In early the 1970’s, when I was just 13 or so, Cryptocaryon irritans (“marine ich”) and Amyloodinium ocellatum (“marine velvet”) were a bit less of a problem for my fish than they are now when I quarantine new fish as an aquarium curator. The reason was a product called Marex from the Aquatronics Corporation (they have long ceased operations). Marex was sort of a wonder drug for us back then – simply adding a single $1.99 dose protected the fish in a 50 gallon aquarium from many diseases plus it killed the unsightly algae that grew all over the tank decorations back in those days! When the company went out of business I moved on to using other products. For the past 25 years, I’ve been using ionic copper measured with a spectrophotometer twice a day to control marine ich and other protozoan diseases. Copper is slow to affect a cure, and the difference between a therapeutic dose and a dose harmful to some fish species is slight. Still, it seemed to be the best method for quarantining or treating active diseases in fish. Thinking back to when I was a youngster, I did some research and discovered that the active ingredient in Marex was chloroquine, and I was familiar with that drug as it was being used by other public aquariums. Acquiring some myself five years ago, I’ve begun incorporating it into my arsenal of aquarium fish disease treatments. A few home aquarists have begun re-exploring its uses as well, often calling it by the shorthand name of “CP” which stands for chloroquine phosphate. This article provides those aquarists with additional background information to enable them to be better able to use this “new” drug if they wish – having options is always a good.

Green chromis with Uronema infection that might have responded to chloroquine if treatment was started soon enough.
Chemical properties
Chloroquine was developed for human medicine in the 1930’s at Bayer laboratories. It was first thought to be too toxic for any practical use, but decades later, it was shown in clinical trials to have significant value as an anti-malarial drug. However, its subsequent wide-spread use allowed the malaria disease organism to become resistant to it, requiring the development of other treatments.
There are at least three forms of the drug available:
Chloroquine diphosphate (Aralen): C18H26ClN3 . 2H3PO4
Chloroquine hydrochloride (Aralen HCL): C18H26ClN3 . 2HCl
Chloroquine sulfate (Plaquenil): C18H26ClN3 . H2O4S
The Chloroquine base also goes by the name; 7-chloro-4-[[4- (diethylamino)-1-methylbutyl]amino] quinolone. The most commonly available version of the drug for aquarium use is the diphosphate salt. This compound is a fine white fine powder that is readily soluble in water. In dry environments it seems to build up a static charge, and the granules tend to become airborne and then stick to nearby objects. This can create problems when weighing out small amounts of the drug, as it tends to stick to the storage container, the weighing pan as well as nearby objects. Always dissolve the prescribed amount of chloroquine in distilled water before adding it to an aquarium.
English pronunciation of the compound varies between “KLOR-oh-kwin” and “Klor-oh-KWEEN”, with the former used by most aquarists, while the latter is listed on some word pronunciation web sites.
Uses and dosages
Chloroquine is typically dosed at a rate of 10 to 20 milligrams per liter (mg/l), with 15 mg/l being considered a “standard dose” (Hemdal 2006). Note: in most instances, solutions measured in “milligrams per liter” are equivalent to “parts per million” or ppm.
The 10 mg/l dose should be used as a quarantine preventative (not for active diseases), or for treating delicate species (although little is known about the sensitivity of different fish species to this medication). A dose of 15 mg/l is considered the normal dose for treating most protozoan infections, while the 20 mg/l dose would be reserved for attempting to eradicate difficult-to-treat Uronema marinum infections.
The first step in preparing to use any drug that will be added to an aquarium at a specific dose is to determine the true water volume of the aquarium. This is often less than an aquarium’s advertised volume (or it could be more if there is a sump attached to the system). The most accurate means to determine the volume of an aquarium system is to measure the amount of water it takes to fill the total system, with all decorations in place. As this is usually not possible to do except when the aquarium is first filled, the following method will give accurate enough results in most instances (this method uses US volume measurements combined with metric dosages):
- Measure (in inches) the length, width and height of the water inside the aquarium from the top of the gravel layer to the water’s surface, and inside the glass front to back and side to side. Multiple these three numbers to get the gross volume in cubic inches and then divide by 231 to determine the volume in gallons (there are 231 cubic inches in a US gallon).
- Deduct an estimated percentage for tank decorations. If you are unsure, the decorations in a typical marine aquarium with artificial coral and rock displace about 15% of the water volume, so you would multiply the gross volume from step 1 by 0.85
- Use the same technique to measure the volume of the gravel layer (if any), but multiply the result by 0.30, as only about 30% of the gravel layer is water, the rest of the volume is the gravel itself.
- Use the same technique to measure the volume of the sump (if any).
- Except for very large systems, the amount of water contained in the filtration system is inconsequential, but you might want to add a couple of gallons to the estimate if the tank uses a large canister filter.
- Add these measurements together to arrive at the estimated net aquarium volume in gallons.
- Once you have estimated the aquarium system volume, multiply the number of gallons by the target dose of the drug (in mg/l or parts per million). Dividing this by 266 will give the number of grams of medication that needs to be added to the water.
- Always run these calculations TWICE to ensure accuracy. If you arrive at different numbers, stop and determine where the mistake was made.
One grave issue when dosing medications occurs if a decimal place is lost through an error in calculation. This can result in a dose many times higher or lower than is called for. Aquarists who are not familiar with using a particular drug may not realize that the dose they have calculated is so far off. For a frame of reference, to dose 100 net gallons of aquarium water with chloroquine at 15 mg/l, you would add 5.6 grams of the drug (100 gal. * 15 mg/l / 266 = 5.639, which rounds down to 5.6 grams of chloroquine).
Home aquarists may have difficulty in measuring minute amounts of a drug to treat small tanks. Avoid guessing or trying to use volume measurements for these weights. Small electronic balances are available for relatively low cost, but may not have sufficient resolution to measure amounts of a drug in the milligram range. One trick to improve accuracy of a measurement is to make a stock solution, and then use a small quantity of that to dose the tank. The reason this works well is that home aquarists generally can measure small volumes of a liquid easier than they can weigh small amounts of a powder. For example, if you need to treat a 10 gallon aquarium with chloroquine at 10 mg/l, you would need to add 376 mg of the drug to the tank, a very small amount to try and weigh out. If you can more easily weigh out a single gram (a nice round amount), you can dissolve that into 12 teaspoons of distilled water, and then add 4 ½ teaspoon of that solution to the 10 gallon tank. For increased accuracy, you can buy a volumetric medicine dosing spoon. These can be used much like a graduated cylinder for measuring accurate amounts of a stock solution. For this example, you would add one gram of chloroquine to 100 milliliters of distilled water, and then add 37.6 ml of that stock solution to the aquarium.
Why the concern about such an accurate dosage when chloroquine has a plus or minus 33% margin of error when using the 15 mg/l dose? The reason is that there are two primary chances for error; in the tank volume calculation and when weighing of the drug itself. Two small errors may more or less cancel each other out, but if the errors are in the same direction, they are additive or subtractive and the dose you add to the aquarium could then be outside reasonable limits.
In addition to controlling protozoan parasites, chloroquine also has some use in eradicating certain metazoan (multi-celled) fish parasites. The Georgia Aquarium has used it to control turbellarian worm infestations at a dose of only 10 mg/l (Tonya Claus, personal communication). These worms have been shown to be resistant to treatment with Praziquantel and formalin, so an alternative treatment such as this is much needed.
A single dose of chloroquine at 15 mg/l was found to be effective at eradicating Aiptasia sp. glass anemones within 48 hours. In one test, no reinfestation of these pest anemones was seen in two months following treatment (personal observation). However, this method cannot be used in aquariums housing other invertebrates as this dose also eradicated algae and sponges that were growing alongside the Aiptasia sp. anemones.
In an effort to isolate the drug from sensitive invertebrates, some aquarists have administered the drug orally to their fish. Chloroquine is very bitter, and if the drug isn’t masked by strong flavors in the food used to bind it with, fish will soon learn to avoid it. In addition, for oral medications to work, the fish still needs to be feeding normally, and acutely ill fish often refuse to feed. Finally, dosage is very difficult to control in oral medication for aquarium fishes. The drug must be mixed into a gelatin food binder at 6 to 10 milligrams of drug per gram of food, and then that has to be fed to the fish at a rate of around 3% of its body weight per day – and few, if any aquarists know the actual weight of their fishes.
Activated carbon has been widely reported to remove chloroquine from aquarium water at the conclusion of a treatment, but be aware that carbon has been implicated in the development of head and lateral line erosion in marine surgeonfish (Hemdal & Odum 2011). If you do decide to use carbon to remove chloroquine, it would be advisable to use a premium pelleted carbon, rinse it well with deionized water prior to use, and remove all of the carbon when finished. The amount of carbon needed to remove all of the chloroquine will be a guess. A starting point would be 4 to 6 grams of well-rinsed carbon per gallon of aquarium water, placed in a fine mesh bag and added to the aquarium’s power filter for 48 hours. If the aquarium will be using delicate invertebrates at the conclusion of the treatment, it would be more prudent to change all of the water first.
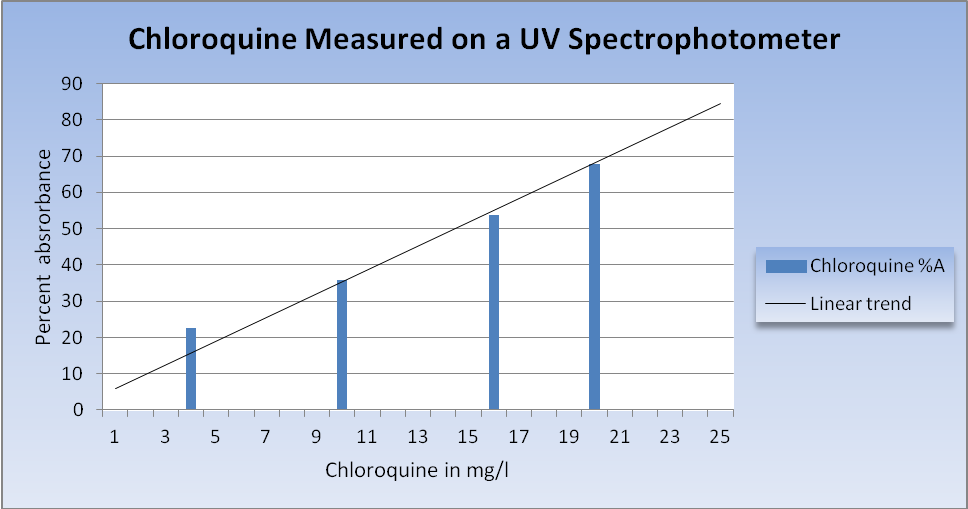
An example of four serial dilutions of chloroquine measured on a Hach UV spectrophotometer. The results are % absorbance (the inverse of the % transmittance) at 329 nm. The linear trend line can be used in subsequent tests to measure the amount of chloroquine in aquarium water.
There is no test kit to measure the chloroquine concentration in water as there is for many copper medications. Public aquariums and laboratories with access to a UV spectrophotometer can use it to measure chloroquine in the water directly. How this works is that at 329 nm, chloroquine in water absorbs ultraviolet light in proportion to its concentration. Using a quartz cuvette that is transparent to UV, a blank sample of untreated water is first measured. Then, a sample of that water is dosed with a serial dilution of chloroquine in the range to be treated, typically 2.5, 5, 10, 20 and 25 mg/l and the percent transmittance is measured for each sample. Once this standard trend line is graphed, the chloroquine concentration of any water sample within that range can be measured. Because other organic compounds can be present in aquarium water that may also absorb UV light, it is best to create a standard curve for each water system prior to treatment.
In one test attempting to measure the ability of carbon to remove chloroquine, a spiked sample actually showed an increase in absorbance at 329 nm after filtering through carbon for 24 hours. Since the chloroquine level couldn’t have risen, it is presumed that something in the carbon dissolved into the water and that obscured the reading. However, this also made it impossible to determine if the carbon actually removed any of the chloroquine, so this aspect remains open to questioning. In a second test, 20 mg of chloroquine was dissolved in a liter of distilled water. This sample was then exposed to 4 g of rinsed activated carbon for a week. Measured at 329 nm, the sample only dropped by a calculated 5 mg/l chloroquine according to the standard curve. Since something in the carbon seems to be obscuring any chloroquine measurements, it is difficult to understand how any of the reports that carbon removes chloroquine could have been substantiated, at least by using a UV spectrophotometer.
Preliminary in vitro study
Two very basic qualitative in vitro tests were conducted to test the efficacy of chloroquine phosphate as a potential treatment against the ciliate Uronema marinum (Hemdal 2010). Uronema is a fairly common ciliate that is difficult to treat as these parasites can burrow into the fish’s skin and therefore isolate themselves from many external bath treatments such as formalin, copper and hyposalinity. These informal tests show that this drug is effective at killing Uronema when it is used as a bath, but it is unknown if enough of the drug would taken up by the fish in order to raise the level in the blood to therapeutic levels.
In the first test, the body of a small parrotfish fish that had succumbed to a Uronema infection was cut in half. One section of the fish was placed in tank water, the second section was placed in tank water dosed with Chloroquine at 40 mg/l (a higher than normal dose). After six hours, the number of Uronema in the treated sample had been markedly reduced, while the numbers in the untreated sample had actually increased.
In a second test, the bodies of two green chromis that had died from acute Uronema infections were exposed to chloroquine at 35 mg/l. A marked reduction of the numbers of the ciliate was seen within three hours, and only one surviving Uronema was seen on the body of one of the fish after eight hours. Using deceased fish for these bio-assays is problematic in that there is difficulty obtaining specimens “as-needed” and room temperature tests longer than 24 hours cannot be performed as the fish flesh begins to putrefy.
Contraindications
At doses typically used to treat fish diseases, chloroquine is also toxic to many invertebrates, algae and bacteria. Seriously high ammonia levels ( > 1 mg/l NH3) are sometimes seen a few days to a week after dosing an aquarium with chloroquine. It is unknown why this is seen in some aquariums but not others. One hypothesis is that the chloroquine has a direct antibiotic effect on the nitrifying bacteria. Another idea is that the chloroquine kills so much microscopic life in the aquarium that the beneficial bacteria are overwhelmed, and an ammonia spike develops. Most likely, it is a combination of both of these factors causing this issue. Always monitor the ammonia levels in aquariums during treatment with chloroquine. Freshwater aquariums should also be monitored for subsequent rise in nitrite levels as well.
Ultraviolet light seems to alter the chemical make-up of chloroquine in water. This is particularly a concern when UV sterilizers are employed. The UV light causes changes in the chloroquine that can turn the aquarium water a murky brown (Tiffany Adams, Shedd Aquarium, personal communication). The presumption is that the effect of the drug is also altered, so UV sterilizers (and probably ozone generators) must be turned off during treatment. Some aquarists go to the extreme of blocking all light entering the aquarium during treatment, but this is not necessary unless the aquarium is open to natural sunlight.
As mentioned, the use of chloroquine to treat malaria in humans has long been known to lose effectiveness as the Plasmodium protist that causes the disease developed a resistance to the drug. Purely speculation, but the same mechanism could cause resistance to aquarium disease-causing protists as well. If this problem ever develops, it will most likely appear in public aquariums or fish importers as they use the drug repeatedly in the same centrally filtered systems. Home aquarists are unlikely to administer the high number of treatments required to cause such a resistance to develop.
The Material Safety Data Sheet (MSDS) for chloroquine phosphate is difficult to interpret. Much of the toxicity data listed were derived from chronic exposure in humans taking the drug for control of malaria; retinal damage, nervous system disruption, and liver damage. Acute exposure of the amounts typically used in home aquariums can cause irritation to the eyes and respiratory tract. Always use gloves, eye protection and a dust mask when handling this material, and keep it away from children and pets.
The Phosphate Connection
Most, if not all of the chloroquine available for aquarium use is in the form of chloroquine diphosphate (as opposed to chloroquine hydrochloride or sulfate). This means that dosing an aquarium with this drug will also add some phosphate (PO4) to the water when the compound dissociates as it dissolves. Theoretically, using the molecular weights of its components, chloroquine will release about 20% of its weight as PO4 . This means that for a typical 20 mg/l dose of chloroquine, one would expect the phosphate level in the aquarium to rise by around 4 mg/l. Empirically, a series of tests on chloroquine at 20 mg/l in distilled water resulted in a concurrent rise in PO4 of 4 to 6.1 mg/l, a bit higher than expected*. A rule of thumb might be that for any dose of chloroquine, you could expect to see a rise in phosphate levels of around 20 to 30% of the total dose of chloroquine. Therefore, a single dose of chloroquine at 10 mg/l would increase the PO4 concentration in the water by about 2 to 3 mg/l. This is would be a major concern in reef aquaria, but as chloroquine is typically used in fish-only aquariums, or quarantine systems, the residual phosphate is less of an issue and can be reduced by water changes.
*Please note that phosphate is difficult to measure, even using a spectrophotometer, and there was a large variation in the measurements taken in these tests, with no real explanation.
Availability
The current major drawback to using chloroquine to treat fish diseases is locating a commercial source of the drug. Public aquariums, buying large quantities, have no difficulty in acquiring it from online companies at around $185 per kilogram. Hobbyists, needing much less of the drug, have not been able to find it easily available in lesser amounts – but that should be changing, now that its use has become more popular again. Until an aquarium manufacturer starts marketing it again, you may be able to acquire it from your veterinarian, or perhaps go in for a “group buy” with other hobbyists. Recent online prices for non-prescription chloroquine vary depending on the amount purchased from .185 cents per gram up to $2.40 per gram. One gram of chloroquine will dose 18 gallons of water at 15 mg/l.
Conclusion
While not a panacea or miracle drug, chloroquine is experiencing resurgence in popularity for use in fish-only aquariums and quarantine systems to treat a variety of problems ranging from Cryptocaryon to Aiptasia anemone infestations. Chloroquine remains active in aquariums for many weeks, seems to have low toxicity to fish and may be removed using activated carbon. In critical applications, treatment levels can be measured with a UV spectrophotometer, and the dose adjusted accordingly.
References
- Hemdal, J.F. Odum, R.A. 2011. The Role of Activated Lignite Carbon in the Development of Head and Lateral Line Erosion in the Ocean Surgeonfish. North American Journal of Aquaculture 73:4, 489-492
- Hemdal, J.F. 2010. Red Band Syndrome. Aquarium Fish International 22(1):26-30
- — 2009. Mortality Rates of Fishes in Captivity. Advanced Aquarist’s Online Magazine. 8(12): http://www.advancedaquarist.com/2009/12/fish2
- — 2006. Advanced Marine Aquarium Techniques. 352pp. TFH publications, neptune City, New Jersey




0 Comments