In Part 1 of this series, we began our review of fluorescent pigments and also discussed the concepts of fluorescence excitation and emission, Stokes shift, photoconversion and so on. A review of that article is recommended if you are unfamiliar with any of these terms. This time, our discussion continues with green, yellow-green and yellow fluorescent pigments and a few hypotheses concerning their function. We’ll also see a couple of cases where light energy of specific bandwidths has induced coloration changes.

This Hawaiian soft coral (Sinularia densa) demonstrates green fluorescence. The reddish coloration is that of zooxanthellae or algal fluorescence.
Green fluorescence is perhaps the most common of any seen in aquaria. Well over half of the 70 or so known fluorescent pigments spanning 9 color categories can be described as ‘greenish.’ Fluorescence of green fluorescent proteins (GFPs) can also combine with other ‘glowing’ pigments to form any number of colors.
Table 4 lists the fluorescent proteins by emission maxima as described in various works:
| Pigment | Emission | 2 | 3 | Excitation | 2 | Found in: | Reference |
|---|---|---|---|---|---|---|---|
| P-510 | 510 | 479 | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-510 | 510 | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-510 | 510 | * | * | 498 | * | Renilla muelleri (sea pansy) | Labas et al., 2002 |
| P-510 | 510 | * | * | 490 | 420 | Heteractis magnifica | Tu et al., 2003 |
| P-510 | 510 | * | * | 440 | * | Montastraea cavernosa | Mazel et al., 2003 |
| P-510-520 | 510-520 | * | * | 440 | * | Montastraea faveolata | Lesser et al., 2000 |
| P-510-520 | 510-520 | * | * | 440 | * | Montastraea cavernosa | Lesser et al., 2000 |
| P-510-623 | 510-623 | * | * | * | * | Montastraea cavernosa @ 40m | Vermeij et al., 2002 |
| P-511 | 511 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-512 | 512 | * | * | 503 | * | Discosoma sp. 3 | Labas et al., 2002 |
| P-512 | 512 | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-512 | 512 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-513 | 513 | 545 | 490 | * | * | Agaricia sp. | Mazel, 1995 |
| P-513 | 513 | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-514 | 514 | 490 | * | 501 | 480 | Acropora aspera | Papina et al., 2002 |
| P-514 | 514 | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-515 | 515 | * | * | * | * | Mycetophyllia lamarckiana | Mazel, 1997 |
| P-515 | 515 | * | * | ~494 | * | Mycetophyllia sp. | Fux and Mazel, unpublished |
| P-515 | 515 | * | * | 505 | * | Montastraea annularis | Mazel, 1997; Manica & Carter, 2000 |
| P-515 | 515±3 | * | * | 505±3 | * | Montastraea cavernosa (mc2/3/4) | Kelmanson & Matz, 2003 |
| P-515 | 515 | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-515 | 515 | * | * | * | * | Colpophyllia natans | Fux and Mazel, unpublished |
| P-515 | 515 | * | * | * | * | Scolymia sp. | Mazel, 1997 |
| P-515 | 515 | * | * | * | * | Plesiastrea verispora (green morph) | Gilmore et al., 2003 |
| P-515 | 515 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-515 | 515 | * | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-515 | 515 | * | * | * | * | Ricordea sp. | Mazel, 1995 |
| P-516 | 516 | * | * | 486 | * | Pocillopora damicornis | Salih et al., 2000 |
| P-516 | 516 | * | * | 506 | ~480 | Montastraea cavernosa (=mc2/3/4 – see P-515) | Labas et al., 2002 |
| P-516 | 516 | * | * | * | * | Lobophyllia hemprichii | Nienhaus et al., 2005 |
| P-517 | 517 | 555 | 485 | 505 | 465 | Acropora tenuis | Papina et al., 2002 |
| P-517 | 517 | 574 | * | 506 | 566 | Ricordea florida | Labas et al., 2002 |
| P-517 | 517 | * | * | 506 | * | Ricordea florida | Mazel, 1995 |
| P-517 | 517 | * | * | 507 | * | Favia favus | Tsutsui et al., 2005 |
| P-517 | 517 | 551 | 483 | * | * | Montastraea annularis | Mazel, 1995 |
| P-518 | 518 | * | * | 508 | 475 | Ricordea florida | Labas et al., 2002 |
| P-518 | 518 | * | * | * | * | Acropora cytheria @ Waikiki Aquarium | Hochberg et al., 2004 |
| P-518 | 518 | * | * | * | * | Acropora digitifera | Hochberg et al., 2004 |
| P-518 | 518 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-518 | 518 | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-518 | 518 | * | * | 503 | * | Family Pectiniidae | Ando et al., 2004 |
| P-518 | 518 | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-519 | 519 | * | * | ~505 | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-519 | 519 | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-519-557 | 519-557 | 590 | * | * | * | Madracis carmabi | Vermeij et al., 2002 |
| P-519-559 | 519-559 | 590 | * | * | * | Madracis senaria | Vermeij et al., 2002 |
| P-519-570 | 519-570 | 590-615 | * | * | * | Madracis pharensis (green tissue) | Vermeij et al., 2002 |
| P-520 | 520 | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-520 | 520 | * | * | 488 | * | Goniopora tenuidens | Salih et al., 1999 |
| P-520-555 | 520-555 | 590 | * | * | * | Madracis carmabi | Vermeij et al., 2002 |
| P-520-558 | 520-558 | 590 | * | * | * | Madracis senaria | Vermeij et al., 2002 |
| 520-570 | 520-570 | 590 | * | * | * | Madracis pharensis (green tissue @ 10m) | Vermeij et al., 2002 |
| 520-570 | 520-570 | 590 | * | * | * | Madracis pharensis (green tissue @ 20m) | Vermeij et al., 2002 |
| 520-570 | 520-570 | 590-620 | * | * | * | Madracis pharensis (green tissue @ 40m) | Vermeij et al., 2002 |
| 520-570 | 520-570 | 590 | * | * | * | Madracis pharensis (green tissue @ 60m) | Vermeij et al., 2002 |
| P-522 | 522 | * | * | 499 | ** | Anemonia sculata var. rufescens | Wiedenmann et al., 2000 |
| P-522 | 522 | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-522-623 | 522-623 | * | * | * | * | Madracis formosa @ 40m | Vermeij et al., 2002 |
| P-530 | 530 | * | * | 450 | ** | Porites astreoides | Mazel, 2003 |
| P-532 | 532 | 590 | * | * | * | Madracis pharensis (brown tissue @ 10m) | Vermeij et al., 2002 |
| P-532 | 532 | * | * | * | * | Madracis pharensis (red tissue @ 10m) | Vermeij et al., 2002 |
| P-533 | 533 | 590 | * | * | * | Madracis pharensis (brown tissue @ 20m) | Vermeij et al., 2002 |
| P-534 | 534 | 575 | * | * | * | Madracis formosa @ 60m | Vermeij et al., 2002 |
| P-534 | 534 | 593 | * | * | * | Montastraea cavernosa @ 60m | Vermeij et al., 2002 |
| P-535 | 535 | 587 | * | * | * | Madracis pharensis (brown tissue @ 60m) | Vermeij et al., 2002 |
| P-537 | 537 | 590 | * | * | * | Madracis pharensis (grey and blue tissue @ 10m) | Vermeij et al., 2002 |
| P-538 | 538 | ~580 | * | 528 | 494 | Zoanthus 2 | Matz et al., 1999 |
| P-538 | 538 | * | * | 525 | 494 | Zoanthus sp. | Yanushevich et al., 2002 |
| P-540 | 540 | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-540 | 540 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-542 | 542 | * | * | * | * | Agaricia undata @ 40m | Vermeij et al., 2002 |
| P-550 | 550 | * | * | 530 | * | Porites murrayensis | Dove et al., 2001 |
| P-557 | 557 | 600 | 545 | * | * | Agaricia sp. | Mazel, 1995 |
| P-559 | 559 | 590 | * | * | * | Madracis senaria @ 60m | Vermeij et al., 2002 |
| P-560 | 560 | 590 | * | * | * | Madracis pharensis @ 10m (grey tentacles) | Vermeij et al., 2002 |
| P-561 | 561 | * | * | 548 | * | Fungia concinna | Karasawa et al., 2004 |
| P-561 | 561 | 587-616 | * | * | * | Madracis senaria @ 40m | Vermeij et al., 2002 |
| CP-562 | * | * | * | 562 | * | Anemonia sulcata, immature P-595 | Wiedenmann et al., 2002 |
| P-565 | 565 | * | * | 490 | * | Agaricia humilis | Mazel et al., 2003 |
| P-565 | 565 | * | * | 548 | * | Cerianthus sp. | Ip et al., 2004 |
| P-573 | 573 | 510 | * | * | * | Ricordea florida | Mazel, 1995 |
| P-574 | 574 | 517 | * | 506 | 566 | Ricordea florida | Labas et al., 2002 |
| P-574 | 574 | 550 | * | * | * | Plesiastrea verispora | Dove et al., 2001 |
| P-575 | 575 | ~630 | * | ~525 | ~570 | Montastraea cavernosa | Mazel, 1997 |
| P-575 | 575 | * | * | 506 | 555 | Montipora (digitata/angulata) | Mazel, unpublished |
| P-575 | 575 | * | * | 557 | * | Dendronephthya | Pakhomov et al., 2004 |
| P-575 | 575 | 630 | * | 520 | * | Scolymia sp. | Mazel et al., 2003 |
| P-575 | 575 | * | * | ~569 | ~540 | Phycoerythrin within symbiotic cyanobacteria in coral | Mazel et al., 2004 |
Comments about Green Fluorescent Proteins
It seems quite clear that GFPs emissions are quite varied and abundant in coral reef animals. At least some GFPs require the presence of oxygen for maturation – the transition from a colorless state to one of fluorescence with the emission peaking in the green portion of the spectrum, yet once mature, oxygen apparently has no further effect (Tsien, 1998). However, modification of environment can cause further color-shifting, resulting in various apparent colorations (Labas, 2002).
Fluorescent Coloration is Not Produced by Zooxanthellae! This is one of the persistent myths within the hobby yet there is no data to support this view. To the contrary, the evidence does support fluorescent pigment production by the host. Kawaguti (1966) shows several electron photomicrographs of pigment granules apparently being produced by a coral cell’s endoplasmic reticulum. With that said, zooxanthellae pigmentation (photopigments including chlorophyll a, c², peridinin and perhaps others) can greatly influence the non-fluorescent coloration ranging from brown to yellow-green.
Possible Functions of GFP-like Compounds
Opinions on possible function (or non-function) of fluorescent proteins are almost as numerous as the number of papers addressing the subject. Here are a few:
Photoprotection? Perhaps the most popular concept is one where pigments act as sunscreens – photoprotectants – against ultraviolet or visible radiation. Fairly early on in the course of ‘serious’ coral reef studies, Kawaguti (1944) was of the opinion that fluorescent pigments in corals act to shield symbiotic zooxanthellae from ‘strong sunlight.’ His idea became mantra for decades and sporadic re-visitations seemed to confirm his thoughts (see Salih et al., 1998; Salih et al., 2000 and others).
Fluorescent pigments can cause a reflection of light, which can be 40-80% higher than that of adjacent non-fluorescent cells (Salih et al., 1998), leading to the conclusion that polyp retraction can produce a highly reflective layer of varying optical density thereby protecting gonads and high-growth areas (polyp tips, ‘anchoring’ basal tissues).
However, Mazel et al. (2003) found no photoprotective quality associated with green fluorescent proteins (with emission spectra peaking at 500nm to 518nm bandwidth).
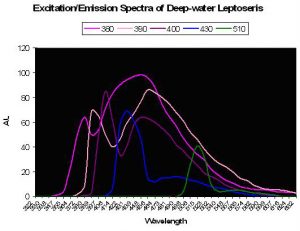
Figure 36. The colored lines represent excitation and emission – the excitation being the initial peak. Fluorescence is noted at excitation wavelengths of 380, 390 and 400 nm. Little fluorescence is noted when the pigment(s) is exposed to bandwidths peaking at 430 and 510nm. After Schlichter et al., 1986.
A Photosynthetic Aid? Schlichter et al. (1985) were perhaps the first to describe fluorescence as a potential aid to photosynthesis. These researchers documented behavior of fluorescent granules and dispersed pigmentation in the deep-water coral Leptoseris fragilis (found at depths of 100-145m, or ~325-475’ at Eilat, Israel on the Red Sea). Normally, this coral appears dark brown since its chromatophores (pigment-containing granules) are beneath a layer of symbiotic zooxanthellae. However, the color changes from to green in less than 1 minute after coral is transferred to daylight conditions. It is thought that host tissue contractions could force zooxanthellae downwards, or move chromatophores upwards through displacement, possibly via a vascular microtubule system within the coral’s tissue. Schlichter states that violet light is absorbed by the host pigment and is fluoresced as bluish-green light that is usable in photosynthesis. These deep-water corals receive only up to 10 µmol·m²·s PAR at noon and would need to efficiently collect light – much radiation making it through a densely packed zooxanthellae layer above the fluorescent pigment could be absorbed and fluoresced in an altered wavelength. Though the paper does not state as much, it could be that fluorescence shifts absorbed wavelengths to a bandwidth normally rarified at this depth thus avoiding photosaturation at those wavelengths usually transmitted through the water column. See Figure 36.
In a later paper, Schlichter et al. (1986) further discuss that pigment granules (chromatophores) fluoresce reddish while the intense turquoise autofluorescence seems dispersed throughout the cytoplasm, and is not in any specialized structure. Interestingly, we could speculate that the red fluorescence is also of benefit to photosynthesis.
Phototaxis: A Beacon for Zooxanthellae? This rather interesting concept involves phototaxis (a movement of an animal or plant towards light) and was proposed by Hollinsworth et al. in 2005. Cultured motile (non-symbiotic) zooxanthellae were exposed to white light which was split into a spectrum and projected on the culture flask. The idea was to determine if these dinoflagellates had a preference for any particular ‘color.’ Apparently they did – they swarmed in the area illuminated by green light. Blue light attracted significantly fewer zooxanthellae. Interestingly, previous observations suggested the motile zooxanthellae would move out of the area irradiated with ultraviolet wavelengths. These observations prompted a hypothesis – green fluorescence may act as a beacon, attracting free swimming zooxanthellae to coral recruits or planulae. Of course, other factors are undoubtedly in involved with corals attracting symbionts – corals are known to release chemical cues to the environment and it has been proven that zooxanthellae can swim against weak currents to reach a host (Pasternak et al., 2006). If we were to mesh these observations, we might arrive at the hypothesis that chemical cues released by the coral act as a navigational beacon to potential symbionts while green fluorescence is akin to landing strip lights.
GFP: A Shading Function for Photoreceptors? This thought was advanced in a paper by Shagin et al. (2004) and briefly discussed the possibility that GFP might shield the photoreceptors of the jellyfish Aequorea victoria. In addition, Burr et al. (2000) advanced the possibility of a protein (hemoglobin) in a nematode as possibly possessing an optical function. (Hemoglobin concentrates densely around Mermis nigrescens’ photoreceptor and is thought to play a role in phototaxis.) Some coral species have photoreceptors as well (Gorbunov and Falkowski, 2002), but to my knowledge no one has advanced a hypothesis linking fluorescence and protection of coral photoreceptors via optical shading. However a shielding function by GFP has been suggested for the non-photosynthetic soft coral Carijoa riisei (Khang and Salih, 2005).
This ends our discussion of potential functions of GFPs, and we will now turn our attention to specific green fluorescent proteins beginning with P-512.
Green-Blue Pigments
P-512
Host: Discosoma, Pigment 3
- Excitation: 503nm
- Emission: 512nm
- Stokes shift: 9nm
- Reference: Labas et al., 2002
- Comments: P-512 is one of several identified in the Discosoma genus. See Figure 37.
P-513
Host: Agaricia sp.
- Excitation: Not listed
- Emission: 513nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: With shoulders at 545 nm and 490 nm. See Figure 38.
Host: Ricordea florida
- Excitation: Not listed
- Emission: 513nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: In addition to the peak fluorescence at 513nm, Ricordea florida reportedly has a single shoulder at ~550-565nm. (R. florida specimens are variously colored green, blue, fluorescent orange, pink, copper, yellow and multiple combinations – Sprung and Delbeek, 1997).
P-514
Host: Acropora aspera
- Excitation: 501nm, shoulder at 480nm
- Emission: 514nm, shoulder at 490nm
- Stokes shift: 13nm
- Reference: Papina et al., 2002
- Comments: Collected off the coast of Okinawa, Japan in 1.5m of water, in August of 2001. This Acropora specimen appeared yellow-green in natural light and fluoresced bluish-purple when excited with UV-A radiation peaking at 365nm. Shifts in pH (5.0-8.0) do not significantly affect excitation and emission wavelengths or fluorescent quantum yield. See Figure 39.
Host: Montastraea cavernosa
- Excitation: ~505nm
- Emission: 514nm
- Stokes shift: ~9nm
- Reference: Kelmanson and Matz, 2003
- Comments: Found in a red M. cavernosa specimen collected in the Florida Keys National Marine Sanctuary.
Host: Entacmaea quadricolor (Actinaria)
- Excitation: N/A
- Emission: 514nm
- Stokes shift: N/A
- Reference: Wiedenmann et al., 2002
- Comments: Precursor to P-611. The fluorescence is stable over a pH range of 4-10.
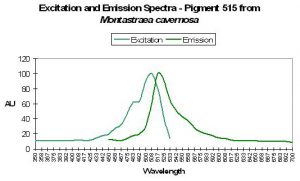
P-515
P-515’s fluorescence can contribute significantly to daylight appearance and is often found in conjunction with other fluorescent pigments (Fux and Mazel, 1999).
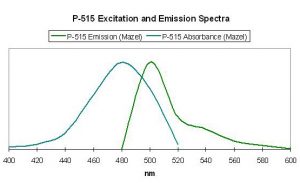
Figure 40. Excitation and emission wavelengths of a pigment found in the stony coral Mycetophyllia. After Mazel, 1997.
Host: Mycetophyllia lamarckiana
- Excitation: ~500-505nm
- Emission: 515nm
- Stokes shift: 10-15nm
- Reference: Mazel, 1997
- Comments: See Figure 40.
Host: Montastraea annularis
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Mazel, 1997
Host: Montastraea sp.
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Fux and Mazel, unpublished
Host: Montastraea cavernosa
- Excitation: 505±3
- Emission: 515±3nm
- Stokes shift: ~10nm
- Reference: Kelmanson and Matz, 2003.
- Comments: M. cavernosa can contain a number of pigments. There are several with similar emissions, and are referred to as P-515±3 by Kelmanson and Matz. See Figure 41.
Host: Scolymia sp.
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Fux and Mazel, unpublished
Host: Mycetophyllia sp.
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Fux and Mazel, unpublished
Host: Colpophyllia sp.
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Fux and Mazel, unpublished
Host: Plesiastrea verispora
- Excitation: Not listed
- Emission: 515nm
- Stokes shift: N/A
- Reference: Gilmore et al., 2003.
- Comments: These Plesiastrea specimens (appearing blue in natural light due to a non-fluorescent chromoprotein) were collected in 5-9m of water at Port Jackson, Australia.
P-516: Precursor of Pigment 581
Commercially available as EosFP.
Host: Montastrea cavernosa
- Excitation: 506nm
- Emission: 516nm
- Stokes shift: 10nm
- Reference: Labas et al., 2002
- Comments: Relative brightness of this green form is 1.28 (when compared to a modified form of A. victoria GFP).
Host: Pocillopora damicornis
- Excitation: 486nm
- Emission: 516nm
- Stokes shift: 30nm
- Reference: Salih et al., 2000.
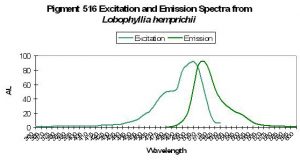
Host: Lobophyllia hemprichii
- Excitation: ~510nm
- Emission: 516nm
- Stokes shift: 6nm
- Reference: Nienhaus et al., 2005
- Comments: These researchers observed this green form (emission peak at 516nm) maturing to red fluorescence (peak at 581nm) when irradiated with ‘blue’ light (actually violet) at ~400nm. This maturation is driven by light energy and not chemical oxidation. See Figure 43 for excitation/emission spectra.
P-517
Host: Ricordea florida
- Excitation: Not listed
- Emission: 517nm
- Stokes shift: N/A
- Reference: Mazel, 1995
Host: Montastraea annularis
- Excitation: Not listed
- Emission: 517nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Emission has shoulders at 551nm and 483nm.
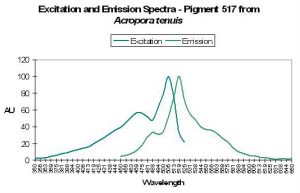
Host: Acropora tenuis
- Excitation: 505nm, shoulder at 465nm.
- Emission: 517nm, 555nm and 485nm.
- Stokes shift: 12nm
- Reference: Papina et al., 2002
- Comments: This A. tenuis specimen was collected in August 2001 at a depth of 1.5m in Okinawa, Japan. It appeared to the observer as brown in natural light, and fluoresced green under a black light. Spectral chart is that of an intact coral. Papina et al. noted no significant shifts of either fluorescent quantum yield or spectral characteristics over a pH range of 5.0 to 8.0. See Figure 44.
P-517 variant: Precursor of Pigment 593
Commercially available as Kikume or KikGR.
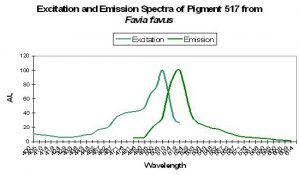
Figure 45. The spectra of a GFP from a stony coral in its immature state. The mature form is red with a peak emission at 593nm. After Tsutsui et al., 2005.
Host: Favia favus
- Excitation: 507nm
- Emission: 517nm
- Stokes shift: 10nm
- Reference: Tsutsui et al., 2005
- Comments: Pigment 517 from Favia favus is photoconvertible to P-593 (green to red). Relative brightness (compared to an engineered GFP from Aequorea victoria) is 1.12. See Figure 45.
Pigment 517 variant: Precursor to Pigment 574
Host: Ricordea florida
- Excitation: 506nm
- Emission: 517nm
- Stokes shift: 11nm
- Reference: Labas et al., 2002
- Comments: See Figure 46.
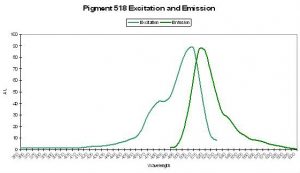
P-518
Host: Ricordea florida
- Excitation: Not listed
- Emission: 518nm
- Stokes shift: N/A
- Reference: Mazel, 1995
Host: Acropora cytheria
- Excitation: Not listed
- Emission: 518nm
- Stokes shift: Not listed
- Reference: Hochberg et al., 2004
- Comments: This particular Acropora cytheria specimen was grown at the Waikiki Aquarium under a combination of artificial light (metal halide lamps) supplemented with afternoon sunlight (J.C. Delbeek, personal communication).
Host: Acropora digitifera
- Excitation: Not listed
- Emission: 518nm
- Stokes shift: Not listed
- Reference: Hochberg et al., 2004
- Comments: Found in an Acropora digitifera specimen on a shallow reef flat (depth of 0.1m; ~4”), Mayoette, Comorros.
Host: Family Pectiniidae
- Excitation: 503nm
- Emission: 518nm
- Stokes shift: 15nm
- Reference: Ando et al., 2004
- Comments: This is an interesting protein – its fluorescence is ‘switchable.’ The green pigment is bleached upon exposure to intense light at ~490nm. The green fluorescence returns upon exposure to violet light at about 400nm. This photoconvertible pigment (from green fluorescence to non-fluorescent) is commercially available as “Dronpa” (“Dron” being a ninja term for ‘vanishing’ and ‘pa’ for photoactivatible) and was isolated from a coral in the family ‘Pectiniidae’ (which includes genera Echinophyllia, Oxypora, Mycedium and Pectinia – Veron, 1986). Relative brightness of this green form is 2.40 (when compared to a modified form of A. victoria GFP).
Host: Ricordea florida
- Excitation: 508nm
- Emission: 518nm
- Stokes shift: 10nm
- Reference: Labas et al., 2002
Host: Montastraea cavernosa
- Excitation: ~505nm
- Emission: 518nm
- Stokes shift: ~13nm
- Reference: Kelmanson and Matz, 2003
- Comments: This pigment was found in a green fluorescent M. cavernosa collected in the Florida Keys.
P-518 could also be an immature green form of the red fluorescent protein Kaede (P-582), although it is difficult to believe the pigment would not mature in shallow water as described by Hochberg et al., 2004. This could simply be a non-photoconvertible form of P-518.
P-519
Host: Montastraea cavernosa
- Excitation: Not listed
- Emission: 519nm
- Stokes shift: N/A
- Reference: Kelmanson and Matz, 2003
- Comments: Pigment 519 was found in a red fluorescent M. cavernosa collected in the Florida Keys. No excitation wavelength is listed; however, its emission spectrum is almost identical to P-518.
P-520: Precursor of Pigment
580.
- Host: Ricordea florida
- Excitation: Not listed
- Emission: 520nm
- Stokes shift: N/A
- Reference: Mazel, 1995.
- Comments: This pigment might be the immature form of the ‘red’ fluorescent P-580. See below.
Host: Montastraea cavernosa
- Excitation: 508nm, with a shoulder at 572nm
- Emission: 520nm, with shoulder at 580nm
- Stokes shift: 12nm and 8nm
- Reference: Labas et al., 2002.
- Comments: Possibly the same pigment found in Ricordea florida by Mazel (1995)?
P-520: Precursor of Pigment
611.
- Host: Entacmaea quadricolor
- Excitation: ~500nm
- Emission: ~520nm
- Stokes shift: 20nm
- Reference: Nienhaus et al., 2003.
P-522
Host: Montastraea cavernosa
- Excitation: Not listed
- Emission: 522nm
- Stokes shift: N/A
- Reference: Kelmanson and Matz, 2003.
- Comments: This pigment was found in a red Montastraea cavernosa collected in the Florida Keys National Marine Sanctuary.
Host: Anemonia sculata
- Excitation: 511nm
- Emission: 522nm
- Stokes shift: 11nm
- Reference: Wiedenmann et al., 2000
- Comments: Note the double-peaked emission in Figure 48.
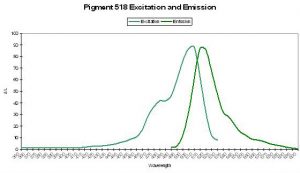
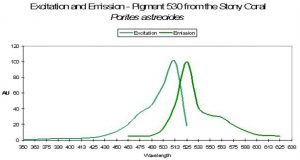
P-530
Host: Porites astreoides
- Excitation: ~500nm
- Emission: 530nm
- Stokes shift: ~30nm
- Reference: Mazel et al., 2003
- Comments: See Figure 49 for excitation and emission spectra.
Yellow-Green Pigments
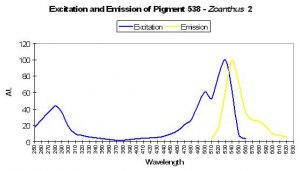
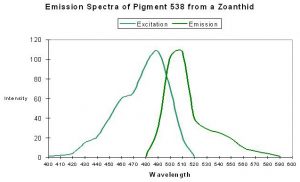
P-538
Host: Zoanthus 2.
- Excitation: 528nm
- Emission: 494nm and 538nm
- Stokes shift: 10nm
- Reference: Matz et al., 1999; Labas et al., 2002
- Comments: P-538 was isolated by Matz et al. (1999) from a Zoanthus species and found in combination with another fluorescent pigment (P-506). Fluorescent Quantum Yield and Relative Brightness are fairly low – 0.42 and 0.38, respectively. See data of Yanushevich et al. (2002) in Figure 50.
P-550
Host: Porites murrayensis
- Excitation: 530nm
- Emission: 550nm
- Stokes shift: 20nm
- Reference: Dove et al., 2001
- Comments: This coral was purple when viewed in natural light due to the presence of a non-fluorescent protein. Maximum host tissue/zooxanthellae absorbance is at 576nm (data not shown).
P-557
Host: Agaricia sp.
- Excitation: Not listed
- Emission: 557nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Emission also has shoulders at 600nm and 545nm. Reports of this particular pigment are practically non-existent, with Mazel’s reports being the only ones of which I am aware. See Figure 52. (Mazel reports yellow Agaricia are common in Bonaire – personal communication).
P-561 (Kusabira-Orange)
Host: Fungia concinna
- Excitation: 548nm
- Emission: 565nm
- Stokes shift: 17nm
- Reference: Karasawa et al., 2004.
- Comments: Commercially available from MBL International under the trade name “Kusabira-Orange”. Wild type P-561 has a fluorescent quantum yield of 0.45, and is not pH sensitive (pKa <5.0). The Japanese name for Fungia is “Kusabira-ishi.” See Figure 53.
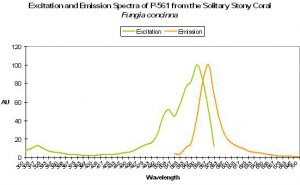
Figure 53. A true orange fluorescent protein found in the popular ‘mushroom’ stony coral (Fungia). After Karasawa et al., 2004.
P-562 (or asCP-562)
Host: Anemonia sculata
- Excitation: 559nm
- Emission: 562nm (immature form)
- Stokes shift: 52nm
- Reference: Wiedenmann et al., 2002
- Comments: This yellow green pigment is found in the anemone (Anemonia sculata) and is technically fluorescent although laboratory instruments are needed to detect it. Under certain conditions, this pigment can transform from yellow-green to red with a very weak emission maximum at 611nm.
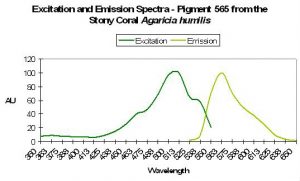
P-565
Host: Agaricia humilis
- Excitation: 490nm
- Emission: 565nm
- Stokes shift: 75nm
- Reference: Mazel et al., 2003.
- Comments: See Figure 54.
Host: Cerianthus sp.
- Excitation: 548nm
- Emission: 565nm
- Stokes shift: 17nm
- Reference: Ip et al., 2004.
- Comments: This pigment is apparently spectrally different from the P-565 found in Agaricia stony corals.
An Early GFP Description
In homage to Siro Kawaguti, one of the first researchers of coral coloration, Table 3 describes some of the properties of fluorescent pigments found in Pacific corals. Though much of Kawaguti’s early works were completed and published in the late 1930’s and 40’s, his ideas, for the most part, have withstood the test of time. His equipment was not as sophisticated as that of today and did not offer great precision, yet his descriptions are remarkable. His works offer perspectives on GFPs and the varieties of coral hosts and complement later research. Note that his work deals with mostly emission wavelengths (as seen in the ‘1st Band’ and ‘2nd Band’ columns) while absorbance (‘Max. Abs. 1’ and ‘2’ columns is rarely described. See Table 5.
| Green Fluorescence | ||||
|---|---|---|---|---|
| Coral | 1st Abs. Band | 2nd Abs. Band | Maxima Abs.1 | Maxima Abs. 2 |
| Fungia actiniformis | 498-521 | 475-484 | 512 | 480 |
| Montipora verrucosa | 505-514 | * | * | * |
| Porites (arboreal) | 515-525 | 495 | * | * |
| Fungia repunda | 504-517 | 476-483 | * | * |
| Montipora ramosa | 498-511 | 486 | 504-507 | * |
| Tridacophyllia lacinata | 488-511 | 467-471 | * | * |
| Pachyseris rugosa | 498-509 | * | * | * |
| Caulastrea tumida | 498-510 | * | * | * |
| Hydnophora microconos | 498-511 | * | * | * |
| Acropora hyacynthus | 498-512 | 474 | * | * |
| Acropora haimei | 498-513 | 471 | * | * |
| Pocillopora acuta | 498-514 | * | * | * |
| Favia sp. | 500-507 | * | * | * |
| Mycedium elephantotus | 491-505 | * | * | * |
| Herpetolitha limax | 493-508 | * | * | * |
| Lobophyllia hemprichii | 494-514 | * | * | * |
| Lobophyllia corymbosa | 501-514 | 473 | * | * |
| Psammocora contigua | 482-498 | 463 | * | * |
| Euphyllia glabrescens | 489-500 | * | * | * |
| Galaxea fascicularis | 488-501 | * | * | * |
| Pocillopora eydouxi | 473-489 | * | * | * |
| Plerogyra sinuosa | 488-507 | * | * | * |
| Echinopora lamellosa | 491-503 | * | * | * |
| Goniopora sp. | 485-501 | 458-466 | * | * |
| Acropora arcuata | 494-503 | * | * | * |
| Cyphastrea mocrophthalma | 482-499 | 462-466 | * | * |
| Tridacophyllia lacinata | 493-504 | * | * | * |
Next time, we’ll look at more fluorescent pigments and discuss how light can cause coloration shifts in at least some pigments. Interested parties can correspond with the author at [email protected].
References
Please see Part 1 (Volume V, Issue IX) for a full listing of references used in this series.








0 Comments