longimanus is found along sheltered rocky shores and salt lakes. T. acroporanus is a known parasite of at least one coral, the Pacific Acropora species A. florida.

Figure 136. ‘Red Bugs’ on an Acropora specimen appear red when they are reflecting light. Photo by the author.

Figure 137. Note the red coloration concentrated in the caudal ramus, and the reflective eye spot. Are these Tegastes paulipes? Photo courtesy of Greg Ho and www.ximinasphotography.com

Figure 138. Same coral as that in Figure 137. Notice the bugs within the Acropora‘s corallites. Are these parasites attempting to enter the coral’s gastrovascualr cavities? Or are they being eaten? Photo courtesy of Greg Ho and www.ximinasphotography.com

Figure 139. A ‘red bug’ showing a reddish-brown coloration in transmitted light. Compare this to photos below. Photo by the author.

Figure 140. The same Tegastes specimen as shown in Figure 139 contains a fluorescent protein and appears green when appropriate excitation light is applied. Has this bug incorporated fluorescent proteins obtained from its coral host? Photomicrograph by the author.

Figure 141. Not all bugs are red, as this ‘black’ Tegastid copepod shows. This unidentified specimen was found on a Montipora coral. Microphotograph by the author.
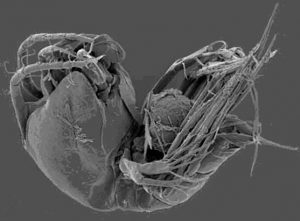
Figure 142. Although a deep-water species, this Smacigastes micheli demonstrates the beauty (or creepiness, depending upon your point of view) of parasitic copepods. From Ivanenko & Defaye 2004.
Tegastes acroporanus
- Host: Acropora florida
- Maximum Reported Size (female): ?
- Maximum Reported Size (male): ?
- Color: Red, according to aquarists’ reports
- Locality: Eniwetok Atoll, Marshall Islands
- Reference: Humes, 1981
- Tegastes gemmeus
- Host: Cyphastrea Celina and Pocillopora capitata (listed as P. verrucosa)
- Maximum Reported Size (female): 0.43mm
- Maximum Reported Size (male): 0.41mm
- Color: Gray with red eye
- Locality: Oahu, Hawaii
- Reference: Humes, 1984
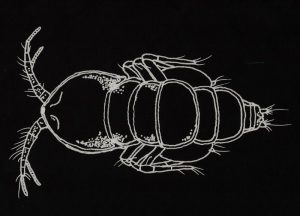
Figure 143. Tegastes gemmeus, a copepod known to infest Hawai’ian corals. This is a typical dorsal view of Tegastes and Parategastes.
Tegastes georgei
- Host: Stylophora sp. and Pocillopora sp.
- Maximum Reported Size (female): ?
- Maximum Reported Size (male): ?
- Color: ?
- Locality: Gulf of Eliat, Red Sea
- Reference: Humes, 1984
Tegastes paulipes
- Host: Pocillopora verrucosa
- Maximum Reported Size (female): 0.42mm
- Maximum Reported Size (male): 0.42mm
- Color: Light pale tan to gray with darker amber areas, red eye, egg sacs gray.
- Locality: Moluccas Islands
- Reference: Humes, 1984
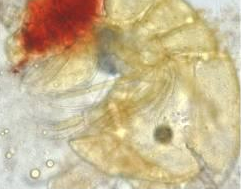
Figure 145. A ‘red bug’ taken from a captive Acropora specimen. Is it Tegastes paulipes? This is quite interesting as T. paulipes is not officially recognized as a parasite of Acropora species. Photo courtesy of Greg Hiller. More details can be found here: http://www.advancedaquarist.com/issues/june2003/feature.htm
Parategastes conexus
- Host: Stereonephthya ulicoides
- Maximum Reported Size (female): 0.41mm
- Maximum Reported Size (male): 0.43mm
- Color: Opaque pale grayish tan, eye red, genital area bright red, egg sacs gray.
- Locality: Moluccas Islands
- Reference: Humes, 1984
Order: Siphonostomatoida; Family: Asterocheridae; Genus: Acontiophorus, Stockmyzon
There isn’t much information about these copepods relative to some other genera. These ‘bugs’ seem to be confined to the Atlantic and Mediterranean, and have not been reported from the Pacific.
Acontiophorus scutatus
- Host: Asterocheres astroidicola
- Reference: Humes and Ho, 1968a
Stockmyzon mucronipes
- Host: Astroides calycularis
- Maximum Reported Size (female): 1.42mm
- Maximum Reported Size (male): 1.08mm
- Color: Translucent, red eye, egg sacs gray
- Locality: Madagascar
- Reference: Humes and Ho, 1968a
Parasitic Copepods in the Reef Aquarium
An aquarium differs radically from a natural environment, and a quite different from a coral reef. Temperature, nutrients, and a number of other factors may contribute to unnatural populations or outbreaks of copepod populations. While these may contribute to abnormal infestations, hobbyists do have a number of methods available to them, including quarantine, chemical control or eradication, biological controls including deliberate manipulation of physical and chemical parameters, as well as selective control by natural predators.
Prevention
The old adage ‘An ounce of prevention is worth a pound of cure’ applies to both quarantine and husbandry procedures. However, the effectiveness of currently accepted pre-treatment and quarantine procedures are yet to be validated for many parasitic copepod genera.
Parameters Possibly Affecting Parasite Populations
Part of a successful control strategy for parasites might include manipulation of physical parameters. We’ll briefly discuss two – temperature and water motion. These are easily (but not necessarily inexpensively) controlled.
Temperature
Temperature is an easily measured parameter of any body of water and its importance in the regulation of biological and biochemical reactions is well recognized. Yet, I saw very little mention of temperature while reviewing a number of hobby-related works on parasites. However, Sparks (1985) mentions temperatures in the lower range of tolerance as important in keeping parasites, disease, etc. in check. Further, Jacoby and Greenwood (1989) reported statistically significant seasonal changes in demersal zooplankton populations in Australia (including Parategastes copepods known to preferentially inhabit certain algal beds – other Parategastes species are parasites of certain coral taxa).
Humes and Lee, 1985 found parasitic copepod Anthessius populations rose in populations of the clam Pecna in water off Hong Kong during the later part of the year. When compared to average environmental data (water and air temps, rainfall) for the area, it appears there is a general trend of more ‘bugs’ during times of warmer water. However, this time is also one of lessened rainfall that could reduce lessened nutrient loadings. This observation is by all means not conclusive, but it is an interesting one. See Figures 147 and 148.
Any seasoned hobbyist has seen blooms of various (usually undesirable) organisms within aquaria and all are due to a domino-effect of change, some undoubtedly triggered by warm water. Although we have lots of subjective evidence, work remains to be done on the effects of temperature and coral parasite populations. Maintaining the temperature as low as 22°C (~72°F) in an aquarium for tropical fishes and invertebrates should present no problems.
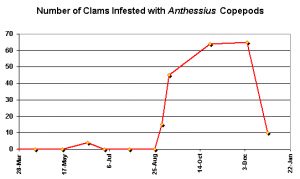
Figure 147. Clams (Pecna viridis) infested with Anthessius copepods. Compare this to the locale’s weather data, below. From Humes and Lee, 1985.
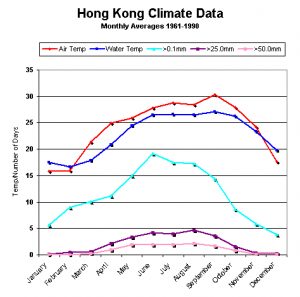
Figure 148. Hong Kong’s climate data by month. Temperatures (air and water are mean values). Rainfall is by number of days in a given month when rainfall exceeds 0.1mm, 25mm and 50mm. Source: Hong Kong Observatory (www.weather.gov.hk/cis/normal/1961_1990/enormal07.htm)
Water Motion
Experienced snorkelers or divers know that natural coral reefs are subjected to much higher water motion than seen in most aquaria. The high water velocity could serve to remove parasites from corals’ tissues where they are hence eaten by fishes. It is really a matter of speculation that low water movement might encourage parasitic infestations, however it is generally accepted that zooxanthellae photosynthesis is proportional (up to a point) to water velocity when sufficient light energy is available. If water motion is low, then the amount of nutrient obtained by the coral from the symbiotic Symbiodinium might also be low. In this case, the coral’s energy reserves could also be limited, thus an infestation by parasitic copepods might have more of an effect.
Isolation from the Host
During preparation of this article hundreds of Tegastes (or Parategastes) parasites were isolated from the host coral, and were examined microscopically for a number of days. Oddly, not one female with egg sacs was observed. These bugs remained isolated from the host for about 4 days before dying. However, plenty of protozoa were observed in the sample, suggesting that environmental conditions (dissolved oxygen, pH, etc.) were not the cause of copepod deaths.
Why were there no females reproducing? Are these particular parasitic copepods unusually long lived making rapid reproduction unnecessary?
Treatments for Parasitic Copepods
The very first question that should be asked is ‘Should I treat?’ The very best scenario is no parasites at all, but a few might do no significant harm. In addition, some parasitic copepods seem to have a high fidelity to specific coral species and the risk of infection of other coral species might be non-existent or minimal. However, infestations resulting in loss of corals’ color, polyp expansion, or general malaise should be taken seriously and followed with a regimented treatment protocol.
Copepods succumb to a variety of chemicals, and a review of older material (Kabata, 1970; Dulin, 1976) suggests use of insecticides such as DDT (!), lindane (!) and Dylox. Fortunately we don’t have to resort to such drastic (and dangerous) methods for control of coral parasites.
Successful treatments for copepods infestations are dependent upon many factors. The resistance of the animal against the treatment procedure is something we know very little, although there is little to suggest that one genus of copepod is more resistant than others to a certain ‘medication.’ If we consider each ‘bug’ is equally susceptible, then two other factors are involved: Dosage and Length of Treatment. These are being refined, as the information below states.
Quarantine Treatments for Red and Black Bugs
There are several products for ‘bug control’ available to hobbyists. With the exception of the ReVive product (obtained as a manufacturer’s sample), these solutions were obtained through normal retail channels. All were tested using the following protocols.
Testing Protocol
Since I do not have access to all the coral parasites, I decided to conduct some bioassays using similar creatures. Note that we should not be concerned with only the target organism, but should examine the effects of the medication on a variety of organisms. Also included are observations made by a select group of hobbyists. These comments should not be considered to be comprehensive, but as results obtained with a specific ‘bug’ species under the conditions of testing.
Protocol: Snails, planarians, and copepods were collected from a freshwater pond and divided into a study group and a control group. These groups were placed into stainless steel pans containing one liter of pond water. Medication was added per the manufacturer’s recommendations. Observations were made continuously during the recommended treatment time, after which at least one water change was made. A final observation was made after 24 hours. These trials were conducted twice, about 1 week apart.
ReVive Coral Cleaner
- Manufacturer: Two Little Fishies
- Contains: Oleum abietis, 5,000 mg/l; Citrus limon, 5,000 mg/l (or 0.5% each)
- pH: 5.27 @ 24.2°C. – Does not adversely affect water pH when following manufacturer’s dosing recommendations.
- 1 capful = 8 milliliters
- Marked Expiration Date: No
This product is effective against crustaceans, including copepods. It is not intended for treatment of whole aquaria.
The label lists two main active ingredients: Oleum abietis (pine or fir oil) and Citrus Limon (lemon peel extract). Pine oil is often used by humans as a decongestant, and it has antiseptic properties. Cold-pressed extracts of lemon peels has various commercial and industrial uses.
The bottle I obtained is not marked with an expiration date. When stored properly (in the dark and refrigerated) it probably has a shelf life of a year or two. When citrus limon degrades, it develops a turpentine odor and this might used as an indicator of product condition.
I found this product to be an effective coral cleaner. A Montipora specimen was soaked in a Revive solution for 16 minutes, although copepods and other crustaceans had abandoned the coral a few minutes after the treatment began. The coral exhibited some mucus discharge (about that seem when most corals are handled) and was soaked in ReVive-free aquarium water before being returned to the aquarium. The Revive solution and debris was poured through a 190 micron mesh, and the collected material was examined with the aid of a microscope. Various amphipods and copepods, plus a number of unidentified animals were observed. See Figure 150. The kill rate of copepods was 100%.
The aforementioned Montipora specimen was chosen for treatment because it had inexplicably lost most of its vibrant orange coloration, and batteries of testing had failed to reveal any environmental (mostly chemical) clues. After about 3 weeks, I noticed that portions of the specimen were regaining its orange fluorescence. Although I cannot guarantee that the cleansing dip was responsible for the return of coloration, I am at a loss to provide an alternate explanation.

Figure 150. A copepod ‘chased’ from a Montipora specimen by the ReVive product. Note the brown dots within the crustacean – are these zooxanthellae, or simply natural coloration? Photomicrograph by the author.
Results of ReVive Treatment (Using freshwater animals, protocol described above):
- Unidentified Crustaceans: 94% kill rate after 15 minute dip
- Snails: Initially stunned by the treatment, but apparently fully recovered when observed at 24 hours.
MelaFix Marine
- Manufacturer: Aquarium Pharmaceuticals
- Contains: Oil of the Meleluca Tree, 12,500 mg/l (or 1.25%)
- pH: 4.16 @ 22.1°C. Does not adversely affect water pH when following manufacturer’s dosing recommendations.
- 1 capful = 11.3 milliliters
- Marked Expiration Date: Yes. Purchased in October 2009. Expiration date is July 2012.
Aquarist Steve Ruddy kindly relayed his experiences with ‘black bugs’ (see Figures 5, 6, and 7) and MelaFix. He found that black bugs abandoned the host coral (a Montipora species – See Figure 4) about 20 minutes after the treatment began. Note: Ruddy’s treatment time of 20 minutes exceeds the manufacturer’s maximum recommended time of 5 minutes. These copepods settled to the bottom of the container and swam in circles, and all motion stopped 30 minutes after treatment started. However, the parasites were not killed during the treatment process, and were still alive some 24 hours after the treatment began.
We should note that Meleluca oil is considered a carcinogen by the State of California, and the bottles of Melafix Marine are so marked.
Results of Melafix Treatment (Freshwater animals used, protocol described above):
- Unidentified Crustaceans: 97.5% kill rate after 15 minute dip*
- Snails: Initially stunned by the treatment, but apparently fully recovered when observed at 24 hours.
*An interesting observation. When comparing the kill rates of different copepods, it is apparent that this product is very effective against some bugs, and not so against others. This point may be moot, as Ruddy found it worked very well when used to drive (but not kill) bugs from a Montipora coral during a quarantine ‘dip’.
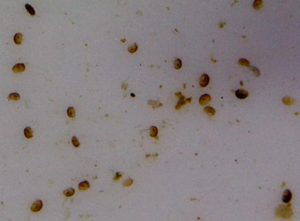
Figure 152. When treated with Melafix, these unidentified copepods (black bugs) abandoned a distressed Montipora specimen. Photo courtesy of Steve Ruddy.
Pro-Coral Cure
- Manufacturer: Tropic Marin
- Contains: Iodine, about 5,600 mg/l (or 0.56%)
- pH: 6.49 @ 22.0°C – Does not adversely affect water pH when following manufacturer’s dosing recommendations.
- Marked Expiration Date: No
This product’s container uses a small push-pump to dispense the iodine solution. I found it to deliver 0.7 milliliters (it is advertised to dispense 1 ml). I bring this up as I found the pump awkward and messy to use and chose to measure the solution using a suction bulb and pipette. For those dispensing the product manually, a dose smaller than recommended seems to work well (see results below).
Results of Pro-Coral Cure Treatment (Freshwater animals used, protocol described above):
- Unidentified Crustaceans: 94% kill rate after 15 minute dip
- Snails: Initially stunned by the treatment, but apparently fully recovered when observed at 24 hours.
- This product was also tested as an agent of flatworm control. We’ll discuss this in a future article.
Product Shelf Life of ‘Coral Dips’
Dosage is based upon the reactive agent content. Most products have a limited shelf life and an expiration date is (or should be) marked on the container. For example, meleluca degrades upon exposure to light, heat, humidity and exposure to air, and the product containing it (MelaFix, Aquarium Pharmaceuticals) has an expiration date on the bottle. This date is there for a reason – don’t ignore it. Store these and other products in dark, cool dry conditions and in a container containing little air (Carson et al., 2006) to avoid considerable changes in the chemical makeup. The only indictor of spoilage of which I am aware is the potential development of a turpentine odor.
Two Little Fishies product (ReVive) has no expiration date.
The Tropic Marin Pro-Coral Cure is not marked with an expiration date. Iodine degradation is often linked to light intensity. Store this product in the dark.
Ethyl and Methyl Alcohols
Ethyl and methyl alcohols are toxic to invertebrates, depending upon the dose of course. In various papers, the copepod expert Arthur Humes mentions using 95% ethyl alcohol to make a 5% solution of alcohol and seawater. He soaked various corals in this solution over timeframes of several hours to overnight, and found endoparasitic copepods would abandon their host. I tried this, and found it true, but it also killed the coral host.
In-Tank Treatments for Red and Black Bugs
In-tank treatments fall into two general categories – biological and chemical. Personally I would prefer a natural means of controlling coral parasites, however, there are many issues surrounding this protocol. I suppose it is an overall lack of success I’ve had over the years when choosing fishes, crabs, nudibranchs and other animals for elimination of some sort of pest.
Biological controls are subject to the independent whims of parasite predators. Long-time hobbyists recognize that individuals of a given fish species have their own personalities and behaviors. For instance, some Copperband Butterflyfishes will destroy Aiptasia colonies, while another Copperband might show little or no interest in a diet of Aiptasia anemones. Other fishes may have specific dietary requirements, and elimination of parasites through predation could result in loss through starvation of the predatory fish. As we have seen, many coral parasites reside within coral tissues and successful bio-removal would involve substantial loss of coral tissues – a case where the cure for parasites is successful due to the loss of the host. However, my experiences will not necessarily be yours. I wish you the best of luck in our endeavors.
Chemical Treatments
Milbemycin Oxime (Interceptor™)
- Manufacturer: Novartis AG
- Treatment protocol developed by Dustin Dorton.
- Comments: Store milbemycin oxime at room temperature.
Interceptor is a medication marketed for treatment of canine scabies, eye worms, roundworms, hookworms, whipworms, and heartworms in dogs, and it has been found to be effective against at least some parasitic copepod taxa.
According to Dorton, 1 Interceptor™ tablet (intended for large dogs weighing 51-100 pounds) weighs one gram and contains 23 milligrams of milbemycin oxime. One tablet is ground to powder and 25 milligrams is added to a small container of aquarium water and mixed (this might take quite a bit of stirring). This water containing 25 mg of the tablet is sufficient to treat 10 gallons of actual water volume in the aquarium. In other words, 1 tablet containing 23 mg of milbemycin oxime will treat 380 gallons, according to Dorton (400 gallons according to my calculation). A local school or university, laboratory, or larger water or wastewater treatment plant will have an analytical scale (or balance). They might perform weight analyses for you.*
- Remove all shrimp and crabs from the aquarium. Remove mechanical filtration and activated carbon. Turn off UV sterilizers and protein skimmers (but water should still circulate through these vessels in order for these volumes of water to be medically treated as well).
- Add a sufficient amount of medication to the aquarium. Allow treatment to proceed for 6 hours, and perform a 25% water change. Resume use of carbon, skimming, etc.
- Repeat this procedure 7 days later, and again after 14 days.
- * Dosage is apparently not as critical as suggested by Dorton, as time has confirmed that higher concentrations (5x or more than that recommended by Dorton) of milbemycin oxime does little, if any, harm to corals.
Milbemycin Oxime and Lufenuron (Sentinel™)
- Manufacturer: Novartis AG
- In addition to milbemycin, this product also contains lufenuron for flea control.
Lufenuron (Program™)
- Manufacturer: Novartis AG
- Not recommended for use as a coral treatment.
Ivermectin (Stromectol™, Ivomec™, HeartGard™, Iverhart Plus™, Tri-Heart Plus™ and Acarexx™)
Ivermectin is a treatment for lice, mites, ear mites and is also an antihelmitic (effective against roundworms, heartworms, and lungworms) in cattle and swine. It is also prescribed for humans as a treatment for Bancroft’s filariasis and scabies.
Wright (2009) suggests this treatment protocol: Dissolve Ivomec (1% solution) in propylene gycol (an alcohol). Dose at 0.75 mg/l per gallon (actual volume). Wright’s article used 34 mg in 45 gallons).
Turn off the protein skimmer, UV sterilizer, and canister filters and disperse the Ivomec solution in a strong stream of water within the aquarium. After 12 hours, filter water using fresh activated carbon and resume use of the protein skimmer and UV sterilization. Repeat this treatment every two weeks for a total of 3 times.
The author (Wright) cautions that this treatment might harm arthropods, mollusks, crustaceans, other invertebrates (I’m assuming he doesn’t mean corals other than the Acropora specimen mentioned in the article) and angelfishes.
MelaFix Marine
- Manufacturer: Aquarium Pharmaceuticals
- See details listed above. API recommends this product for whole-tank treatment, but these claims have not been evaluated by this writer.
Conclusions
Not all copepods that associate with scleractinians are harmful. However, our lack of knowledge forces us to make an assumption that corals crawling with copepods are in danger.
- There are hundreds, perhaps thousands of coral parasites. It seems there are specialist parasites found in or on specific coral species, while others are generalists and might infest and feed on a number of coral species. Although there is some indication within the hobby that ‘bugs’ might adopt a new home coral species if their host dies, it will take years of work to establish how parasites behave in artificial environments.
- It is not uncommon for hobbyists to mix Atlantic corals (sea whips, sea fans, Manicina, etc.) together with Pacific coral species. We understand nothing of how parasites from different locales interact with foreign potential hosts.
- Parasitic copepods may introduce bacterial pathogens.
- Many parasites are internal and are not readily apparent, where endoparasites living in the gastrovascualr cavities remain hidden from view. Stock (1988) discusses the ease which Pacific endoparasites are coaxed from host coral tissues. Internal parasites of Atlantic/Caribbean corals are more difficult to remove than those of their Pacific counterparts – indeed, this researcher states that parasites of the Family Corallovexia (and perhaps Corallonoxia) were apparent only after host tissues were chemically dissolved. Does this mean that current treatment protocols are incapable of purging host tissues of their copepod parasites? Without further documentation, this question must remain unanswered.
- Some copepod parasites consume coral tissues, including symbiotic zooxanthellae, which are then incorporated into the parasites’ bodies. Presumably, the parasite forms a symbiotic relationship with Symbiodinium sp. Thus some parasites not only consume coral tissues but rob corals of the benefits of their algal symbionts. Large numbers of external parasites could also shade zooxanthellae and cause of loss of beneficial nutrient recycling.
- The number of bugs on a coral is only part of the equation. It is the degree of trauma caused by the parasite, where the amount of damage is compared to the amount of tissue. Simply dividing corals into the categories of ‘large polyp’ and ‘small polyp’ could be insufficient in estimating potential impacts of parasites. In the case of SPS corals, the hobbyist should consider the porosity of the coral skeleton. Perforate skeletons are porous while imperforate skeletons lack these channels that often contain soft living coral tissues. For instance, Montipora and Porites corals are generally highly porous and superficial tissue damage is less likely to become a health issue. On other hand, some corals (such as Pocillopora meandrina) have non-porous, rock-hard skeletons and damage to the paper-thin layer of soft tissues simply covering the skeleton might have a far greater impact than the same amount of damage to a perforate coral. Acropora species are usually listed as possessing imperforate skeletons. However, those hobbyists with a lot of experience in fragging Acroporas have noted living tissue channels running within the skeleton. Hence, Acroporas defy a simple sorting and must be judged on a case-by-case basis. This could explain why ‘bugs’ are not much of an issue with soft corals.
- Specific chemical treatments may not be a universal cure for coral parasites. There is evidence that a prescribed treatment will eliminate some ‘bugs’ but only irritate others.
- Quite a few photographs exist of ectoparasites Tegastes, Parategastes, and others. Despite the large number of parasitic Xarifid copepods, no photographs of these bugs have been provided by hobbyists. This suggests endoparasites (despite being larger in size and in number of species) are not abundant in number in important aquarium coral species, or have simply been overlooked due to their hidden and/or tenacious nature. Current pre-treatment methods are encouraged, yet we do not know if they are effective against endoparasitic copepods.
- Biological controls for some copepods are available, but these are sometimes a hit-or-miss proposition.
- Without the presence of a host, some copepods (specifically the Tegastes(?) bug seen in Figure 7) will survive for only a few days (~48 hours, based on limited observations).
- Hobbyists using medications for in-tank treatments should expect losses of livestock. This could be a result of sensitivity of the organism(s) to the toxic nature of the chemical, or perhaps as through secondary effects. To my knowledge, no one has investigated the responses of nitrifying bacteria to medications mentioned in this article. Nitrifying bacteria populations are generally more easily upset than their carbonaceous counterparts.
This article is but a small tool for use by serious hobbyists in answering many questions. Hobbyists are encouraged to photo-document individuals observed during parasitic outbreaks, as well as compare notes on those environmental factors possibly encouraging explosion of parasite populations.
For now, prevention of aquarium infestation has to rely to two simple procedures: Treatment to removal existing parasites followed by a quarantine period to prevent introduction of any surviving egg masses or adults. Once the corals are in the display aquarium, treatment and control of parasite infestations becomes much more complex in nature. Medications are simply a band aid for treatment of environmental factors aiding in an explosion of parasite populations and it unclear if present treatment protocols are effective against all parasitic genera. Investigations of biological controls are encouraged, but hobbyists should accept results are not guaranteed for either eradication of parasites or long-term survival of the predator.
Next time, we’ll examine copepod parasites found on soft corals, gorgonians and Tridacna clams.
Acknowledgements
Many thanks to Steve Ruddy of Coral Reef Ecosystems (www.coralreefecosystems) for supplying photographs, preserved specimens, and comments during the preparation of this article. Ken Lunde (KonaDog) obtained permission from Greg Ho for use of his photographs (www.ximinasphotography.com). Jake Adams and www.CoralIdea.com again provided images of Atlantic corals. Justin Medwieg (www.madfragsonline) shared some of his wonderful coral photographs.
Please direct questions to the comments section below.
| Coral | Parasite | Size, Color | Reference |
|---|---|---|---|
| Acrhelia horrescens | Xarifia plectrata | 1mm; gray w/ red eye | Humes, 1985 |
| Acrhelia horrescens | Anchimolgus abbreviatus | Humes, 1991 | |
| Acrhelia horrescens | Anchimolgus tenuipes | Humes, 1991 | |
| Acropora abrotanoides | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora abrotanoides | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora abrotanoides | Xarifia sabiuraensis | 1.6mm | Humes, 1985 |
| Acropora abrotanoides | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora convexa | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora convexa | Xarifia sabiuraensis | 1.6mm | Humes, 1985 |
| Acropora convexa | Scyphuliger longicaudus | Kim, 2003 | |
| Acropora ‘corymbosa’ group | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora ‘corymbosa’ group | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora ‘corymbosa’ group | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora ‘corymbosa’ group | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora ‘corymbosa’ | Xarifia linearis | 1.4mm | Nair, 1983 |
| Acropora ‘corymbosa’ group | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora ‘corymbosa’ group | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora ‘corymbosa’ group | Xarifia ablusa | 1mm | Humes, 1985 |
| Acropora corymbosa | Scyphuliger pilosus | Kim, 2003 | |
| Acropora corymbosa | Scyphuliger pennatus | Kim, 2003 | |
| Acropora cymbicyanthus | Scyphuliger tenuatis | Humes, 1990 | |
| Acropora cytheria | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora cytheria | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora cytheria | Xarifia tenuis | 1.4mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia ablusa | 1mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia linearis | 1.4mm | Nair, 1983 |
| Acropora digitifera (?) | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora digitifera (?) | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora elseyi | Xarifia ablusa | 1mm | Humes, 1985 |
| Acropora elseyi | Xarifia fastigata | 1.5mm | Humes, 1985 |
| Acropora elseyi | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora exigua | Alteuthellopsis corallina | Humes, 1981b | |
| Acropora exigua | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora exigua | Ecphysarion lobophorum | Humes and Ho, 1968 | |
| Acropora exilis | Scyphuliger latus | Kim, 2003 | |
| Acropora exilis | Scyphuliger aristoides | Humes, 1993 | |
| Acropora exilis | Scyphuliger paucisurculus | Kim, 2003 | |
| Acropora florida | Ecphysarion lobophorum | Humes & Stock, 1973 | |
| Acropora florida | Tegastes acroporanus | Humes, 1981 | |
| Acropora florida | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora florida | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora florida | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora florida | Xarifia pectinea | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora florida | Xarifia sabiuraensis | 1.6mm | Humes, 1985 |
| Acropora florida | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora florida | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora formosa | Xarifia basilica | >3mm; opaque brown-gray, red eye | Humes, 1985 |
| Acropora formosa | Xarifia bullifera | 1.35mm; opaque gray, red eye | Humes, 1985 |
| Acropora formosa | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora formosa | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora gemmifera | Xarifia species | Humes, 1985 | |
| Acropora gravida | Xarifia anomala | Humes, 1994 | |
| Acropora gravida | Xarifia breviramea | Humes, 1994 | |
| Acropora gravida | Xarifia gerlachi | Humes, 1994 | |
| Acropora gravida | Xarifia pectinea | Humes, 1994 | |
| Acropora gravida | Xarifia sabiuraensis | Humes, 1994 | |
| Acropora gravida | Xarifia trituberata | Humes, 1994 | |
| Acropora gravida | Xarifia tumorisa | Humes, 1994 | |
| Acropora hebes | Xarifia indica | 1.5mm | Nair, 1983 |
| Acropora hebes | Xarifia laccadivensis | 1.6mm | Nair, 1983 |
| Acropora hebes | Xarifia robusta | 1.7mm | Nair, 1983 |
| Acropora humilis | Xarifia ablusa | 1mm | Humes, 1985 |
| Acropora humilis | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora humilis | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora humilis | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora humilis | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora humilis | Xarifia linearis | 1.4mm | Nair, 1983 |
| Acropora humilis | Xarifia longicauda | 1.4mm | Nair, 1983 |
| Acropora humilis | Xarifia pectinea | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora humilis | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora humilis | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia basilica | >3mm; opaque brown-gray, red eye | Humes, 1985 |
| Acropora hyacinthus | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia infrequens | 1.5mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia pectinea | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora hyacinthus | Xarifia sabiuraensis | 1.6mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora hyacinthus | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora hyacinthus | Scyphuliger concavipes | Humes, 1991 | |
| Acropora hyacinthus | Scyphuliger manifestus | Humes, 1991 | |
| Acropora hyacinthus | Scyphuliger eumorphus | Humes, 1993 | |
| Acropora intermeda | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora intermeda | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora intermedia | Xarifia pectinea | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora intermedia | Xarifia sabiuraensis | 1.6mm | Humes, 1985 |
| Acropora intermedia | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora intermedia | Xarifia tumorisa | 1.3mm | Humes, 1985 |
| Acropora intermedia | Scyphuliger karangmiensis | Kim, 2007 | |
| Acropora millepora | Xarifia breviramea | 1.74mm | Humes, 1985 |
| Acropora palifera | Xarifia anomala | 1.25mm | Humes, 1985 |
| Acropora palifera | Xarifia exuta | 2.5mm | Humes, 1985 |
| Acropora palifera | Xarifia guttulifera | 2.4mm | Humes, 1985 |
| Acropora palifera | Xarifia mucronata | 2.4mm | Humes, 1985 |
| Acropora palifera | Schedomolgus exciliculus | Humes, 1993 | |
| Acropora palifera | Ecphysarion spinulatum | Humes, 1993 | |
| Acropora palifera | Unicispina latigentalis | Humes, 1993 | |
| Acropora palmata | Corallovexia similis | Stock, 1975 | |
| Acropora palmata | Corallovexia sp. | Herriot & Immerman, 1979 | |
| Acropora patula | Lipochrus acroporinus | ||
| Acropora patula | Xarifia pectinea | Humes, 1985 | |
| Acropora patula | Xarifia sabiuraensis | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora patula | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora patula | Xarifia tumorisa | 1.6mm | Humes, 1985 |
| Acropora patula | Schedomolgus idanus | 1.3mm | Humes, 1985 |
| Acropora pectinata | Xarifia sp. | Misaki, 1978 | |
| Acropora pectinata | Xarifia sp. | Misaki, 1978 | |
| Acropora rambleri | Lipochrus species | Humes, 1993 | |
| Acropora rambleri | Xarifia ablusa | Humes, 1985 | |
| Acropora rambleri | Xarifia breviramea | 1mm | Humes, 1985 |
| Acropora rambleri | Xarifia pectinea | 1.74mm | Humes, 1985 |
| Acropora rambleri | Xarifia sabiuraensis | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora rambleri | Xarifia trituberata | 1.6mm | Humes, 1985 |
| Acropora rosaria | Lipochrus acroporinus | 1.6mm | Humes, 1985 |
| Acropora rosaria | Xarifia ablusa | Humes, 1985 | |
| Acropora rosaria | Xarifia fastigata | 1mm | Humes, 1985 |
| Acropora rosaria | Xarifia rosariae | 1.5mm | Humes, 1985 |
| Acropora rosaria | Ecphysarion ampullulum | 1.7mm | Humes, 1985 |
| Acropora rosaria | Lipochrus acroporinus | Humes, 1993 | |
| Acropora sarmentosa | Xarifia pectinea | Humes & Dojiri, 1982 | |
| Acropora sarmentosa | Xarifia tumorisa | 1mm; gray w/ red eye | Humes, 1985 |
| Acropora sp. | Xarifia anomala | 1.3mm | Humes, 1985 |
| Acropora sp. | Xarifia gerlachi | 1.25mm | Humes, 1985 |
| Acropora sp. cf. A. teres | Xarifia gerlachi | ~2mm | Humes, 1985 |
| Acropora squarrosa (millepora) | Xarifia tumorisa | ~2mm | Humes, 1985 |
| Acropora squarrosa (millepora) | Scyphuliger placidus | 1.3mm | Humes, 1985 |
| Acropora squarrosa (millepora) | Scyphuliger humesi | Kim, 2004 | |
| Acropora squarrosa (millepora) | Scyphuliger vicinus | Kim, 2004 | |
| Acropora syringodes | Xarifia species | Humes, 1991 | |
| Acropora valida | Xarifia breviramea | Humes, 1985 | |
| Alveopora catalai | Anchimolgus multidentatus | 1.74mm | Humes, 1985 |
| Alveopora mortensi | Xarifia mediolobata | Kim, 2003 | |
| Alveopora mortensi | Xarifia radians | 2.6mm | Humes, 1985 |
| Alveopora mortensi | Odontomolgus mundulus | 2.3mm | Humes, 1985 |
| Alveopora sp. | Xarifia brevicauda | Humes, 1974 | |
| Astreopora sp. | Alteuthellopsis corallina | 1.35mm; opaque, red-orange intestine, red eye | Humes & Ho, 1968 |
| Astreocheres astroidicola | Acontiophorus scutatis | Humes, 1981b | |
| Astroides calycularis | Stockmyzon murinipes | ||
| Astroides calycularis | Stockmyzon mucronipes | Bandera and Huys | |
| Colpophyllia natans | Corallavexia kristenseni | Bandera & Huys, 2008 | |
| Colpophyllia natans | Corallavexia mixtibrachium | Stock, 1975 | |
| Colpophyllia natans | Corallovexia mediobrachium | Stock, 1975 | |
| Cyphastrea chalcidium | Xarifia villosa | Herriot & Immerman, 1979 | |
| Cyphastrea ocellina | Tegastes gemmus | 1.2mm | Humes, 1985 |
| Dendrogyra sp. | Corallovexia sp. | Stock, 1975 | |
| Dichocoenia sp. | Corallovexia sp. | Stock, 1975 | |
| Diploria clivosa | Corallovexia mediobrachium | Humes, 1984 | |
| Cyphastrea sp. | Diallagomolgus sp. | Herriot & Immerman, 1979 | |
| Diploria labyrinthformis | Corallavexia brevibrachium | Humes, 1994 | |
| Diploria strigosa | Corallavexia mediobrachium | Stock, 1975 | |
| Echinopora gemmacea | Xarifia dispar | Stock, 1975 | |
| Echinopora horrida | Xarifia echinoporae | 1.5mm | Humes, 1985 |
| Echinopora horrida | Anchimolgus exsertus | 2.3mm | Humes, 1985 |
| Echinopora lamellosa | Xarifia dispar | Humes, 1991 | |
| Echinopora lamellosa | Xarifia echinoporae | 1.5mm | Humes, 1985 |
| Echinopora lamellosa | Anchimolgus tridentatus | 2.3mm | Humes, 1985 |
| Echinopora sp. | Xarifia dispar | Kim, 2003 | |
| Eunicella singularis | Stockmyzon mucronipes | 1.5mm | Humes, 1985 |
| Euphyllia glabrescens | Xarifia gracilipes | Bandera & Huys, 2008 | |
| Eusmilia fastigata | Corallonoxia baki | ~2mm | Humes, 1985 |
| Favia | Amarda sp. | ||
| Favia favus | Cerioxynus alatus | Stock, 1975 | |
| Favia sp. | Orstomella faviae | Humes, 1994 | |
| Favia sp. | Rakotoa proteus | Humes, 1994 | |
| Favia sp. | Anchimolgus sp. | 2.3mm; bright red, dark red eye | Humes & Ho, 1968 |
| Favia sp. | Andrianellus exsertidens | Humes, 1994 | |
| Favia sp. | Stockia sp. | Humes, 1994 | |
| Favites flexuosa | Xarifia torigera | Humes, 1994 | |
| Favites halicora | Cerioxynus faviticolus | Humes, 1994 | |
| Favites pentagona | Cerioxynus moluccensis | 2.3mm; pale brown w/ red eye | Humes, 1985 |
| Favites pentagona | Rakotoa ceramensis | Humes, 1994 | |
| Favites virens | Cerioxynus bandensis | Humes, 1994 | |
| Fungia concinna | Anchimolgus maximus | Humes, 1979 | |
| Fungia echinata | Xarifia species | Humes, 1994 | |
| Fungia echinata | Anchimolgus latens | Kim, 2003 | |
| Fungia echinata | Anchimolgus pandus | Humes, 1985 | |
| Fungia echinata | Schedomolgus tener | Humes, 1978 | |
| Fungia fungites | Schedomolgus dumbensis | Humes, 1978 | |
| Fungia fungites | Odontomolgus scitulus | Humes, 1973 | |
| Fungia paumotensis | Anchimolgus orectus | Kim, 2003 | |
| Fungia paumotensis | Anchimolgus punctilis | Humes, 1973 | |
| Fungia species | Zazaranus fungicolus | Humes, 1978 | |
| Fungia species | Odontomolgus flammeus | Humes, 1978 | |
| Fungia species | Anchimolgus hastatus | Humes, 1985 | |
| Galaxea astreata | Xarifia species | Kim, 2007 | |
| Galaxea fascicularis | Xarifia exserens | Kim, 2007 | |
| Galaxea fascicularis | Anchimolgus compressus | Humes, 1985 | |
| Galaxea fascicularis | Anchimolgus contractus | 2.3mm; opaque gray, red eye | Humes, 1985 |
| Galaxea fascicularis | Anchimolgus moluccanus | Humes, 1996 | |
| Galaxea fascicularis | Anchimolgus nastuas | Humes, 1979 | |
| Gardineroseris planulata | Anchimolgus angustus | Humes, 1996 | |
| Gardineroseris planulata | Xarifia clavellata | Humes, 1996 | |
| Gardineroseris planulata | Xarifia filata | Humes, 1992 | |
| Gardineroseris planulata | Xarifia rasilis | 0.8mm | Humes, 1985 |
| Gardineroseris planulata | Odontomolgus mucosus | ~1mm; Gray, red eye | Humes, 1985 |
| Gardineroseris planulata | Odontomolgus pumulis | 0.9mm; gray w/ red eye | Humes, 1985 |
| Gardineroseris planulata | Odontomolgus unioviger | Kim, 2006 | |
| Gardineroseris planulata | Anchimolgus eparmatoides | Humes, 1992 | |
| Gardineroseris planulata | Anchimolgus gibberulus | Kim, 2006 | |
| Gardineroseris planulata | Anchimolgus stellus | Humes & Stock, 1972 | |
| Goniastrea retiformis | Alteuthellopsis corallina | Humes, 1992 | |
| Goniastrea retiformis | Amarda curvus | Humes, 1972 | |
| Goniastrea retiformis | Amarda goniastraea | Humes, 1981b | |
| Goniastrea retiformis | Odontomolgus parvus | Kim, 2007 | |
| Goniopora minor | Wedanus formosanus | Humes, 1995 | |
| Goniopora pedunculata | Xarifia hadra | Kim, 2007 | |
| Goniopora pedunculata | Xarifia scutipes | Cheng et al., 2008 | |
| Goniopora species | Xarifia resex | 1.7mm | Humes, 1985 |
| Goniopora species | Anchimolgus conformatus | 2mm | Humes, 1985 |
| Goniopora species | Anchimolgus mimeticus | 1.4mm | Humes, 1985 |
| Goniopora species | Odontomolgus campulus | Humes & Stock, 1973 | |
| Goniopora stokesi | Anchimolgus brevarius | Humes, 1995 | |
| Goniopora stokesi | Anchimolgus gigas | Humes, 1995 | |
| Goniopora tenuidens | Xarifia hadra | Humes, 1995 | |
| Goniopora tenuidens | Xarifia resex | Humes & Stock, 1995 | |
| Goniopora tenuidens | Xarifia scutipes | 1.7mm | Humes, 1985 |
| Goniopora tenuidens | Wedanus inconstans | 1.4mm | Humes, 1985 |
| Gyrosmilia interrupta | Xarifia apertipes | 2mm | Humes, 1985 |
| Halomitra pileus | Odontomolgus fultus | Humes, 1978 | |
| Heliofungia actiniformis | Odontomolgus decens | Humes, 1985 | |
| Hydnophora exesa | Xarifia comptula | Humes & Stock, 1972 | |
| Hydnophora exesa | Xarifia curtata | Humes and Stock, 1972 | |
| Hydnophora exesa | Panjakus hydnophorae | 2.5mm | Humes, 1985 |
| Hydnophora microconus | Panjakus iratus | Up to 3.5mm | Humes & Ho, 1968 |
| Hydnophora microconus | Panjakus saccipes | Humes and Stock, 1973 | |
| Hydnophora microconus | Anchimolgus paragensis | Kim, 2005 | |
| Hydnophora rigida | Panjakus eumeces | Humes, 1991 | |
| Hydnophora sp. | Humesiella corallicola | Kim, 2007 | |
| Hydnophora sp. | Panjakus sp. | Kim, 2005 | |
| Hydnophora sp. | Panjakus hydnophorae | 1.5mm | Sebastian & Pillai, 1973 |
| Hydnophora tenella | Panjakus hydnophorae | Humes, 1994 | |
| Leptoria phrygia | Xarifia species | Humes and Stock, 1973 | |
| Leptoria tenuis | Panjakus directus | Humes and Stock, 1973 | |
| Leptoria tenuis | Panjakus necopinus | Humes, 1985 | |
| Lobophyllia corymbosa | Orstomella lobophylliae | Humes, 1995 | |
| Lobophyllia costata | Orstomella lobophylliae | Humes, 1995 | |
| Manicina areolata | Corallavexia longibrachium | 1.4mm; bright red, dark red eye | Humes & Ho, 1968 |
| Meandrina meandrites | Corallovexia sp. | 1.4mm; bright red, dark red eye | Humes & Ho, 1968 |
| Meandrina meandrites | Corallonoxia baki | Stock, 1975 | |
| Merulina ampliata | Alteuthellopsis corallina | Herriot & Immerman, 1979 | |
| Merulina ampliata | Amardopsis merulinae | Stock, 1975 | |
| Merulina ampliata | Xarifia species | Humes, 1981b | |
| Merulina ampliata | Odontomolgus bulbalis | Humes 1994 | |
| Montastrea brasiliana | Corallavexia ventrospina | Humes, 1985 | |
| Montastrea cavernosa | Corallavexia dorsospinosa minor | Humes. 1991 | |
| Montastrea cavernosa | Corallavexia dorsospinosa | Stock, 1975 | |
| Montastrea cavernosa | Corallovexia sp. | Stock, 1975 | |
| Montastrea curta | Cerioxynus montastreae | Stock, 1975 | |
| Montipora caliculata | Haplomolgus incolumis | Herriot & Immerman, 1979 | |
| Montipora composita | Xarifia anopla | Humes, 1994 | |
| Montipora composita | Xarifia heteromeles | Humes, 1991 | |
| Montipora compressa | Odontomolgus forhani | Humes, 1985 | |
| Montipora foliosa | Xarifia species | ~1mm | Humes, 1985 |
| Montipora ramosa | Xarifia pectinea (only 1 found) | Humes & Stock, 1972 | |
| Montipora ramosa | Xarifia temnura | Humes, 1985 | |
| Montipora sinensis | Xarifia temnura | 1mm; gray w/ red eye | Humes, 1985 |
| Montipora sp. | Xarifia anopla | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Montipora sp. | Xarifia extensa | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Montipora sp. | Xarifia species | Humes, 1985 | |
| Montipora stellata | Xarifia species | 2.5mm | Humes, 1985 |
| Montipora undata | Xarifia anopla | Humes, 1985 | |
| Montipora undata | Xarifia heteromeles | Humes, 1985 | |
| Montipora undata | Xarifia syntoma | Humes, 1985 | |
| Montipora undata | Haplomolgus subdeficiens | ~1mm | Humes, 1985 |
| Montipora undata | Xarifia temnura | 0.8mm | Humes, 1985 |
| Montipora verilli | Alteuthellopsis corallina | Humes, 1978 | |
| Montipora verrucosa | Tegastes gemmeus | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Montipora verrucosa | Xarifia apertipes | Humes, 1984 | |
| Mussusmillia hispida | Harpacticoida | Nogueira, 2003 | |
| Mycetophyllia lamarckiana | Corallovexia sp. | Humes, 1984 | |
| Mycetophyllia lamarckiana | Corallovexia sp. #2 | Humes, 1985 | |
| Oulophyllia crispa | Cerioxynus oulophillia | Herriot & Immerman, 1979 | |
| Oxypora sp. | Xarifia species | Herriot & Immerman, 1979 | |
| Pachyseris rugosa | Xarifia acicularis | Humes, 1994 | |
| Pachyseris speciosa | Xarifia exigua | Humes, 1985 | |
| Pachyseris speciosa | Xarifia laminellispinosa | Gray, red eye | Humes, 1985 |
| Parahalometra robusta | Xarifia species | 0.8mm; light pale brownish, red eye | Humes & Ho, 1968 |
| Parahalometra robusta | Anchimolgus convexus | 1.9mm | Humes & Ho, 1968 |
| Pavona angularis | Odontomolgus actinophorus | Humes, 1985 | |
| Pavona angulata | Xarifia longipes | Humes, 1978 | |
| Pavona angulata | Odontomolgus actinophorus | Humes and Stock, 1973 | |
| Pavona cactus | Xarifia finitima | 1.5mm | Humes, 1985 |
| Pavona cactus | Odontomolgus actinophorus | Humes and Stock, 1973 | |
| Pavona danai | Odontomolgus actinophorus | 1mm; gray, red eye | Humes, 1985 |
| Pavona danai | Odontomolgus pavonus | Humes and Stock, 1973 | |
| Pavona danai | Anchimolgus gracilipes | Humes and Stock, 1973 | |
| Pavona sp. | Xarifia diminuta | Kim, 2007 | |
| Pavona varians | Xarifia finitima | Kim, 2007 | |
| Pavona venusta | Odontomolgus actinophorus | 1.35mm | Humes, 1985 |
| Pavona sp. | Odontomolgus rhadinus | Humes & Stock, 1973 | |
| Pectinia lactuca | Mandobius regalis | 1mm; gray, red eye | Humes, 1985 |
| Physogyra lichensteini | Xarifia gradata | Humes and Stock, 1973 | |
| Physogyra lichensteini | Xarifia minax | Humes, 1994 | |
| Platygyra astreiformis | Panjakus platygyrae | 1.3mm | Humes, 1985 |
| Platygyra lamellina | Panjakus platygyrae | 1.4mm | Humes, 1985 |
| Platygyra daedala | Panjakus daedala | Humes, 1994 | |
| Platygyra daedala | Alteuthellopsis corallina | Humes and Stock, 1973 | |
| Platygyra ryukyuensis | Panjakus fastigatus | Humes and Stock, 1973 | |
| Platygyra ryukyuensis | Panjakus parvipes | Humes, 1981b | |
| Platygyra ryukyuensis | Andrianellus papillipes | Kim, 2005 | |
| Platygyra sinensis | Xarifia species | Kim, 2005 | |
| Platygyra sp. | Alteuthellopsis corallina | Kim, 2007 | |
| Platygyra sp. | Xarifia dispar | Humes, 1985 | |
| Platygyra sp. | Panjakus sp. | Humes, 1981b | |
| Platygyra daedala | Andrianellus exsertidens | 1.5mm | Humes, 1985 |
| Plerogyra sp. | Gelastomolgus | Humes & Stock, 1973 | |
| Pocillopora damicornis | Alteuthellopsis corallina | Humes, 1994 | |
| Pocillopora damicornis | Xarifia fimbriata | Humes, 1994 | |
| Pocillopora damicornis | Xarifia fissilis | Humes, 1981b | |
| Pocillopora damicornis | Xarifia jugalis | ~1.5mm | Humes, 1985 |
| Pocillopora damicornis | Xarifia obesa | ~2mm; gray w/ red eye | Humes, 1985 |
| Pocillopora damicornis | Xarifia quinaria | 1.4mm; gray w/ red eye | Cheng et al., 2007 |
| Pocillopora damicornis | Xarifia sectilis | 1.4mm | Humes, 1985 |
| Pocillopora damicornis | Xarifia serrata | Humes, 1994 | |
| Pocillopora damicornis | Anchimolgus partenuides | 1.7mm; gray w/ red eye | Humes, 1985 |
| Pocillopora damicornis var. caespitosa | Xarifia fimbriata | 1.3mm | Humes, 1985 |
| Pocillopora damicornis var. caespitosa | Xarifia imparilis | Kim, 2007 | |
| Pocillopora damicornis var. caespitosa | Xarifia jugalis | ~1.5mm | Humes, 1985 |
| Pocillopora damicornis var. caespitosa | Xarifia quinaria | 1.5mm; gray w/ red eye | Humes, 1985 |
| Pocillopora danae | Xarifia obesa | 1.4mm; gray w/ red eye | Humes, 1985 |
| Pocillopora eydouxi | Xarifia comata | 1.3mm; gray w/ red eye | Humes, 1985 |
| Pocillopora eydouxi | Xarifia fimbriata | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Pocillopora eydouxi | Xarifia imparilis | ~1.2mm | Humes, 1985 |
| Pocillopora eydouxi | Xarifia jugalis | ~1.5mm | Humes, 1985 |
| Pocillopora eydouxi | Xarifia maldivensis | 1.5mm; gray w/ red eye | Humes, 1985 |
| Pocillopora eydouxi | Xarifia obesa | 1.4mm; gray w/ red eye | Humes, 1985 |
| Pocillopora eydouxi | Xarifia sectilis | 1.4mm | Humes, 1985 |
| Pocillopora ligulata | Xarifia tenta | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Pocillopora sp. | Tegastes georgei | 1.7mm; gray w/ red eye | Humes, 1985 |
| Pocillopora sp. | Xarifia fimbriata | 1mm; gray to tan w/ red eye | Humes, 1985 |
| Pocillopora sp. | Xarifia maldivensis | Humes, 1981 | |
| Pocillopora sp. | Xarifia obesa | ~1.5mm | Humes, 1985 |
| Pocillopora sp. | Xarifia serrata | 1.4mm | Humes, 1985 |
| Pocillopora sp. cf. verrucosa | Xarifia comata | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Pocillopora sp. cf. verrucosa | Xarifia obesa | 1.3mm | Humes, 1985 |
| Pocillopora sp. cf. verrucosa | Xarifia serrata | ~1.2mm | Humes, 1985 |
| Pocillopora verrucosa | Tegastes paulipes | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Pocillopora verrucosa | Xarifia comata | 1.3mm | Humes, 1985 |
| Pocillopora verrucosa | Xarifia gibberula | Humes, 1984 | |
| Pocillopora verrucosa | Xarifia imparilis | ~1.2mm | Humes, 1985 |
| Pocillopora verrucosa | Xarifia obesa | 1.3mm gray w/ red eye | Humes, 1985 |
| Pocillopora verrucosa | Xarifia serrata | 1.5mm; gray w/ red eye | Humes, 1985 |
| Pocillopora verrucosa | Xarifia tenta | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Pocillopora verrucosa | Panjakus bidentis | 1.3mm | Humes, 1985 |
| Porites (andrewsi?) | Monomolgus unihastatus | 1mm; gray to tan w/ red eye | Humes, 1985 |
| Porites (nigrescens?) | Monomolgus unihastatus | Kim, 2004 | |
| Porites latistella | Xarifia longa | Humes and Stock, 1973 | |
| Porites lobata | Euryte verecunda | Humes and Stock, 1973 | |
| Porites lobata | Hemicyclops regalis | Cheng et al., 2007 | |
| Porites lobata | Monomolgus torulus | Humes, 1992 | |
| Porites lobata | Kombia incrassata | Humes, 1994 | |
| Porites lutea | Orstomella yaliuensis | Humes, 1984 | |
| Porites lutea | Xarifia species | Humes, 1984 | |
| Porites lutea | Kombia curvata | Chen et al., 2009 | |
| Porites monticulosa | Kombia imminens | Humes, 1985 | |
| Porites somaliensis | Kombia angulata | Humes & Stock, 1973 | |
| Porites sp. | Kombia avitus | Nair & Pillai, 1986 | |
| Porites nigrescens | Monomolgus baculigeres | Humes, 1979 | |
| Psammocora sp. | Monomolgus psammocorae | Kim, 2007 | |
| Psammocora sp. | Kombia angulata | Humes, 1979 | |
| Psammocora contigua | Xarifia diminuta | Humes and Stock, 1973 | |
| Psammocora contigua | Xarifia imitans | Humes, 1962 | |
| Psammocora contigua | Odontomolgus actinophorus | 1.35mm | Humes, 1985 |
| Psammocora contigua | Numboa porosa | Humes, 1997 | |
| Psammocora contigua | Odontomolgus rhadinus | 1.1mm; gray w/ red eye | Humes, 1985 |
| Psammocora digitata | Xarifia formosa | Humes and Stock, 1973 | |
| Psammocora digitata | Xarifia imitans | Humes & Ho, 1967 | |
| Psammocora logianensis | Dumbeana undulatipes | 1.2mm; gray w/ red eye | Humes, 1996 |
| Psammocora logianensis | Emunoa proknta | 1.1mm; gray w/ red eye | Humes, 1996 |
| Psammocora logianensis | Lipochaetes extrusis | Humes, 1996 | |
| Psammocora samoensis | Odontomolgus exilipes | Humes, 1995 | |
| Psammocora samoensis | Odontomolgus geminus | Humes, 1995 | |
| Psammocora sp. | Xarifia diminuta | Kim, 2003 | |
| Psammocora sp. cf. contigua | Xarifia diminuta | Kim, 2003 | |
| Psammocora togianensis | Numboa porosa | Humes, 1997 | |
| Scapophyllia cylindrica | Xarifia simplex | 1.35mm | Humes, 1985 |
| Seriatopora caliendrum | Xarifia reducta | 1.35mm | Humes, 1985 |
| Seriatopora hystrix | Xarifia eminula | 1.8mm; gray w/ red eye | Humes, 1985 |
| Seriatopora hystrix | Xarifia levis | 1.1mm | Humes, 1985 |
| Seriatopora hystrix | Xarifia obesa | Opaque gray, red eye | Humes & Ho, 1968 |
| Seriatopora hystrix | Xarifia reducta | ~1mm; tan w/ red eye | Humes, 1985 |
| Seriatopora hystrix | Xarifia umbonata | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Seriatopora hystrix | Xarifia varialabrata | 1.1mm | Humes, 1985 |
| Seriatopora hystrix | Anchiomolgus tenuipes | 1.2mm; gray w/ red eye | Humes, 1985 |
| Seriatopora hystrix | Anchimolgus noumensis | 0.8mm; light pale brown w/ red eye | Humes, 1985 |
| Seriatopora octoptera | Xarifia reducta | Kim, 2003 | |
| Seriatopora sp. | Xarifia reducta | Kim, 2003 | |
| Stereonephthya ulicoides | Parategastes conexus | Humes, 1993 | |
| Stylophora mordax | Xarifia decorata | 1.1mm | Humes, 1985 |
| Stylophora mordax | Xarifia lissa | Humes, 1984 | |
| Stylophora pistillata | Tegastes georgei | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Stylophora pistillata | Xarifia decorata | 1.4mm; opaque, red-brown intestine, red eye | Humes, 1985 |
| Stylophora pistillata | Xarifia dissona | Humes, 1981 | |
| Stylophora pistillata | Xarifia lissa | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Stylophora pistillata | Xarifia obesa | 1.45mm; Gray, intestine dk. Brown, red eye | Humes, 1985 |
| Stylophora pistillata | Xarifia sectilis | 1.4mm | Humes, 1985 |
| Stylophora pistillata var. palmata | Xarifia obesa | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Stylophora sp. | Xarifia decorata | 1.7mm; gray w/ red eye | Humes, 1985 |
| Stylophora sp. | Xarifia lissa | ~1.5mm; Gray to tan w/ red eye | Humes, 1985 |
| Stylophora sp. | Xarifia species | 1.5mm; opaque, intestine red-brown, red eye | Humes & Ho, 1968 |
| Stylophora subseriata | Xarifia serrata | 1.4mm | Humes, 1985 |
| Tubastrea aurea | Xarifia insolita | Humes, 1985 | |
| Tubastrea aurea | Xarifia insolita | 1.3mm | Humes, 1985 |
| Tubastrea sp. | Xarifia species | Humes, 1985 | |
| Turbinaria danae | Xarifia lacerans | Cheng et al., 2007 | |
| Turbinaria danae | Xarifia unicata | Humes, 1985 | |
| Turbinaria sp. (T. elegans?) | Xarifia hamata | ~1mm; gray w/ red eye | Humes, 1985 |
| Wedanus inconstans | Goniopora minor | 1.6mm; gray w/ red eye | Humes, 1985 |
| Parasite | Coral |
|---|---|
| Xarifia plectrata | Acrhelia horrescens |
| Anchimolgus abbreviatus | Acrhelia horrescens |
| Anchimolgus tenuipes | Acrhelia horrescens |
| Xarifia anomala | Acropora abrotanoides |
| Xarifia breviramea | Acropora abrotanoides |
| Xarifia sabiuraensis | Acropora abrotanoides |
| Xarifia trituberata | Acropora abrotanoides |
| Xarifia anomala | Acropora convexa |
| Xarifia sabiuraensis | Acropora convexa |
| Scyphuliger longicaudus | Acropora convexa |
| Xarifia anomala | Acropora ‘corymbosa’ group |
| Xarifia breviramea | Acropora ‘corymbosa’ group |
| Xarifia gerlachi | Acropora ‘corymbosa’ group |
| Xarifia infrequens | Acropora ‘corymbosa’ group |
| Xarifia linearis | Acropora ‘corymbosa’ |
| Xarifia trituberata | Acropora ‘corymbosa’ group |
| Xarifia tumorisa | Acropora ‘corymbosa’ group |
| Xarifia ablusa | Acropora ‘corymbosa’ group |
| Scyphuliger pilosus | Acropora corymbosa |
| Scyphuliger pennatus | Acropora corymbosa |
| Scyphuliger tenuatis | Acropora cymbicyanthus |
| Xarifia gerlachi | Acropora cytheria |
| Xarifia infrequens | Acropora cytheria |
| Xarifia tenuis | Acropora cytheria |
| Xarifia ablusa | Acropora digitifera (?) |
| Xarifia anomala | Acropora digitifera (?) |
| Xarifia breviramea | Acropora digitifera (?) |
| Xarifia gerlachi | Acropora digitifera (?) |
| Xarifia infrequens | Acropora digitifera (?) |
| Xarifia linearis | Acropora digitifera (?) |
| Xarifia trituberata | Acropora digitifera (?) |
| Xarifia tumorisa | Acropora digitifera (?) |
| Xarifia ablusa | Acropora elseyi |
| Xarifia fastigata | Acropora elseyi |
| Xarifia tumorisa | Acropora elseyi |
| Alteuthellopsis corallina | Acropora exigua |
| Xarifia breviramea | Acropora exigua |
| Ecphysarion lobophorum | Acropora exigua |
| Scyphuliger latus | Acropora exilis |
| Scyphuliger aristoides | Acropora exilis |
| Scyphuliger paucisurculus | Acropora exilis |
| Ecphysarion lobophorum | Acropora florida |
| Tegastes acroporanus | Acropora florida |
| Xarifia anomala | Acropora florida |
| Xarifia breviramea | Acropora florida |
| Xarifia gerlachi | Acropora florida |
| Xarifia pectinea | Acropora florida |
| Xarifia sabiuraensis | Acropora florida |
| Xarifia trituberata | Acropora florida |
| Xarifia tumorisa | Acropora florida |
| Xarifia basilica | Acropora formosa |
| Xarifia bullifera | Acropora formosa |
| Xarifia infrequens | Acropora formosa |
| Xarifia tumorisa | Acropora formosa |
| Xarifia species | Acropora gemmifera |
| Xarifia anomala | Acropora gravida |
| Xarifia breviramea | Acropora gravida |
| Xarifia gerlachi | Acropora gravida |
| Xarifia pectinea | Acropora gravida |
| Xarifia sabiuraensis | Acropora gravida |
| Xarifia trituberata | Acropora gravida |
| Xarifia tumorisa | Acropora gravida |
| Xarifia indica | Acropora hebes |
| Xarifia laccadivensis | Acropora hebes |
| Xarifia robusta | Acropora hebes |
| Xarifia ablusa | Acropora humilis |
| Xarifia anomala | Acropora humilis |
| Xarifia breviramea | Acropora humilis |
| Xarifia gerlachi | Acropora humilis |
| Xarifia infrequens | Acropora humilis |
| Xarifia linearis | Acropora humilis |
| Xarifia longicauda | Acropora humilis |
| Xarifia pectinea | Acropora humilis |
| Xarifia trituberata | Acropora humilis |
| Xarifia tumorisa | Acropora humilis |
| Xarifia anomala | Acropora hyacinthus |
| Xarifia basilica | Acropora hyacinthus |
| Xarifia breviramea | Acropora hyacinthus |
| Xarifia gerlachi | Acropora hyacinthus |
| Xarifia infrequens | Acropora hyacinthus |
| Xarifia pectinea | Acropora hyacinthus |
| Xarifia sabiuraensis | Acropora hyacinthus |
| Xarifia trituberata | Acropora hyacinthus |
| Xarifia tumorisa | Acropora hyacinthus |
| Scyphuliger concavipes | Acropora hyacinthus |
| Scyphuliger manifestus | Acropora hyacinthus |
| Scyphuliger eumorphus | Acropora hyacinthus |
| Xarifia anomala | Acropora intermeda |
| Xarifia breviramea | Acropora intermeda |
| Xarifia pectinea | Acropora intermedia |
| Xarifia sabiuraensis | Acropora intermedia |
| Xarifia trituberata | Acropora intermedia |
| Xarifia tumorisa | Acropora intermedia |
| Scyphuliger karangmiensis | Acropora intermedia |
| Xarifia breviramea | Acropora millepora |
| Xarifia anomala | Acropora palifera |
| Xarifia exuta | Acropora palifera |
| Xarifia guttulifera | Acropora palifera |
| Xarifia mucronata | Acropora palifera |
| Schedomolgus exciliculus | Acropora palifera |
| Ecphysarion spinulatum | Acropora palifera |
| Unicispina latigentalis | Acropora palifera |
| Corallavexia similis | Acropora palmata |
| Corallovexia sp. | Acropora palmata |
| Lipochrus acroporinus | Acropora patula |
| Xarifia pectinea | Acropora patula |
| Xarifia sabiuraensis | Acropora patula |
| Xarifia trituberata | Acropora patula |
| Xarifia tumorisa | Acropora patula |
| Schedomolgus idanus | Acropora patula |
| Xarifia sp. | Acropora pectinata |
| Xarifia sp. | Acropora pectinata |
| Lipochrus species | Acropora rambleri |
| Xarifia ablusa | Acropora rambleri |
| Xarifia breviramea | Acropora rambleri |
| Xarifia pectinea | Acropora rambleri |
| Xarifia sabiuraensis | Acropora rambleri |
| Xarifia trituberata | Acropora rambleri |
| Lipochrus acroporinus | Acropora rosaria |
| Xarifia ablusa | Acropora rosaria |
| Xarifia fastigata | Acropora rosaria |
| Xarifia rosariae | Acropora rosaria |
| Ecphysarion ampullulum | Acropora rosaria |
| Lipochrus acroporinus | Acropora rosaria |
| Xarifia pectinea | Acropora sarmentosa |
| Xarifia tumorisa | Acropora sarmentosa |
| Xarifia anomala | Acropora sp. |
| Xarifia gerlachi | Acropora sp. |
| Xarifia gerlachi | Acropora sp. cf. A. teres |
| Xarifia tumorisa | Acropora squarrosa (millepora) |
| Scyphuliger placidus | Acropora squarrosa (millepora) |
| Scyphuliger humesi | Acropora squarrosa (millepora) |
| Scyphuliger vicinus | Acropora squarrosa (millepora) |
| Xarifia species | Acropora syringodes |
| Xarifia breviramea | Acropora valida |
| Anchimolgus multidentatus | Alveopora catalai |
| Xarifia mediolobata | Alveopora mortensi |
| Xarifia radians | Alveopora mortensi |
| Odontomolgus mundulus | Alveopora mortensi |
| Xarifia brevicauda | Alveopora sp. |
| Alteuthellopsis corallina | Astreopora sp. |
| Acontiophorus scutatis | Astreocheres astroidicola |
| Stockmyzon murinipes | Astroides calycularis |
| Stockmyzon mucronipes | Astroides calycularis |
| Corallavexia kristenseni | Colpophyllia natans |
| Corallavexia mixtibrachium | Colpophyllia natans |
| Corallovexia mediobrachium | Colpophyllia natans |
| Xarifia villosa | Cyphastrea chalcidium |
| Tegastes gemmus | Cyphastrea ocellina |
| Corallovexia sp. | Dendrogyra sp. |
| Corallovexia sp. | Dichocoenia sp. |
| Corallovexia mediobrachium | Diploria clivosa |
| Diallagomolgus sp. | Cyphastrea sp. |
| Corallavexia brevibrachium | Diploria labyrinthformis |
| Corallavexia mediobrachium | Diploria strigosa |
| Xarifia dispar | Echinopora gemmacea |
| Xarifia echinoporae | Echinopora horrida |
| Anchimolgus exsertus | Echinopora horrida |
| Xarifia dispar | Echinopora lamellosa |
| Xarifia echinoporae | Echinopora lamellosa |
| Anchimolgus tridentatus | Echinopora lamellosa |
| Xarifia dispar | Echinopora sp. |
| Stockmyzon mucronipes | Eunicella singularis |
| Xarifia gracilipes | Euphyllia glabrescens |
| Corallonoxia baki | Eusmilia fastigata |
| Amarda sp. | Favia |
| Cerioxynus alatus | Favia favus |
| Orstomella faviae | Favia sp. |
| Rakotoa proteus | Favia sp. |
| Anchimolgus sp. | Favia sp. |
| Andrianellus exsertidens | Favia sp. |
| Stockia sp. | Favia sp. |
| Xarifia torigera | Favites flexuosa |
| Cerioxynus faviticolus | Favites halicora |
| Cerioxynus moluccensis | Favites pentagona |
| Rakotoa ceramensis | Favites pentagona |
| Cerioxynus bandensis | Favites virens |
| Anchimolgus maximus | Fungia concinna |
| Xarifia species | Fungia echinata |
| Anchimolgus latens | Fungia echinata |
| Anchimolgus pandus | Fungia echinata |
| Schedomolgus tener | Fungia echinata |
| Schedomolgus dumbensis | Fungia fungites |
| Odontomolgus scitulus | Fungia fungites |
| Anchimolgus orectus | Fungia paumotensis |
| Anchimolgus punctilis | Fungia paumotensis |
| Zazaranus fungicolus | Fungia species |
| Odontomolgus flammeus | Fungia species |
| Anchimolgus hastatus | Fungia species |
| Xarifia species | Galaxea astreata |
| Xarifia exserens | Galaxea fascicularis |
| Anchimolgus compressus | Galaxea fascicularis |
| Anchimolgus contractus | Galaxea fascicularis |
| Anchimolgus moluccanus | Galaxea fascicularis |
| Anchimolgus nastuas | Galaxea fascicularis |
| Anchimolgus angustus | Gardineroseris planulata |
| Xarifia clavellata | Gardineroseris planulata |
| Xarifia filata | Gardineroseris planulata |
| Xarifia rasilis | Gardineroseris planulata |
| Odontomolgus mucosus | Gardineroseris planulata |
| Odontomolgus pumulis | Gardineroseris planulata |
| Odontomolgus unioviger | Gardineroseris planulata |
| Anchimolgus eparmatoides | Gardineroseris planulata |
| Anchimolgus gibberulus | Gardineroseris planulata |
| Anchimolgus stellus | Gardineroseris planulata |
| Alteuthellopsis corallina | Goniastrea retiformis |
| Amarda curvus | Goniastrea retiformis |
| Amarda goniastraea | Goniastrea retiformis |
| Odontomolgus parvus | Goniastrea retiformis |
| Wedanus formosanus | Goniopora minor |
| Xarifia hadra | Goniopora pedunculata |
| Xarifia scutipes | Goniopora pedunculata |
| Xarifia resex | Goniopora species |
| Anchimolgus conformatus | Goniopora species |
| Anchimolgus mimeticus | Goniopora species |
| Odontomolgus campulus | Goniopora species |
| Anchimolgus brevarius | Goniopora stokesi |
| Anchimolgus gigas | Goniopora stokesi |
| Xarifia hadra | Goniopora tenuidens |
| Xarifia resex | Goniopora tenuidens |
| Xarifia scutipes | Goniopora tenuidens |
| Wedanus inconstans | Goniopora tenuidens |
| Xarifia apertipes | Gyrosmilia interrupta |
| Odontomolgus fultus | Halomitra pileus |
| Odontomolgus decens | Heliofungia actiniformis |
| Xarifia comptula | Hydnophora exesa |
| Xarifia curtata | Hydnophora exesa |
| Panjakus hydnophorae | Hydnophora exesa |
| Panjakus iratus | Hydnophora microconus |
| Panjakus saccipes | Hydnophora microconus |
| Anchimolgus paragensis | Hydnophora microconus |
| Panjakus eumeces | Hydnophora rigida |
| Humesiella corallicola | Hydnophora sp. |
| Panjakus sp. | Hydnophora sp. |
| Panjakus hydnophorae | Hydnophora sp. |
| Panjakus hydnophorae | Hydnophora tenella |
| Xarifia species | Leptoria phrygia |
| Panjakus directus | Leptoria tenuis |
| Panjakus necopinus | Leptoria tenuis |
| Orstomella lobophylliae | Lobophyllia corymbosa |
| Orstomella lobophylliae | Lobophyllia costata |
| Corallavexia longibrachium | Manicina areolata |
| Corallovexia sp. | Meandrina meandrites |
| Corallonoxia baki | Meandrina meandrites |
| Alteuthellopsis corallina | Merulina ampliata |
| Amardopsis merulinae | Merulina ampliata |
| Xarifia species | Merulina ampliata |
| Odontomolgus bulbalis | Merulina ampliata |
| Corallavexia ventrospina | Montastrea brasiliana |
| Corallavexia dorsospinosa minor | Montastrea cavernosa |
| Corallavexia dorsospinosa | Montastrea cavernosa |
| Corallovexia sp. | Montastrea cavernosa |
| Cerioxynus montastreae | Montastrea curta |
| Haplomolgus incolumis | Montipora caliculata |
| Xarifia anopla | Montipora composita |
| Xarifia heteromeles | Montipora composita |
| Odontomolgus forhani | Montipora compressa |
| Xarifia species | Montipora foliosa |
| Xarifia pectinea (only 1 found) | Montipora ramosa |
| Xarifia temnura | Montipora ramosa |
| Xarifia temnura | Montipora sinensis |
| Xarifia anopla | Montipora sp. |
| Xarifia extensa | Montipora sp. |
| Xarifia species | Montipora sp. |
| Xarifia species | Montipora stellata |
| Xarifia anopla | Montipora undata |
| Xarifia heteromeles | Montipora undata |
| Xarifia syntoma | Montipora undata |
| Haplomolgus subdeficiens | Montipora undata |
| Xarifia temnura | Montipora undata |
| Alteuthellopsis corallina | Montipora verilli |
| Tegastes gemmeus | Montipora verrucosa |
| Xarifia apertipes | Montipora verrucosa |
| Harpacticoida | Mussusmillia hispida |
| Corallovexia sp. | Mycetophyllia lamarckiana |
| Corallovexia sp. #2 | Mycetophyllia lamarckiana |
| Cerioxynus oulophillia | Oulophyllia crispa |
| Xarifia species | Oxypora sp. |
| Xarifia acicularis | Pachyseris rugosa |
| Xarifia exigua | Pachyseris speciosa |
| Xarifia laminellispinosa | Pachyseris speciosa |
| Xarifia species | Parahalometra robusta |
| Anchimolgus convexus | Parahalometra robusta |
| Odontomolgus actinophorus | Pavona angularis |
| Xarifia longipes | Pavona angulata |
| Odontomolgus actinophorus | Pavona angulata |
| Xarifia finitima | Pavona cactus |
| Odontomolgus actinophorus | Pavona cactus |
| Odontomolgus actinophorus | Pavona danai |
| Odontomolgus pavonus | Pavona danai |
| Anchimolgus gracilipes | Pavona danai |
| Xarifia diminuta | Pavona sp. |
| Xarifia finitima | Pavona varians |
| Odontomolgus actinophorus | Pavona venusta |
| Odontomolgus rhadinus | Pavona sp. |
| Mandobius regalis | Pectinia lactuca |
| Xarifia gradata | Physogyra lichensteini |
| Xarifia minax | Physogyra lichensteini |
| Panjakus platygyrae | Platygyra astreiformis |
| Panjakus platygyrae | Platygyra lamellina |
| Panjakus daedala | Platygyra daedala |
| Alteuthellopsis corallina | Platygyra daedala |
| Panjakus fastigatus | Platygyra ryukyuensis |
| Panjakus parvipes | Platygyra ryukyuensis |
| Andrianellus papillipes | Platygyra ryukyuensis |
| Xarifia species | Platygyra sinensis |
| Alteuthellopsis corallina | Platygyra sp. |
| Xarifia dispar | Platygyra sp. |
| Panjakus sp. | Platygyra sp. |
| Andrianellus exsertidens | Platygyra daedala |
| Gelastomolgus | Plerogyra sp. |
| Alteuthellopsis corallina | Pocillopora damicornis |
| Xarifia fimbriata | Pocillopora damicornis |
| Xarifia fissilis | Pocillopora damicornis |
| Xarifia jugalis | Pocillopora damicornis |
| Xarifia obesa | Pocillopora damicornis |
| Xarifia quinaria | Pocillopora damicornis |
| Xarifia sectilis | Pocillopora damicornis |
| Xarifia serrata | Pocillopora damicornis |
| Anchimolgus partenuides | Pocillopora damicornis |
| Xarifia fimbriata | Pocillopora damicornis var. caespitosa |
| Xarifia imparilis | Pocillopora damicornis var. caespitosa |
| Xarifia jugalis | Pocillopora damicornis var. caespitosa |
| Xarifia quinaria | Pocillopora damicornis var. caespitosa |
| Xarifia obesa | Pocillopora danae |
| Xarifia comata | Pocillopora eydouxi |
| Xarifia fimbriata | Pocillopora eydouxi |
| Xarifia imparilis | Pocillopora eydouxi |
| Xarifia jugalis | Pocillopora eydouxi |
| Xarifia maldivensis | Pocillopora eydouxi |
| Xarifia obesa | Pocillopora eydouxi |
| Xarifia sectilis | Pocillopora eydouxi |
| Xarifia tenta | Pocillopora ligulata |
| Tegastes georgei | Pocillopora sp. |
| Xarifia fimbriata | Pocillopora sp. |
| Xarifia maldivensis | Pocillopora sp. |
| Xarifia obesa | Pocillopora sp. |
| Xarifia serrata | Pocillopora sp. |
| Xarifia comata | Pocillopora sp. cf. verrucosa |
| Xarifia obesa | Pocillopora sp. cf. verrucosa |
| Xarifia serrata | Pocillopora sp. cf. verrucosa |
| Tegastes paulipes | Pocillopora verrucosa |
| Xarifia comata | Pocillopora verrucosa |
| Xarifia gibberula | Pocillopora verrucosa |
| Xarifia imparilis | Pocillopora verrucosa |
| Xarifia obesa | Pocillopora verrucosa |
| Xarifia serrata | Pocillopora verrucosa |
| Xarifia tenta | Pocillopora verrucosa |
| Panjakus bidentis | Pocillopora verrucosa |
| Monomolgus unihastatus | Porites (andrewsi?) |
| Monomolgus unihastatus | Porites (nigrescens?) |
| Xarifia longa | Porites latistella |
| Euryte verecunda | Porites lobata |
| Hemicyclops regalis | Porites lobata |
| Monomolgus torulus | Porites lobata |
| Kombia incrassata | Porites lobata |
| Orstomella yaliuensis | Porites lutea |
| Xarifia species | Porites lutea |
| Kombia curvata | Porites lutea |
| Kombia imminens | Porites monticulosa |
| Kombia angulata | Porites somaliensis |
| Kombia avitus | Porites sp. |
| Monomolgus baculigeres | Porites nigrescens |
| Monomolgus psammocorae | Psammocora sp. |
| Kombia angulata | Psammocora sp. |
| Xarifia diminuta | Psammocora contigua |
| Xarifia imitans | Psammocora contigua |
| Odontomolgus actinophorus | Psammocora contigua |
| Numboa porosa | Psammocora contigua |
| Odontomolgus rhadinus | Psammocora contigua |
| Xarifia formosa | Psammocora digitata |
| Xarifia imitans | Psammocora digitata |
| Dumbeana undulatipes | Psammocora logianensis |
| Emunoa proknta | Psammocora logianensis |
| Lipochaetes extrusis | Psammocora logianensis |
| Odontomolgus exilipes | Psammocora samoensis |
| Odontomolgus geminus | Psammocora samoensis |
| Xarifia diminuta | Psammocora sp. |
| Xarifia diminuta | Psammocora sp. cf. contigua |
| Numboa porosa | Psammocora togianensis |
| Xarifia simplex | Scapophyllia cylindrica |
| Xarifia reducta | Seriatopora caliendrum |
| Xarifia eminula | Seriatopora hystrix |
| Xarifia levis | Seriatopora hystrix |
| Xarifia obesa | Seriatopora hystrix |
| Xarifia reducta | Seriatopora hystrix |
| Xarifia umbonata | Seriatopora hystrix |
| Xarifia varialabrata | Seriatopora hystrix |
| Anchiomolgus tenuipes | Seriatopora hystrix |
| Anchimolgus noumensis | Seriatopora hystrix |
| Xarifia reducta | Seriatopora octoptera |
| Xarifia reducta | Seriatopora sp. |
| Parategastes conexus | Stereonephthya ulicoides |
| Xarifia decorata | Stylophora mordax |
| Xarifia lissa | Stylophora mordax |
| Tegastes georgei | Stylophora pistillata |
| Xarifia decorata | Stylophora pistillata |
| Xarifia dissona | Stylophora pistillata |
| Xarifia lissa | Stylophora pistillata |
| Xarifia obesa | Stylophora pistillata |
| Xarifia sectilis | Stylophora pistillata |
| Xarifia obesa | Stylophora pistillata var. palmata |
| Xarifia decorata | Stylophora sp. |
| Xarifia lissa | Stylophora sp. |
| Xarifia species | Stylophora sp. |
| Xarifia serrata | Stylophora subseriata |
| Xarifia insolita | Tubastrea aurea |
| Xarifia insolita | Tubastrea aurea |
| Xarifia species | Tubastrea sp. |
| Xarifia lacerans | Turbinaria danae |
| Xarifia unicata | Turbinaria danae |
| Xarifia hamata | Turbinaria sp. (T. elegans?) |
| Wedanus inconstans | Goniopora minor |
References
- Bandera, M. and R. Huys, 2008. Proposal for new genus for Asterocheres mucronipes Stock 1960 (Copepoda, Siphonostommatoida, Asterocheridae), an associate of the scleractinian coral Astroides calcycularis (Pallas, 1766) in the Strait of Gibraltar. Zool. J. Linnean Soc., 152:635-653.
- Borneman, E., 2004. It’s a small world, after all. http://reefkeeping.com/issues/2004-06/eb/index.php
- Carson, C., K. Hammer and T. Riley, 2006. Meleluca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clinical Microbiol. Rev., 19(1):50-62.
- Cheng, Y.-R., J.-S. Ho and C.-F. Dai, 2009. Orstomella yaliuensis, n. sp., a xarifid copepod (Crustacea) parasitic in the polyps of hump coral Porites lutea Milne Edwards and Haime off Taiwan. Syst. Parasitol. 74:17-21.
- Cheng, Y.-R., J.-S. Ho and C.-F. Dai, 2007. Two new species of Xarifia Humes, 1950 (Copepoda, Xarifiidae) associated with corals of Taiwan. Crustaceana, 80, 9: 1135-1145.
- Cheng, Y.-R. and C.-F. Dai, 2009. The infection process of the reef coral Stylophora pistillata by the parasitic copepod, Xarifia obesa. Coral Reefs, First Online.
- Cheng, Y.-R. and C.-F. Dai, 2009. Endosymbiotic copepods may feed on zooxanthellae from their coral host, Pocillopora damicornis. Coral Reefs, First Online.
- Coma, R., M. Ribes, C. Orejas, and J.-M. Gili, 1999. Prey capture by a benthic coral reef hydrozoan. Coral Reefs, 18:141-145.
- Delbeek, J.C. and J. Sprung, 2005. The Reef Aquarium, Volume 3: Science Art and Technology. Ricordia Publishing, Coconut Grove, Florida. 679 pp.
- Delbeek, J.C. and J. Sprung, 1994. The Reef Aquarium, Volume 1: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Ricordia Publishing, Coconut Grove, Florida. 544 pp.
- Doignon, G., D. Deheyn and F. Fiers, 2004. Telestacicola xenophiothricis n. sp. (Copepoda, Poecilostomatoida), a remarkably well adapted commensal of the brittlestar Ophiothrix purpurea (Echinodermata). Belg. J. Zool., 134 (2/1): 67-73.
- Dorton, D., 2004. A “cure” for red bugs. http://www.canreef.com/vbulletin/archive/index.php/t-23461.html
- Dulin, M., 1976. Diseases of Marine Aquarium Fishes. TFH Publications, neptune City. 128 pp.
- Gollner, S., V. Ivaneko, and P. Arbizu, 2008. A new species of deep-sea Tegastidae (Crustacea: Copepoda: Harpacticoida) from 9°50´N on the East Pacific Rise, with remarks on its ecology. Zootaxa 1866: 323-326.
- Gosliner, T., D. Behrens, and G. Williams, 1996. Coral Reef Animals of the Indo-Pacific. Sea Challengers Press, Monterey.
- Ho J.-S., Cheng, Y.-R., and C.-F. Dai, 2008. The infection of a parasitic copepod, Xarifia obesa on corals. Proc. 11th Coral Reef Symp.
- Humes, A. and J.-S. Ho, 1968a. Lichomolgid copepods (Cyclopoida) associated with corals in Madagascar. Bull. Mus. Comp. Zool., 136(11):353-413.
- Humes, A. and J.-S. Ho, 1968b. Xarifiid copepods (Cyclopoida) parasitic in corals in Madagascar. Bull. Mus. Comp. Zool., 136(11):415-460.
- Humes, A., 1974. Cyclopoid copepods associated with the coral genera Favia, Favites, Platygyra, and Merulina in New Caledonia. Pac. Sci., 28(4):383-399.
- Humes, A., 1979. Cyclopoid copepods (Lichomolgidae) associated with the scleractinian Cyphastrea in New Caledonia. Pac. Sci., 33(2):195-206.
- Humes, A., 1981a. A new species of Tegastes (Copepoda: Harpacticoida) associated with a scleractinian coral at Eniwetok Atoll. Proc. Biolog. Soc. Washington. 94(1): 254-263.
- Humes, A., 1981b. Harpacticoid copepods associated with cnidarian in the Indo-west Pacific. J. Crustacean Biol., 1(2): 227-240.
- Humes, A., 1984. Harpacticoid copepods associated with cnidarians in the tropical Pacific. Zoologica Scripta, 13(3):209-221.
- Humes, A., 1985. A review of Xarifiidae (Copepoda, Procilostomada) parasites of scleractinian corals in the Indo-Pacific. Bull. Mar. Sci. 36, 467-632.
- Humes, A., 1986. Two new species of Cerioxynus (Copepoda: Poecilostomatoida) parasitic in corals (Scleractinia: Faviidae) in the south Pacific. Syst. Parasitol., 8(3): 187-198.
- Humes, A., 1991. Mandobius regalis gen. et. sp. n. (Copepoda: Poecilostomatoida: Lichomolgidae) associated with the coral Pectinia lactuca in New Caledonia. Zool. Scripta, 20(3).
- Humes, A., 1993. Catalogue of copepods associated with marine invertebrates at Nosy Bé, northwestern Madagascar, and vicinity. Rev. Hydrobiol. trop. 26 (4): 293-304.
- Humes, A., 1994. How many copepods? Hydrobiological, 292/293: 1-7.
- Humes, A., 1995. Three new species of Hemicyclops (Copepoda: Poecilostomatoida: Clausidiidae) from northwestern Madagascar. Bull. Museum Nat. Hist. 17 (1-2): 41-162.
- Humes, A., 1996. New genera of Copepoda (Poecilostomatoid) from the scleractinian coral Psammocora in New Caledonia. 1 Zool. J. Linnean Soc., 118(1): 59-82.
- Humes, A. and Y.-S. Lee, 1985. The Poecilostome copepod Anthessius mytilicolus Reddiah, 1966, associated with the mussel Pecna viridis (L.) at Hong Kong. Asian Mar. Biol. 2:85-92.
- Humes, A. and J. Stock, 1972. A Revision of the Family Lichomolgidae Kossmann, 1877, Cyclopoid Copepods Mainly Associated with Marine Invertebrates. Smithsonian Contributions to Zoology, No. 127. Smithsonian Institution Press, Washington. 380 pp.
- Hiller, G., 2003, An aquarist’s experiences with a species of Acropora parasites. http://www.advancedaquarist.com/issues/june2003/feature.htm
- Ho, M-J., Y-R. Cheng and C-F. Dai, 2008. The infection of a parasitic copepod, Xarifia obesa on corals. Proc. 11th Int. Coral Reef Symp. (Abstract)
- Ivanenko, V., F. Ferrari and H-U. Dahms, 2008. Nauplii of Tegastes falcatus (Norman, 1869) (Harpacticoida, Tegastidae), a copepod with an unusual naupliar mouth and mandible. J. Crustacean Biology 28(2):270-280.
- Ivanenko V. and A. Smurov, 1996. About finding of bacteria on symbiotic copepod Scottomyzon gibberum Scott, – a possible vector of pathogenic microorganisms. Doklady Akademii Nauk. 346(4): 573-575.
- Ivanenko, S., 2009. Stockmyzon crassus (Stock, 1966). In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=383234 on 2009-11-17
- Kabata, Z., 1970. Diseases of Fishes: Crustacea as Enemies of Fishes. T.F.H. Publications, Jersey City, NJ. 171 pp.
- Jacoby, C. and J. Greenwood, 1989. Emergent zooplankton in Moreton Bay, Queensland, Australia: seasonal, lunar, and diel patterns in emergence and distribution with respect to substrata. Mar. Ecol. Prog. Ser., 51: 131-154.
- Kabata, Z., 1970. Diseases of Fishes. Book 1: Crustacea as Enemies of Fishes. T.F.H. Publications, Jersey City, NJ. 171 pp.
- Levenson, M., undated. Red Bugs – No more! http://www.melevsreef.com/redbugs.html
- Misaki, H., 1978. Two new species of Xarifia (Copepoda, Cyclopoida) parasitic on the coral Acropora pecrinata at Saibiura. Bull. Mar. Park Res. Sta. 2:105-114.
- Nogueira, J., 2003. Fauna living in colonies of Mussismilia hispa (Verrill) (Cnidaria: Scleractinia) in four south-eastern Brazil Islands. Brazilian Archives of Biology and Technology. 46(3): 421-432.
- Robertson, R., 1970. Review of the predators and parasites of stony corals, with special reference to symbiotic prosobranch gastropods. Pacific Science, 24: 43-54.
- Sebastian, M.J. and N. Krishna Pillai, 1973. Humesiella corallicola n.g., n.sp., a cyclopoid copepod associated with coral on the south east coast of India. Hydrobiologia, 42 (1): 143-152.
- Shimek, R., 2002. A Spineless Column: Bitty bugs – Copepods in the reef aquarium. Reefkeeping.com/issues/2002-10/rs/index.php
- Sparks, A., 1985. Synopsis of Invertebrate Pathology, Exclusive of Insects. Elsevier Science Publishers, Amsterdam. 423 pp.
- Sprung, J. and J.C. Delbeek, 1997. The Reef Aquarium, Volume 2: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Ricordia Publishing, Coconut Grove, Florida. 546 pp.
- Stocks, J., 1988. Copepods associated with reef corals: A comparison between the Atlantic and the Pacific. Hydrobiologica 167/168:545-547.
- Walter, T. Chad, 2009. Asterocheres astroidicola Conradi, Bandera & López-González, 2006. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=362327 on 2009-11-17
- Walter, T. Chad, 2009. Schedomolgus dumbensis Kim I.H., 2003. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=362026 on 2009-11-17
- Walter, T. Chad, 2009. Xenomolgus Humes & Stock, 1972. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=348183 on 2009-11-17
- Walter, T. Chad, 2009. Amarda goniastreae Humes, 1985. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=348656 on 2009-11-17
- Walter, T. Chad, 2009. Panjakus auriculatus Humes & Dojiri, 1979. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=354142 on 2009-11-17
- Walter, T. Chad, 2009. Panjakus Humes & Stock, 1972. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id==347665 on 2009-11-17
- Walter, T. Chad, 2009. Ecphysarion ampullulum Humes, 1993. In: Walter, T.C., Boxshall, G. (Eds) (2009). World Copepoda database. Accessed through the World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=351232 on 2009-11-17
- Wilkens, P., 1990. Invertebrates: Stone and False Corals, Colonial Anemones.
- Engelbert Pfriem Verlag, Wuppertal, Germany.
- Wilkens, P. and J. Berkholz, 1986. Invertebrates: Tube-, Soft- and Branching Corals. Engelbert Pfriem Verlag, Wuppertal, Germany.
- Wright, K.M. 2009. Ivermectin Treatment of Red Bug Disease in Acropora Coral. Exotic DVM 11(1):7



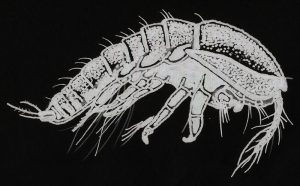
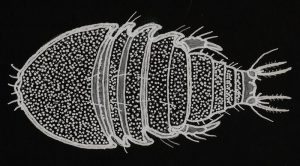


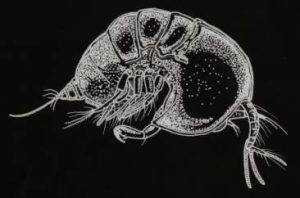




0 Comments