Tridacnids, generally called giant clams, are common additions to reef aquariums, most of which belong to the genus Tridacna . However, there are two tridacnids available from time to time, which are not in the same genus with the rest of the bunch. These are Hippopus hippopus and Hippopus porcelanus , the former of which is far more likely to be seen for sale than the latter. Regardless, folks are generally unfamiliar with both of these species, so I’ll fill you in.
Hippopus hippopus
Commonly called the hippopus clam, strawberry clam, horse’s hoof clam, or bear paw clam, this species is typically found on reef flats, often in grassy areas, living on sandy to slightly muddy bottoms, or on coral fragments and gravels (Barnes 1984 and Govan et al . 1988). Shelley (1988) reported that juveniles are usually lightly byssally attached to the substrate, and that they typically stay attached until they are larger than 14cm. However, Pasaribu (1988) reported that hippopus clams are generally not attached to the substrate. This depends on size and substrate though, with juveniles being attached if possible, and larger clams living unattached and relying more upon their own weight to keep them in place. They also live from the intertidal zone down to about 6m.
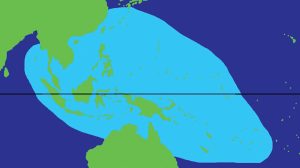
Theirnatural range extends from the eastern Indian Ocean from Myanmar eastward across the Pacific Ocean to the Marshall Islands and to Fiji and Tonga, and from as far north as Japan down to the Great Barrier Reef, New Caledonia, and western Australia. However, hippopus has been heavily over-fished and is rare to extinct in many parts of this range (Wells 1997 and Raymakers et al . 2003).
Unlike the Tridacna species, hippopus’ mantle, without exception, extends only to the upper margin of the shell. It does not extend laterally out of the shell, as is seen with the Tridacna species. The inhalent siphon, where water is drawn into the body chamber, has no tentacles around its margin, either. However, I have seen small protrusions around the margins of some specimens, and sometimes the siphon’s margin can also be somewhat jagged in appearance or rather convoluted/frilly. The exhalent siphon, where water exits, is typically flattened and disc-like, forming a low cone with a circular opening.
The inhalent siphon of H. hippopus lacks fringing tentacles, making it easy to distinguish which species of Hippopus it is.
This species can come in a few colors, but the vast majority has a predominantly yellowish-brown or olive-green mantle with white to cream or golden splotches and/or thin stripes. Others may be more gray in color, but that’s about it. They often have considerable areas of rather translucent tissue with lighter or no color, as well.
Theshell is usually grayish-white, sometimes with a faint tinge of yellow or orange. However, unlike other tridacnids, it’s oftentimes covered by irregularly-shaped red blotches. The shell commonly becomes highly encrusted by other organisms though, so these markings typically aren’t visible on larger ones in the wild. It can also be very elongated with respect to the hinge, which is typically a bit more than 1/2 the length of the shell, and may be closer to 2/3 of its length for larger specimens. This allows the shell to gape open very widely. The shells are also strongly inflated, and hippopus is usually quite fat even at small sizes.
The shells can also have a variable number of folds/ribs, often having as many as 13 or 14, in a great range of sizes. However, typically only 5 to 8 of these are more pronounced than the others, and can be relatively convex and rounded, or more straight-angled and box-like. In addition, the larger folds also usually have small riblets on their surfaces, so that one large fold may appear be made of several smaller ones. They also lack the scale-like scutes commonly seen on Tridacna species like T. maxima and T. squamosa , but they’re sometimes covered by small semi-tubular structures. These are especially common on smaller clams, and when present they can give the shell something of a prickly appearance.
The shell halves are symmetrical to each other and can close tightly. They’re topped by rather unusual inter-digitating projections though, which are often quite squarish when the clams are young, and become more rounded as the clams grow. There are usually 8 to 12 of these. The byssal opening in the bottom of the shell is usually absent or quite small for juveniles, often being nothing more than a short slot that is closed completely later. This is expected, as hippopus typically maintains only a weak byssal attachment when young, if any at all, and always lives unattached when larger. The area around the byssal opening is rather unique, too, as it’s ringed with numerous small, inter-digitating projections/teeth that are absent in Tridacna species.
The byssal opening of both Hippopus species is quite different than those of the Tridacna species, as it bears numerous inter-digitating projections.
They can also get quite large, as Rosewater (1965) reported that hippopus could grow to 40cm in length, with more recent references indicating even larger sizes. For example, Fossa & Nilsen (2002) say the maximum size is 50cm, while Knop (1996) says the record holder is housed at the Marine Science Institute of the University of the Philippines and measures in at 54cm. However, Fossa & Nilsen also reported that 40cm is usually the maximum size, and Smith (1898) wrote about an “unusually large specimen” from the Philippines that was only 34cm in length. So, don’t expect one to reach such huge sizes in an aquarium.
Hippopus porcellanus
Commonly called the porcelain clam or China clam, this species is hard to come by in the U.S. A few years ago I bought one from Clams Direct, and the owner told me it was the first one he’d ever seen despite importing clams for over 7 years. I have never seen one offered at a shop, either. However, while visiting the C.V. Dinar coral and clam farm in Indonesia I saw that they had reared quite a few of them, all of which were going to the Japanese aquarium market. For that matter, most all of the best clams they had were going to Japan, too. After living there for two years, I can tell you that they get the best stuff across the board. They pay for it, too…
The C.V. Dinar facility in Indonesia is raising quite a few Hippopus clams. Most of them were H. hippopus , but there were several H. porcelanus , too.
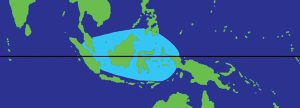
Anyway, like hippopus, porcellanus is typically found on reef flats, often in grassy areas, living on sandy bottoms (Pasaribu 1988 and Delbeek & Sprung 1994). Pasaribu (1988) also reported that porcellanus clams are “generally” not attached to the substrate, and that they also live from the intertidal zone down to only 6m. However, porcellanus has a far smaller natural distribution than hippopus, originally being found only in eastern Indonesia, the southern Philippines, Palau, and Papau New Guinea (Kinch 2002), and later in Malaysia (Raymakers et al . 2003). However, as you might guess, they’ve also been heavily over-fished and are rare to extinct in parts of this range (Wells 1997 and Raymakers et al . 2003).
Also like hippopus, the mantle of porcellanus, without exception, extends only to the upper margin of the shell. The exhalent siphon is also typically flattened and disc-like, forming a low cone with a circular opening, too. However, unlike hippopus, the inhalent siphon always has large, branching tentacles around it. The majority of these clams have a predominantly yellowish-brown or olive-green mantle with white to cream or golden splotches and/or thin stripes. Some may also be more grayish, though. They also commonly have areas of rather translucent tissue with little or no color.
The inhalent siphon of H. porcelanus is always ringed by tentacles, making it easy to distinguish which species of Hippopus it is.
The shell is white when clean, and its appearance is the origin of the common names. “China clam” refers to the look of the shell, not the country it hails from. And, while juveniles have shells that are commonly rather fan-shaped, with the hinge being about 1/2 its length, they do usually become slightly elongated as they get larger. The hinge becomes elongated, sometimes to 1/3 the length of the shell, and the shell also becomes moderately to strongly inflated. Like hippopus, porcellanus is often rather fat.
The shell also typically has 5 to 7 folds, with 5 or 6 being more developed. These are still usually quite low though, and there are no scutes on them, making their shells look similar to that of Tridacna derasa at times. So, despite being closely related species, porcellanus’ shells can look quite different from those of hippopus.
The upper margin of each valve is usually topped by 5 or 6 smoothly rounded, inter-digitating projections, which are symmetrical to those on the other valve. This allows them to close themselves up very tightly when they want to. And, like hippopus, porcellanus typically maintains only a weak byssal attachment when possible, and only when young, living unattached when larger. Accordingly, the byssal opening is usually non-existent or very small for juveniles, being only a short slit at best, which becomes completely closed later. The area around their byssal opening also has numerous small, inter-digitating projections/teeth.
As far as size goes, Rosewater was the first to describe this species (1982), but according to Lucas (1988), he only had small species to examine. Lucas had access to larger specimens and reported a maximum size of 40cm.
Aquarium Care
With the basics covered, now we can get to caring for these species in aquaria, starting with water quality. The requirements for keeping these two clams (and the rest of the tridacnids, too) falls right in with what’s considered “standard” for reef aquariums in general. Temperatures between 25° and 28°C are optimal, as is a pH of 8.1 to 8.3. Alkalinity should optimally be kept in the range of 9 to 12dKH , and calcium should be maintained at 380 to 450ppm, etc. About the only thing in particular to note is that as these clams grow, they add new shell material to the entire inner surface of the shell, not just the top edge. So, even a slow growing clam can use more calcium than you might expect, and having several in an aquarium can deplete calcium and alkalinity surprisingly quickly.
Other than that, having sufficient lighting is really the key to keeping them healthy. Light falling upon the soft mantle tissue and the symbiotic zooxanthellae harbored inside is the primary means by which all tridacnids get their energy in the wild, and the same goes for life in aquariums, too. So, you absolutely must give them sufficient lighting if you expect to keep them alive.
As mentioned above, both of these clams are not found at depths greater than 6m (about 20 feet). That’s quite shallow compared to their Tridacna cousins, with the exception of Tridacna crocea . Crocea also lives from the intertidal zone to about 6m, but the rest of the species can be found significantly further down. For example, the popular T. maxima has been found at depths of 15m, and T. derasa at 25m. Obviously quite a difference.
What this means to hobbyists is that these two clams will need even more light than most other tridacnids in order to thrive. In my experience and based on information taken from the literature, which is covered in detail in Fatherree (2006), they can get by with less light than T. crocea , but their growth rate will be greatly reduced. Thus, even high-output fluorescent lighting will only suffice in relatively shallow tanks, or if a specimen is placed on the rockwork near the water’s surface in a deeper tank. I would highly recommend squeezing as many bulbs into a canopy/fixture as possible at that, mounting the bulbs so that they are as close to the water as possible (without causing heat problems).
It’s also important to note that there can be a great deal of variability between individuals of any given species of tridacnid. There are genetic differences that can make one clam more fit than another under the same conditions, two individuals may be carrying different strains of zooxanthellae, and so on. So, some specimens may be able to get by at times with less light than others, or further down in deeper tanks, but I implore you to not take chances.
With that said, experience has shown that metal halide lighting is really the way to go, preferably a combination system comprised of metal halide and fluorescent lighting. A standard 175w white to blue-white metal halide bulb should be sufficient for keeping these on the bottom of any small to medium size tanks, as in anything less than 45cm deep (or no deeper than this in a larger tank). But, I’d go ahead and move up to 250w, or even 400w bulbs if a specimen will be any further away from the surface, though.
Next, we get to whether or not you need to feed these in an aquarium. All tridacnids are filter-feeders that ingest a variety of particulates stripped from surrounding waters. However, their zooxanthellae can cover a great deal of their nutritional needs, and they’re also able to absorb nutrients directly from seawater. In fact, if provided with enough light, tridacnids of any size can completely forgo the need to filter feed and can thrive in particulate-free water as long as there are enough dissolved nutrients present. I assure you that any claims to the contrary are false.
I dedicated an entire chapter to tridacnid nutrition in Fatherree (2006), which covers how they “work” in great detail, but I’ll keep it simple here by saying that, in aquariums, basically everything is taken care of by having good lighting and simply feeding the fishes. Some fish food is left uneaten and becomes detritus, which tridacnids can filter out, and it also releases other nutrients into the water as it decomposes. But, most food is eaten by the fishes, which produce solid wastes that can also become detritus, and also give off dissolved nutrients that can be absorbed by a tridacnid, too. So, when you feed the fishes, you’re feeding the clams, as well. For what it’s worth, going all the way back to the early 90’s, I’ve kept many tridacnids in well-stocked aquaria topped with metal halide lighting without providing any sort of particulate food whatsoever.
Still, the real question is about stocking levels, i.e. whether or not there are enough fishes in your aquarium/enough fish food going into you aquarium to support one or more clams. It is indeed possible to have too low a fish load (or too high a clam load depending on how you look at it) in a tank, which would mean that the amount of fish waste being produced would not be enough to support the needs of the clam(s). So, my advice is to refrain from taking any chances and use a quality phytoplankton product if you have any doubts. Basically, assuming water quality and lighting are sufficient, you should see shell growth if things are okay, while a lack of growth means they aren’t.
You also need to think about water flow, as these two species live in shallow waters on reefs and near-reef environments that are regularly exposed to strong currents and wave activity. To the contrary, water movement in aquariums is typically nothing like that on the reef or nearby, as the flow in most aquariums tends to be quite linear and constant. While it’s best to expose them to a low velocity surge and/or to turbulent flow, putting them in a position where a pump just blasts them with a strong, non-stop linear current is not recommended. Basically, any sort of current that causes a specimen to chronically retract its mantle and keep the shell even partially shut won’t do. Thus, you can put one anywhere you like with respect to current, as long as it stays open fully.
Next, we get to placing these in the right spot. I think it’s best to place any specimen of any sort on the same kind of substrate that you’d find it living on in its natural habitat, but this should be pretty easy since both of these can be found on sand, gravel, and coral rubble in the wild. So, these can be placed essentially anywhere in an aquarium, as long as lighting and current are acceptable.
Okay, with these things that you should do covered, now I’ll run through some of the things that you should not do when it comes to the placement of a specimen. As is the case with any species of tridacnid, never place one in a tight crevice between rocks and such, as this may restrict their ability to open fully, and also increases the risk of them falling down into the rockwork if they move around too much. Never stick one in a hole in a rock that might restrict their ability to open fully, either. And, if you do place one in a large enough hole, never let a lot of detritus settle into the hole around the clam. If detritus does accumulate around one, you should blow it out regularly using a turkey baster or powerhead, etc.
Also, if a small specimen has attached itself to a sizeable piece of rock, shell, etc., never try to move the clam and the piece it’s attached to by grabbing only the clam. That’s a good way to injure the organ it uses to attach to such things, and you should always pick up the clam and the piece together and be very careful when handling the two. Likewise, you should never try to pull one off anything that it’s attached to as you can rip the thread-producing byssal organ right out of it. And lastly, don’t move a specimen repeatedly over a short period of time. It can be stressful enough trying to adapt to changes in lighting and current when they’re moved into an aquarium, and quickly moving them from one place to another to another can be too much at times. Such activities can lead to slowed growth, a greater susceptibility to disease due to stress, or even outright kill them. If you must move one more than once, be sure to give it plenty of time between each move, as in a week
or two at the least.
Well, that’s all I’ve got. While you might never see a porcellanus for sale, there’s always the chance you will, and hippopus is easy enough to acquire if you want one. So, keep all of this information in mind and do what it takes to keep them alive and well should you make a purchase.
References
- Barnes, R. 1984. Morphogenesis of a nearshore windward fringing reef, Orpheus Island . Unpublished Honours Thesis, Department of Geography, James Cook University.
- Fossa, S.A. and A.J. Nilsen. 2002. The Modern Coral Reef Aquarium, Volume 4 . Birgit Schmettkamp Velag, Bornheim, Germany. 480pp.
- Govan, H. P.V. Nichols, and H. Tafea. 1988. Giant clam resource investigations in Solomon Islands. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific . ACIAR Monograph Number 9, Canberra. 274pp.
- Delbeek, J.C. and J. Sprung. 1994. The Reef Aquarium: Volume One . Ricordea Publishing, Coconut Grove, FL. 544pp.
- Fatherree, J.W. 2006. Giant Clams in the Sea and the Aquarium . Liquid Medium. Tampa, FL. 227pp.
- Kinch, J. 2002. Giant clams: their status and trade in Milne Bay Province, Papau New Guinea. TRAFFIC Bulletin 19(2):1-9.
- Knop, D. 1996 . Giant Clams: A Comprehensive Guide to the Identification and Care of Tridacnid Clams . Dahne Verlag, Ettlingen, Germany. 255pp.
- Lucas, J.S. 1988. Giant clams: description, distribution, and life history. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific . ACIAR Monograph Number 9, Canberra. 274pp.
- Pasaribu, B.P. 1988. Status of giant clams in Indonesia. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific . ACIAR Monograph Number 9, Canberra. 274pp.
- Raymakers, C., S. Ringuet, N. Phoon, and G. Sant. 2003. Review of the exploitation of Tridacnidae in the South Pacific, Indonesia and Vietnam. TRAFFIC Bulletin, Brussels, Belgium.
- Rosewater, J. 1965. The family Tridacnidae in the Indo-Pacific. Indo-Pacific Mollusca 1:347-396.
- Rosewater, J. 1982. A new species of Hippopus (Bivalvia: Tridacnidae). Nautilus 96:3-6.
- Shelley, C.C. 1988. Growth rates of Hippopus hippopus from Orpheus Island, Great Barrier Reef. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific . ACIAR Monograph Number 9, Canberra. 274pp.
- Wells, S. 1997. Giant Clams: Status, Trade and Mariculture, and the Role of CITES in Management. IUCN – The World Conservation Union. Gland, Switzerland and Cambridge, UK. 77pp.










0 Comments