Editor’s Note: This article originally appeared in the French website Recifs.org.
Attention people! Please get closer! Are you dreaming of an aquarium full of red, green and blue corals? Are you dreaming of Acropora that become even more colorful at home than when you drooled in front of them when they were still in the holding tanks of your favorite local fish store? If yes, please read on…
Still with us? So, you must still be believing in Santa Claus? Please, don’t leave yet. We may not give you a miracle recipe that will keep your corals from turning brown, but we will review today’s knowledge about the coloration of the guests in our tanks. You won’t believe how much it has progressed in the last three years, even if only a few of us read about it. So, if the words pocilloporin, GFP and chromophor do not mean a lot to you yet, please take the time to read on, we will try to help you discover a research domain that is in full expansion these days. Do not be scared, we will not forget about the three questions that are of importance to all of us:
- Why do our corals, once in our aquariums, sometimes lose their original colors?
- From where are our corals getting such varied and vibrant colors?
- What use are they getting out of those colors?
This first article will review a few great principles. Color is a subjective feeling resulting from the interaction between light and our sensory system. In order to understand color, it is necessary to understand light’s physical nature and the properties of our visual system. This initial article will only be a review of all this. Nevertheless, we need to motivate you and we will attempt to give an answer to the first of the previous three questions. For the readers still wondering, the others questions will be answered in their respective articles. The order we used for those questions was absolutely not random! It is just echoing the order in which scientific discoveries were made.
Enough about the future, let’s start by the beginning. Yes, brown is a color… Even if golden brown is a color, it is easy to say that it is not one that we like to see in our aquariums, especially when a specimen was originally acquired because of its “one of a kind blue”. Before attempting to understand the origin of this metamorphose, let’s review a few notions of physics. Those familiar with wavelengths, who understand why a car is red, and how we can make it look black without a drop of paint, please skip directly to the next paragraph!
There are no colors without light. But what is light? There are many ways of describing light, and we will take this opportunity to go through those that will be useful to you in your aquarist’s life.
Light is an electromagnetic wave and can be described by the distance separating two oscillations (its wavelength; we will use the nanometer as a unit, or 10-9m) and its number of oscillations per second (its frequency; we will use the Hertz as a unit, Hz).
Our eyes react as electromagnetic waves sensors and are able to detect wavelengths between about 400 and 750 nanometers. The total amount of wavelengths between those two limits makes the spectrum of visible light. Below 400 nm are the ultra-violets; above are the infra-reds.

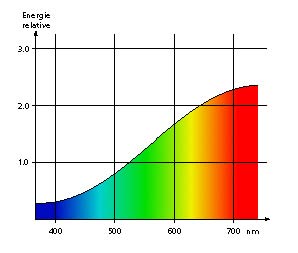
The eye’s sensitive surface – the retina – is in fact made of multiple types of sensors (photoreceptors) capable of different reactions and transmitting data to the brain depending on the wavelength that hits them. It is because of this ability that we can see in color. The following diagram illustrates the link between wavelengths and the perception that we have of them. Our retina is made of three types of photoreceptors, reacting to blue, green and red. A fish’s retina can be made of up to six types, each reacting to ultra-violets, purple, blue, green, yellow and red. They must be enjoying the colors of the coral reefs much better than we do!
White light, in which we live everyday, is just the perception that we have of the superposition of all the wavelengths inside the visible spectrum. But if all that is around us is not plain white, it means that all surfaces do not react the same way when they are lit. When all the wavelengths are evenly absorbed by a material, its color appears to be gray. The higher the absorption goes, the darker the gray becomes, until it appears black when all light is trapped. What about color? The car passing by in the street appears red because, when white light arrives on the body; all wavelengths are absorbed by the paint, except the reds, that are reflected towards our eyes.
This type of pigment can only reflect what is thrown at it. This will give us the answer to our question: how do we transform a red car in a black car? Simply by lighting it with a blue or yellow monochromatic source, as it happens in a tunnel lit by sodium bulbs. This light source contains no, or very little, red wavelengths. This way very little light is reflected towards our eyes and excites the photoreceptors: the car appears black.
Things are, of course, never that simple, the colors that we see are nearly never monochromatic. Even the primary colors (blue, red and green) can me made of a mix of different wavelengths. If we see two seemingly identical colors, it doesn’t mean that they match the same wavelengths. Simply, the green color of an object could be caused either by the reflection of a green wavelength, or by the reflection of different mixes of blue and yellow (as is the case for plants, for example).
So far so good? We still need your attention for a little bit, this will allow you to better understand what we observe in our aquariums. So far, we have seen that the colors we perceive correspond to the substraction of a part of the wavelength making up the visible spectrum and to the interaction of the resulting spectrum – the non-absorbed wavelengths – with our visual system. But some bodies can also use the energy they receive in order to make light. If light is emitted without any heating, it is called luminescence, as opposed to incandescence, the light produced by heating. Light does not only possess the properties of a wavelength, it can also be considered as an energy flux. This energy is carried by particles without any mass, the photons. There is a simple relation between those energy particles and wavelength because the energy of a photon gets greater as the wavelength gets shorter.
Many corals are characterized as fluorescent, which is a possible form of luminescence. Their pigments, using the energy brought by specific light wavelengths, produce their own light in a wavelength that is longer than the original one (and naturally less energetic). You are observing this phenomenon when looking at your aquarium under actinic lighting. This truly is fluorescence and not phosphorescence, as these light emissions stop as soon as you stop lighting the animals. In opposition to what we have seen previously, these animals’ colors are not a consequence of the reflection of a subset of the wavelengths emitted by a light source, but are a consequence of a light emission by the corals’ pigments.
In certain cases, the animal doesn’t even need an external energy source. Some animals are indeed able to use their own energy sources in order to produce light. This is called bioluminescence. This is the case of the well-known glow-worm, but also of many living marine organisms, like the Aequorea victoria jelly fish. Remember this name, because, quite curiously, it is because of this animal that scientific knowledge could progress that much. We will not say much more right now, as this subject will be at the heart of the coming articles on coral coloration.
We cannot end this overview about light without talking about the different means available that help compare two light sources.
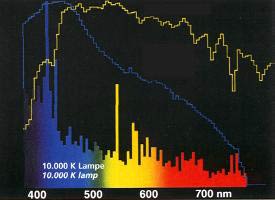
You most probably already know the concept of color temperature through your acquisition of metal halide bulbs. Why use color temperature instead of wavelengths? This simply allows us to compare spectrums. We already previously mentioned that most of the light surrounding us is not monochromatic but is composed of a set of wavelengths. When we are talking about a light source, it should be necessary to use a chart describing the varied wavelengths making up this light source:
You have to admit that this is not very practical. Luckily for us, physics can help us, as it has been proved that when a “black body” is emitting light by incandescence, its spectrum only depends on its temperature. In other word, this means that when we know the temperature, we also know the light spectrum. We could assume that two 10,000 K (in Kelvin, K = °C +273) light sources should have the same wavelengths spectrums. The problem is that a black body is defined as an opaque body, totally isolated, and that light bulbs are very far from being defined as such.
In the case of a black body like our sun, the spectrum is continuous, when it is not for light bulbs. This is due to the fact that the bulb’s content is made of different types of metal halides, each emitting its own spectrum.
The color temperature of a light bulb, or more precisely its extrapolated value, gives us more information about its dominating wavelength than about its real spectrum. The table below can give you a few educated clues. You will note that heated at 5,500 K, a black body emits about the same energy in all wavelengths, and that is light spectrum is usually called “daylight”. Below this value, light tends to become yellow, above it becomes blue – even if our eyes do not necessarily sense this.
Here are some values:
- Candle light: 600 K
- 75 W common light bulb: 2850 K
- 150 W common light bulb: 3,000 K
- Halogen light bulb: 3,400 K
- Daylight: 5,500/6,500 K
- Cloudless sky: from 10,000 to 20,000 K
Not only is it important to know how to judge light’s quality, in fact its spectrum, it is also important to judge:
- the quantity of light generated by a source (in all directions),
- the quantity of light reaching the target surface
Because things are never really simple when light is concerned, there are many units used that relate to the different light properties related here.
The Watt and Watt/m2 refer to the amount of energy consumed by the source and the amount of energy emitted by a given surface.
The lumen and the lux (1 lux = 1 lumen/m2) originated from industrial standards and are linked to a reference light source (540×1012 Hertz and 1/683 Watt). Our eyes are particularly sensitive to this wavelength (yellow-green). Lumen and lux are then better used for the qualification of commercial light sources targeting our visual comfort, and are less useful when studying biological phenomenons like photosynthesis (read below).
The micro-Einstein (µE) quantifies the amount of photons emitted or received by a body. One Einstein is equivalent to one mole of photons (6.023×1023 photons). This unit doesn’t take into consideration the energy carried by the photons (this energy depends on the wavelength). Why should we be interested by this unit? There are two answers.
The first answer will help us to slightly deflate the ego of the person who proudly bought a 14,000 K metal halide bulb: “Yeah… Right… You know… What matters to corals is the PAR. Your bulb, how many micro-Einsteins does it have?” If your friend has a clue about the PAR, then start asking him about the PUR (more on that later).
Seriously, the right answer is that the Einstein is a good indicator of the photosynthetic activity of plants. The biological mechanisms in place during the luminous phase of photosynthesis do not depend on the photons’ energy, but on their number. This is exactly what the Einstein displays. The PAR (Photosynthetic Available Radiation, unit µE/m2/s) measures the number of photons reaching a surface, all this in the wavelengths of the visible light (between 400 and 700nm). It is indeed in this portion of the spectrum that we can find the different absorption peaks of the photosynthetic pigments. As these pigments do not absorb energy in a equal manner on all that 400-700nm range, but only at certain precise wavelengths, some prefer using the PUR (Photosynthetic Usable Radiation) in order to quantify the number of photons truly used by the photosynthetic cells. The PUR is thus defined by the light source (emitted spectrum, intensity) and by the studied pigments (because of their absorption spectrum). This one is probably better left to specialists…
Now that we have reviewed the major physics and biology principles that explain why we can see our corals as colorful animals, let’s move on to a more practical question. Why do our corals change coloration once established in our aquariums?
It would be good to immediately discard the changes due solely on visual aspects. We have learned previously that the spectrum of a light source has a direct impact on color rendition (red car/black car). This means that changing a 10,000K bulb for a 20,000K bulb will result immediately in the change of the animals’ perceived color. Fluorescence will be stimulated, and the yellow, red and brown dominant colors will be “erased”. These are not the changes that we will discuss now, we will study the changes that happen in the mid to long term, using an identical light source.
You all know that nearly all the corals we maintain in our aquariums are hermatypic and that their tissues host symbionts, the zooxanthellae. These symbionts are unicellular algae (phototrophic dinoflagellates) and, as such, are able to transform luminous energy into chemical energy in order to manufacture sugars from inorganic CO2. A part of the products synthesized by the zooxanthellae is transferred to coral cells where they are used as fuel and a carbon source for the coral’s own needs.
This particularity is advantageous for us as it helps us maintain animals in our aquarium without the need to feed them much. On the other hand, it has the inconvenient of turning our corals into brown animals in certain maintenance conditions. There are multiple species of zooxanthellae – in fact tens of species and sub-species – but they all are golden-brown, a consequence of the privileged absorption of the blue/green wavelengths by the photosynthetic apparatus – brown is in fact a very dark red! The visible color of a coral will be the result of the mix between the colors of its pigments, the dominating brown of the zooxanthellae, and the respective densities of these two components. The whole will depend on the two organisms and the subtle laws governing the coral/zooxanthellae symbiosis. It has been shown recently that a third organism – a cyanobacteria in fact – was a participant of the symbiosis in Monstastrea cavernosa and has an impact on the final color. Today, we do not know whether this type of association is very common.
The coral polyps have two metabolic paths available for their growth:
- They can use the zooxanthellae, therefore, indirectly, light and CO2. Under those conditions the coral nearly turns into a phototrophic organism
- They can use their own cellular machinery, therefore, like any other animal, it is imperative that they find an external energy source and feed on it.
In their natural habitat, these two possibilities are believed to be simultaneously employed in variable proportions based on lighting conditions, amount of organic matter in the water column and genetic particularities of each species. For most of the animals that we host, it is the zooxanthellae path that is mainly fulfilling the function.
Without any drastic change in the natural conditions, a balance is reached between the symbiotic algae and their host; the coral’s color is the result of this balance. If corals are not all brown, it means that the natural conditions allow them to maintain sufficiently low amounts of zooxanthellae in order not to mask the color of their pigments. To say the least, for a coral, a move from a reef to an aquarist’s aquarium is quite a change. Turning brown would be the result of a newly established balance through the increase of the zooxanthellae’s numbers, and therefore by the masking of the coral’s pigments. Please note that this newly established balance is achieving its goals pretty well, as you may have noticed that corals that turned brown in your tank do not have any particular growth issues. We now need to understand the mechanisms leading to this increase of the zooxanthellae/pigment ratio. There are simple answers to this question, but, unfortunately, they are not completely satisfactory. The most obvious answer concerns the influence of the lighting. Light, through zooxanthellae and photosynthesis, is the main source of building materials for the coral. If the zooxanthellae become less efficient because of any lack of available photons, an increase of their numbers makes sure that the coral will maintain the initial amount of building material. Some authors report that great modifications of the lighting conditions result in an increase of the number of the zooxanthellae’s photosynthetic pigments in a few hours, and more generally in an increase of their metabolic activity. Turning brown is a relatively slow phenomenon, which is more in line with the observed delays when the number of zooxanthellae is significantly increasing (it takes a few days). What we are apparently witnessing in our aquarium is the following chain of events:
- insufficient lighting (insufficient PAR)
- decrease of the photosynthetic activity
- decrease of the coral’s energy resources
- increase of the number of zooxanthellae and masking of the coral’s pigments
- the coral recovers its energy balance
Attractive isn’t it? There are nevertheless a few questions hanging, leading us towards more complex events. The starting point of this chain of events is based on the hypothesis that our aquariums are insufficiently lit in order to maintain nominal levels of photosynthesis. If you remember the quick review course we went through in the beginning, you know that the PAR gives us a good knowledge about the energy available for photosynthesis. It has been measured that the PAR of 6,500 K bulbs is much higher than the PAR of 10,000K or 20,000K bulbs, but from the strict point of view of color preservation, the latter provide much better results. This could mean that less photosynthesis results in more colors. We will see in a future article that these observations are not necessarily incompatible, but are at least pleading for the existence of more complex mechanisms. In the same fashion, you may have observed during your aquarist’s life that two fragments of the same mother colony, each placed in two different aquariums, but with the same lighting setup, will not always display the same colors; one could turn brown while the other is keeping its original color.
Another interesting explanation is provided by the supporters of the great role of food in the energy balance of hermatypic corals. Observing that hermatypic corals can strive in our aquarium without targeted feeding does not prove that food doesn’t play any role in the natural habitat. Even if it has been proved that zooxanthellae are able to provide more than 150% of corals’ energy needs for Acropora and Stylophora, it has also been reported that the same species are great plankton predators. It is therefore possible that a good amount of the energy resources is provided by food, with the zooxanthellae being only an additional source. When the coral is placed within an aquarium, the chain of events could very well be the following:
- removal of the energy source provided by food
- increase of the zooxanthellae metabolic activity
- increase of the number of photosynthetic pigments within the zooxanthellae
- increase of the number of zooxanthellae
- recovery of the coral’s energy balance with resources provided only by photosynthesis, and a depressed owner of a brown Acropora
Once again, this hypothesis is quite attractive, but it is not bullet-proof. If, generally, our aquariums are not ready to provide all the preys that small-polyp corals (SPS) can eat, they are not void of any dissolved nutrients. It has been proved that corals are able to directly absorb dissolved nutrients (Dissolved Organic Matter, DOM, phosphates, nitrates) through their cell membranes. A great resource of available food is therefore available to the corals in our aquariums.
Greater than normal concentrations of dissolved nutrients could very well be an explanation for the brown coloration of our corals. These nutrients are indeed excellent “fertilizers” and could help the multiplication of zooxanthellae and therefore induce the masking of the coral pigments. In that case, we are stuck, and there are only a few options:
- do not feed the corals, and take the risk that they will turn brown because of the decrease of their energy resources
- feed the corals, and take the risk that they will turn brown because of a DOM increase
Yes… We told you before… There is no miracle recipe warrantying the maintenance of SPS corals with colors identical to the original ones. Well, with a good water quality and an optimized lighting system, you will make sure that everything should go your way. Well… What is an optimized lighting system? If, with 250 W, your corals turn brown, you will get 400 W. What about the next time you see brown? Will you get (bank account and significant other permitting) two times 400 W? Why not? But are you sure that this is all really in the best interest of your corals? What use could they have of all this luminous energy, if, after all, after turning brown, the corals in our aquariums stay quite healthy?
If thing are pretty clear about the zooxanthellae’s pigments – they are the photosynthesis’ vectors – what are the corals’ pigments used for? Are they coming from the food or synthesized by the polyps? What are those pigments?
This is a promise; we will partly give some answers to those questions in a follow-up article: Colors by the Thousands – The Actors of Color
References
- http://www.cea.fr/fr/pedagogie/Ondes/index.htm
- http://physique.paris.iufm.fr/lumiere/
- http://isitv.univ-tln.fr/~lecalve/oceano/fiches/fiche3E.htm
- http://semsci.u-strasbg.fr/rouedes.htm
- http://www.sylvania.com/forum/pdfs/faq0017-0800.pdf
- http://www.chez.com/divirion/web_cou/cou_01.htm
- http://www.animalnetwork.com/fish2/aqfm/1998/june/features/2/default.asp
- http://www.animalnetwork.com/fish2/aqfm/1998/nov/features/1/default.asp
- http://reefkeeping.com/issues/2002-03/atj/index.htm
- http://www.athiel.com/aquatic/riddle3.htm
- http://quasimodo.versailles.inra.fr/inapg/cours/uvneurones/2000/conf07.htm
- http://www.ghc.be/~couleurs/corps_noir.htm
- http://scio.free.fr/ondes/corpsnoir.php3





0 Comments