
It was my intent for the articles examining metals to be the last in the series on coral nutrition, but my schedule has been much too busy for me to devote the time required to complete the second round of observations on the importance of fatty acids in coral diets. Hence, our discussion of coral nutrition this month is an initial examination of the importance in maintaining proper metal concentrations within the reef aquarium.

This Porites coral skeleton is composed of mostly calcium carbonate (in the form of aragonite) but also contains many ‘trace’ elements.
In this article, some rather surprising information is presented – specifically the effects of elevated calcium concentrations or possible benefits of elevated strontium levels. We’ll also examine what is known about natural concentrations of metals in seawater. The information presented here may provide some useful background when examining results generated by some rather sophisticated analytical devices now available for hobbyists’ benefit (such as Triton Lab’s ICP-OES.)
But first, let’s discuss why your best efforts to maintain proper metal concentrations might be for naught. Be aware that some of the metals discussed might not be necessary or even desirable!
Importance of Salinity When Testing for Metals
Years ago, when I worked at a commercial coral farm, I spent a great deal of time on the telephone discussing water quality issues with hobbyists. With some chagrin, I recall one particularly anal aquarist that constantly fretted over an issue he held in paramount importance: A ‘low’ magnesium concentration. His calls were like clockwork, and my power of persuasion was apparently not sufficient to convince him that resolution of his issue was simple. For reasons unknown, he refused to maintain a normal salinity level of ~35 parts per thousand. With low salinity comes low metal concentrations, hence his low magnesium level was due to low salinity (had he reported other metals, he would have found they were likely low as well.) The lesson is simple: Consider salinity levels when estimating metal concentrations in the reef aquarium, and compare measurements made on samples of consistent salinity.
But testing is for nothing if we ignore the importance of our next topic: The Boundary Layer.
Importance of Water Motion: The Boundary Layer
For our purposes, all benthic (submerged) objects are surrounded by a layer of stagnant water called the Boundary Layer. The thickness of this stagnant layer depends upon the velocity of the water moving around or across the object – the quicker the flow, the thinner the boundary layer becomes. Why is this important? It seems that availability of substances (metals, nutrients, etc.) is dependent upon their diffusion through the boundary layer to the coral. Hence, low flow and a thick boundary layer might limit the availability of a metal (especially one found in low concentration) to a coral. Contrarily, a thin boundary layer mediated by water motion allows diffusion of a metal to the coral. The take-home message: Availability of a metal to the coral is dependent upon water motion sufficient to create a thin boundary layer. It follows that a coral with a complex shape (say, a highly branched Acropora specimen) will require more water motion to create a thin boundary layer than a coral with a simple shape (a Porites lutea, for instance.) It is a simple fact: Poor water motion can limit the availability of metals found in low concentrations to corals.

Figure 1. Electronic water velocity meters are expensive but essential for use in water motion experiments.
Measurement of water motion is difficult, and common yet inexpensive methods include the timing of objects (such as bubbles or floating objects) as they travel over given distances to arrive at velocity. More expensive and exact methods include velocity measurements through use of electronic meters operating on Faraday’s Principle, where water motion distorts a magnetic field in a measurable manner. See Figure 1.
Once a metal has passed through the boundary layer and is absorbed by the coral, it faces a second obstacle: The Organic Matrix.
Deposition of Metals: The Organic Matrix
The Organic Matrix is a sheath of living tissue coating the skeleton and is where metals are crystalized and deposited into the skeleton. It is generally accepted that its structure is lattice-like and is composed of protein, lipid and the carbohydrate chitin. This lattice is of a certain size and allows those atoms of relatively small size to pass through, while preventing those of relatively large size to be excluded. Some researchers believe that strontium is a simple substitution for calcium since these atoms are of the same charge and diameter, suggesting strontium is not really required for coral skeleton growth. Rasmussen et al. (1992) state strontium (along with lead and barium) is compatible with the crystal structure of aragonite (the major form of calcium carbonate in coral skeletons.) However, other research suggests strontium in elevated concentrations can actually increase skeletal growth. We’ll discuss this later in this article.
Importance of Environmental Conditions
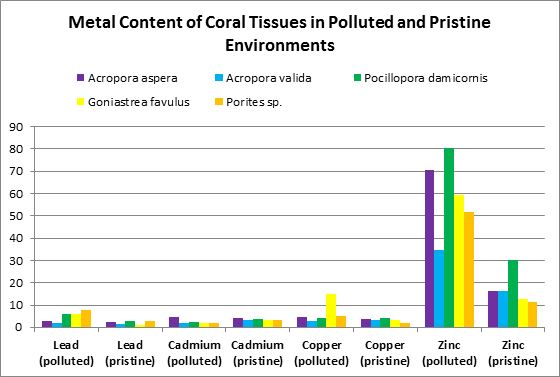
Ambient water quality plays an important part in both tissue and skeletal metal content. Runoff may provide an influx of dissolved and particulate-bound metals. Harbors and marina waters may contain elevated metal levels due to corrosion of boat motor sacrificial anodes and discarded metallic garbage. See Figures 2, 3 and 4 for a comparison metals found in coral tissues in ‘pristine’ and ‘polluted’ sites.
Other researchers (Bastidas and Garcia, 1996) examined metal content of Montastrea annularis from Venezuelan reefs with ‘high’ and ‘low’ sedimentation rates.
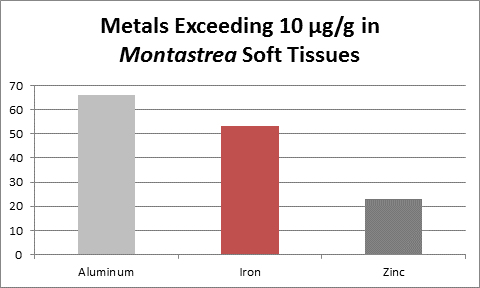
Figure 4. These metals are from a coral from Venezuelan reefs with low sedimentation rates. Not shown is calcium – it was found at a concentration of 31.5% (315,000 mg/kg.)
A Quick Lesson on the Metric System (mg/kg and mg/l compared to ppm)
I’ll take this opportunity to explain some of the terminology used in this article since not all hobbyists routinely use the metric system.
For dry materials (such as coral skeletons):
1 part per million is equivalent to 1 milligram per kilogram. To explain, there are 1,000 milligrams in a gram, and there are 1,000 grams in a kilogram. Hence, 1,000 milligrams (in a gram) times 1,000 grams (in a kilogram) = 1,000,000 milligrams in a kilogram, so 1 milligram per kilogram is equivalent to 1 part per million.
For discussing concentrations of solids (suspended or dissolved) in liquids:
1 part per million is roughly equivalent to 1 milligram per liter. To explain, 1 liter (1,000 milliliters) of pure water at certain given conditions weighs 1 kilogram. There are 1,000 milliliters in a liter, and 1 milliliter weighs one milligram. Hence, 1,000 milligrams in a gram times 1,000 grams (in a liter of pure water), so 1milligram per liter is equivalent to 1 part per million. Note that a liter of seawater weighs more than a liter of pure water, but, for practical purposes, is usually disregarded.
Metals Found in Coral Skeletons
The coral corallum (or skeleton) is composed of a number of metals and non-metals. See Figures 5 and 6 for details.
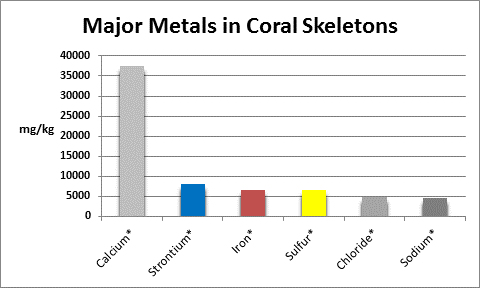
Figure 5. Metals found in coral skeletons in high concentrations (greater than ~0.5%, or ~5,000 mg/kg, or 5,000 ppm, if you will.) A ‘*’ denotes testing methods are available to serious hobbyists. Sodium is by calculation from chloride results.
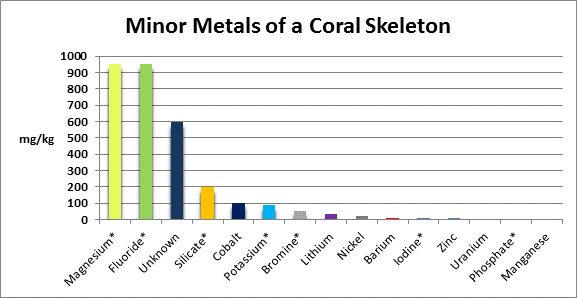
Figure 6. Metals found in relatively minor amounts in coral skeletons. A ‘*’ denotes testing methods are available to serious hobbyists. ‘Unknown’ components were acid insoluble.
Metals and ‘Trace Elements’ Found in Artificial Seawater Mixes
My first thought was to include results of studies that determined the concentrations of metals and compounds in many of the top-selling artificial seawater mixes. This effort was deemed to be too laborious, so I’ll simply provide links to these studies:
- Atkinson, M. and C. Bingman, 1999.The composition of several synthetic seawater mixes. Aquarium Frontiers Online. March 1999. http://www.animalnetwork.com/fish2/aqfm/1999/mar/features/1/default.asp
- Marulla and O’Toole, 2005. Feature Article: Inland Reef Aquarium Salt Study, Part I. www.advancedaquarist.com/2005/11/aafeature1
- Marulla and O’Toole, 2005. Feature Article: Inland Reef Aquarium Salt Study, Part I. www.advancedaquarist.com/2005/12/aafeature1
Review of these references should provide a reasonable (if perhaps dated) background on the salt you use.
Things Competing for Metals In A Reef Aquarium
Obviously, many things absorb metals in an aquarium other than corals. Algae (green, brown, red, calcareous), snails, urchins, and others utilize metals and might concentration them in their tissues.
Not so obvious factors include metal absorption by compounds utilized for phosphorus removal (such as granular ferrous oxide, or GFO).
See Riddle, 2012 for details: http://www.advancedaquarist.com/2012/2/chemistry
This report suggests that GFO removes at least metals from aquarium water.
Activated carbon (Craig Bingman, internet communication) can remove metals complexed with humic acids especially in freshwater systems. Humic acids (tannins, lignins, fulvic acids) are a complex mixture of decomposing organic matter. Humic acids, in high concentrations, lend a ‘tea’ or ‘coffee’ color to water and lesser concentrations appear as ‘yellowing’ of water. Metals can be complexed by humic acids thus lending a detoxifying effect. Some, if not all, artificial seawater formulae contain an artificial agent (EDTA or ethylenediaminetetraacetic acid) that binds with many metals. EDTA is photodegradable thus it ‘releases’ complxed metals over a period of time.
Other organic compounds competing for trace elements would include amino acids, especially those with a negative chare such as aspartic acid.
Elements Found in Coral Soft Tissues and Skeletons
The following listing of elements found in coral skeletons is grouped according to their classification (alkali metals, transition metals, and so on,) It is certain that not all elements listed are necessary (or even desireable) for skeletal growth. Concentrations and chemical species are from Adey and Loveland’s Dynamic Aquaria (1991.)
Alkaline Earth Metals
Alkaline Earth metals include barium, beryllium, calcium, magnesium, radium, and strontium.
Barium (Ba):
- Atomic Weight: 137.33
- Concentration in seawater: 0.05 mg/kg
- As: Ba++
Barium is incorporated into coral skeletons. Its radius is1.47 A, much larger than calcium (1.18 A) and slightly larger than strontium (1.31 A.) It is possible that, like strontium, its radius and valence are similar to those of calcium and its incorporation is a ‘mistake.’
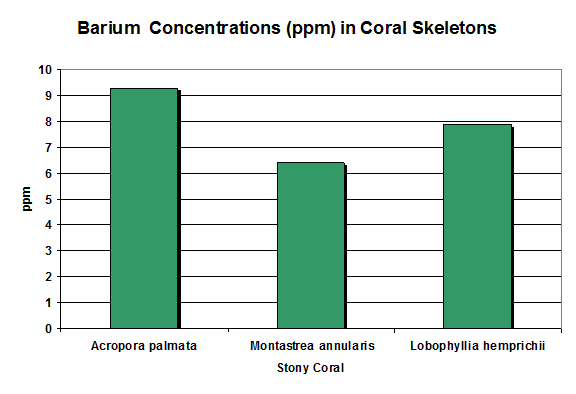
Figure 7. In the overall picture, barium concentrations are fairly consistent. Pingitore et al., 1989 and Ohde and Kitano, 1981 (Lobophyllia).
Rasmussen et al. (1992) state barium (along with lead and strontium) is compatible with the crystal structure of aragonite (the major form of calcium carbonate in coral skeletons.) One pound is in 50,000 gallons of seawater. Barium concentrations exceeding 1 mg/l can be toxic in marine environments.
No barium was found by an analysis of a Goniopora skeleton in the 1990’s (Riddle, unpublished.)
Beryllium (Be):
- Atomic Weight: 9.0122
- Concentration in seawater:?
- As: BeOH+
An analysis of a Goniopora skeleton I had performed in the 1990’s revealed no beryllium (but the method was atomic absorption, and the detection limit was probably 1-2 mg/kg.) No further information is available (at least to me) on beryllium and corals. It is non-essential for plants and animals.
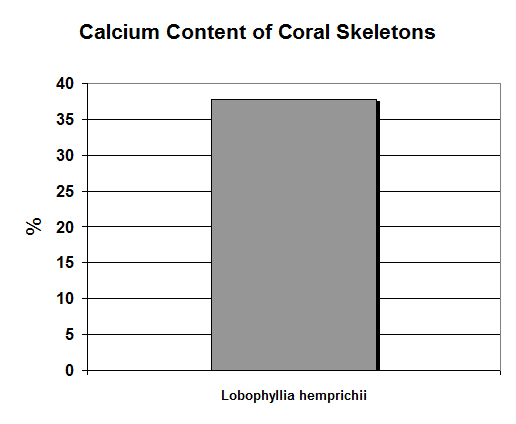
Figure 8. Calcium concentrations can be determined by the sequential hardness titration test. Note that this scale is in %: This equates to 37,000 mg/kg!
Calcium (Ca):
- Atomic Weight: 40.08
- Concentration in seawater: 400 mg/kg
- As: Ca++
Calcium is perhaps the most tested metal in reef aquaria, and for good reason – under proper conditions, it is quickly incorporated into coral skeletons. It is the fifth most abundant element in seawater, and 1 pound is in 300 gallons.
It would be difficult to overstate the important of calcium in the calcification process or for many critical life processes.
Effects of Increased Calcium Concentrations
Swart (1980) investigated the effects of increased concentrations of various metals on coral calcification. He increased calcium concentrations by 100 and 200 mg/l (presumably to 500 and 600 mg/l.) Corals tested included Acropora squamosa, Pocillopora damicornis, Acropora cuneta collected from inner flat and outer reef flats. A Porites lutea was collected from a reef slope. Growth increased over controls in 57% of these experiments. When calcium was elevated by 400 mg/l (presumably to 800 mg/l) growth decreased in every case save one (and in this case growth was about 1% over the control.) See Figure 9 for results of those experiments involving calcium.
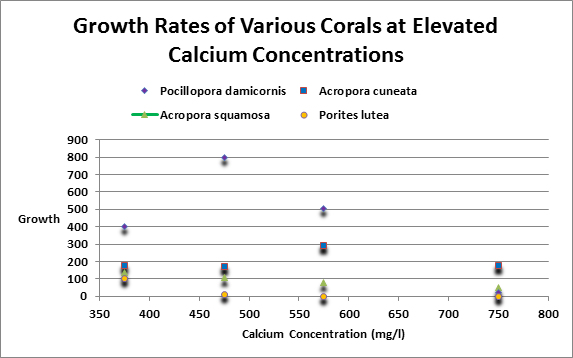
Figure 9. In some cases – but not all! – Elevated calcium concentrations can retard coral growth rates, according to Swart, 1980.
We can speculate that elevated calcium concentrations could have an impact on the absorption of magnesium or perhaps other major and minor metals.
Some artificial seawater formulae have high Ca content of about 450 mg/l; some less.
Magnesium (Mg):
- Atomic Weight: 24.305
- Concentration in seawater: 1,272 mg/kg
- As: Mg++
Magnesium is found in natural seawater at a concentration of about 1,272 mg/l, or about 1 pound in 94 gallons. It is the third largest component of seawater (after sodium and chlorine.) It is an important part of coral (stony, soft, and alcyonarium (sea whips and fans) skeletons or spicules, calcareous algae, and bivalve shells. Chlorophylls (Chl a, b, c2) contain magnesium and several enzymes important in photosynthesis are activated by magnesium.
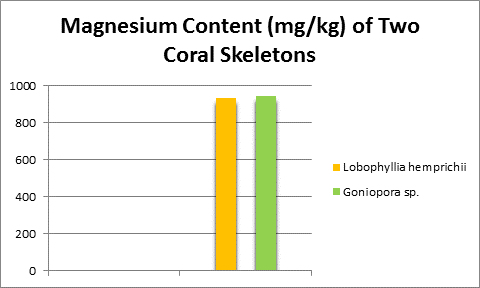
Figure 10. Many, if not all, coral skeletons contain substantial amounts of magnesium. Its concentration is about that of seawater.
Increased magnesium levels above the normal amount should be avoided as it has been shown to be antagonistic to the calcification process (See Figure 11, 12 and 13.) Swart (1980) found reduced growth rates in corals when the magnesium concentration was raised by 100 mg/l (presumably to ~1,400 mg/l.) When the level was elevated by 200 mg/l (~1,500 mg/l?) no growth was observed. Magnesium is possibly a substitute for calcium as it has the appropriate charge and radius (Pingitore et al., 1989.)
Rasmussen et al. (1992) state that, due to structural incompatibility, magnesium is only weakly incorporated into the aragonite skeleton (relative to calcium concentrations.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
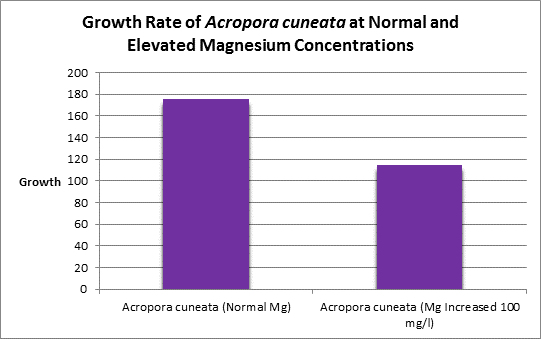
Figure 11. Elevated magnesium concentrations decreased the growth rate of this Acropora species.
Radium (Ra):
- Atomic Weight: 226
- Concentration in seawater: 0.2-3 x 10-10
- As:?
Radium. Do we care? Should we? Very little information is available.
Strontium (Sr):
- Atomic Weight: 87.62
- Concentration in seawater: 8 -13 mg/kg, depending upon the reference.
- As: Sr++
Strontium is possibly just a substitute for calcium as it has the appropriate charge and radius (Sr radius =1.31, Calcium radius = 1.18; Pingitore et al., 1989.) It is present in seawater at 1 pound in 9,223 gallons.
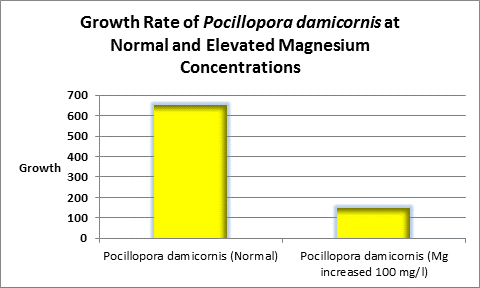
Figure 12. In this experiment, increased level of magnesium decreased the rate of coral growth in the ‘weedy’ stony coral Pocillopora damicornis.
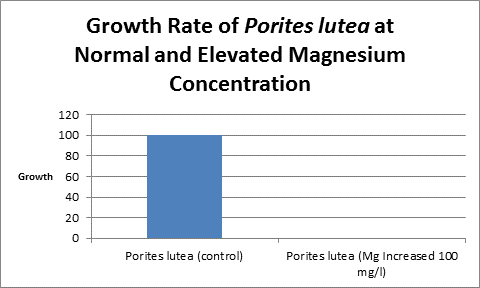
Figure 13. And the trend continues. Porites lutea showed no measurable growth when magnesium was elevated by 100 mg/l.

Figure 14. Magnesium is a part of water’s total hardness, and can be determined by this calculation: Total harness minus calcium hardness = magnesium hardness.
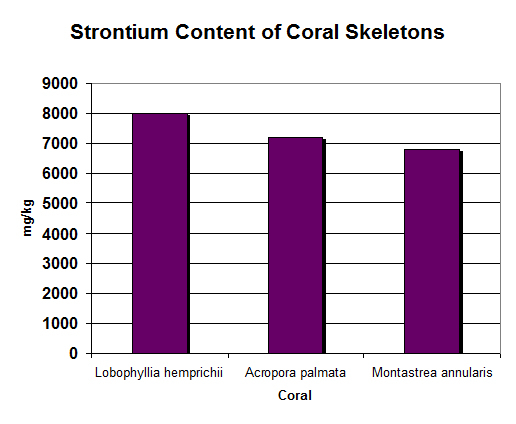
Figure 15. Strontium in coral skeletons is remarkably similar in the hundreds of tests performed. Concentrations of ~7,000 – 8,000 mg/kg are entirely normal.
Effect of Increased Strontium Concentrations
Swart (1980) manipulated (raised) strontium concentrations and observed coral growth rates. Results were mixed. When the strontium level was doubled (presumed to be ~25 mg/l), coral growth increased in only ~38% of the cases. However, when strontium was raised to ten times that of natural seawater (~130 mg/l?), growth rates increased in 63% of the trials. Test kits are available for checking strontium concentrations. See Figure X.
Transition Metals
Transition Metals include 39 elements. For our purposes, Transition Metals include cobalt, cadmium, chromium, copper, iron, manganese, molybdenum, mercury, nickel, silver, thorium, titanium, uranium, vanadium, and zinc and we’ll examine what evidence we have.
Cadmium (Cd):
- Atomic Weight: 112.41
- Concentration in seawater: 0.1 µg/l
- As: CdCl2
Cadmium is a non-essential element for almost all plants and animals (but see below) and it can be a cumulative toxin. Fortunately, there is not much of it in natural seawater and hopefully, in your aquarium.
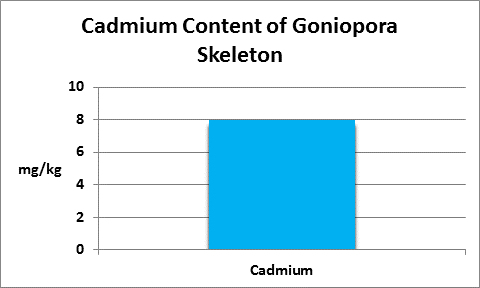
Figure 17. Cadmium is not essential for almost all life forms, in fact it can be toxic. This Goniopora concentrated it in its skeleton. Why?
It is possible for cadmium to be present in tap water due to degradation of galvanized plumbing. According to my old reference, the EPA has set the Maximum Concentration Level (MCL) at 10 µg/l. The solubility of cadmium is controlled by carbonate equilibria (the softer the water, the higher the solubility.) Rasmussen et al. (1992) state that, due to structural incompatibility, cadmium is only weakly incorporated into the aragonite skeleton (relative to calcium incorporation.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
Wiens (2000) found that cadmium at a concentration of 3 parts per billion could be toxic to the ‘difficult’ soft coral Dendronephthya klunzingeri. It is toxic to some fish at 200 parts per billion. However, there is a biological use: Some diatoms use cadmium as a substitute in the important metalloenzyme carbonic anhydrase, which splits carbonates into carbon dioxide for use in photosynthesis.
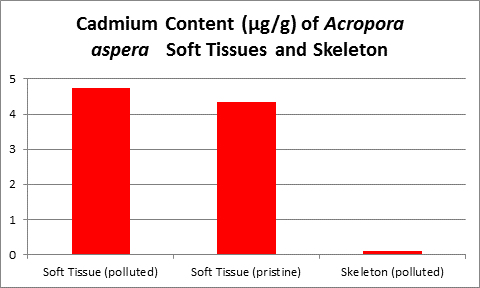
Figure 18. Cadmium content of wet soft tissue including zooxanthellae and skeleton of an Acropora. From McConchie and Harriott (1992.)
McConchie and Harriott (1992) found cadmium concentrations up to 32 to 40 times higher in coral soft tissues than in skeletons.
Chromium (Cr):
- Atomic Weight: 51.996
- Concentration in seawater: 0.3 µg/l
- As: Cr(OH)3, CrO24-
Chromium is non-essential for plants, but humans require trace amounts.
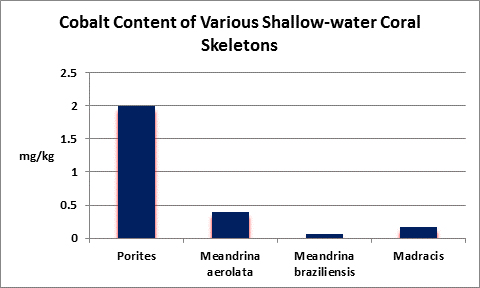
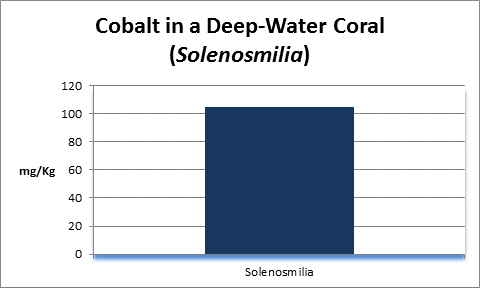
Cobalt (Co):
- Atomic Weight: 58.933
- Concentration in seawater: 0.05 µg/kg
- As: Co++
Cobalt is essential for human life processes, in fact Vitamin B12 contains it. It is not essential for plants. In natural seawater, cobalt occurs at a concentration of 0.00005 mg/l, more or less. You’ve got a really big aquarium if it contains 1 pound of cobalt.
Rasmussen et al. (1992) state that, due to structural incompatibility, cobalt is only weakly incorporated into the aragonite skeleton (relative to calcium concentrations.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
In terrestrial plants, cobalt can bind to important binding sites thus preventing uptake of other important cations.
The Hach Company markets a colorimetric cobalt test, but it is pricey and its detection limit is far below that content of natural seawater.
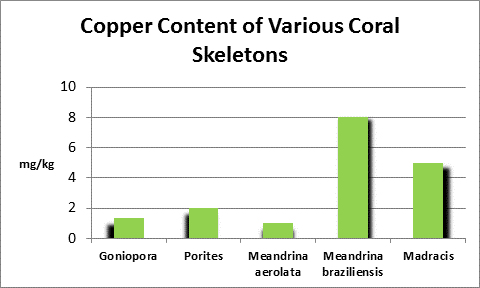
Copper (Cu):
- Atomic Weight: 79.904
- Concentration in seawater: 0.001 – 0.01 mg/kg
- As: CuCO3, CuOH+
Copper is an essential element for plants and animals. Copper forms the backbone of copper dismutase enzymes, which detoxify harmful oxygen radicals. There are other bio-benefits of copper – deposits in polychaete worms’ jaws lend strength (See Simkiss, 1964.)
Some copper compounds are toxic by ingestion or absorption. To protect the general public from toxic concentrations of coper caused by dissolving copper water pipes, the US EPA has established rules for larger water systems to follow: the 90th percentile action level is 1.3 mg/L copper. In plain English, if 10% of representative samples exceed the concentration of 1.3 mg/l copper, the water system must under law take corrective actions. Unfortunately for many hobbyists, the preferred method is addition of phosphate (usually in the form of sodium hexametaphosphate.) Since phosphorus is sometimes the limiting element in algae growth, more reactive or ortho-phosphate could potentially promote unwanted algal growths.
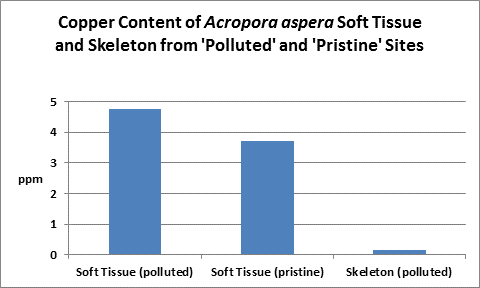
Figure 23. Copper content of wet soft tissue including zooxanthellae and skeleton. From McConchie and Harriott (1992.)
An elevated copper level (>0.5 mg/l) is known to completely inhibit fertilization in the stony corals Goniastrea aspera, Favites chinensis, and Platygyra ryukyensis. Fertilization rates were reduced by ½ when copper is at 0.1 mg/l. No fertilization inhibition was noted at 0.01 mg/l copper.
Copper can be chelated with organic substances such as lignins (substances found in plants and act as natural chelators.) This might account for low toxicity of high amounts (5-15 mg/l) of copper found in coral tissues from highly polluted areas (such as marinas, where boat motor sacrificial anodes containing copper and other elements dissolve.) McConchie and Harriott (1992) found copper concentrations up to 39 to 82 times higher in coral soft tissues than in skeletons.
At least some corals can withstand very high copper concentrations for surprisingly long periods of time (days.) Researchers noted the corals used in the experiment produced copious amounts of mucus. It is unknown if the copper complexed with the musus and detoxified it, or if it acted to decrease diffusion across the boundary layer.
Mills (1999) noted that copper deficiencies in tobacco plants resulted in low activity of copper/zinc dismutase enzyme.
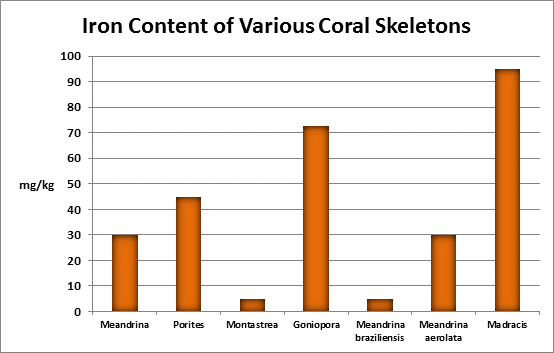
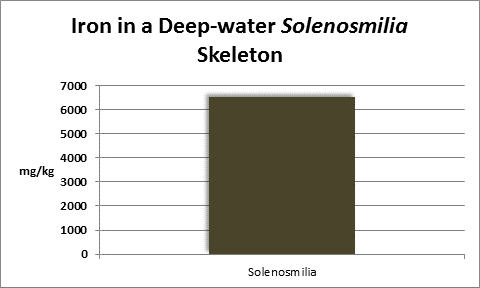
Iron (Fe):

Figure 24. Many kits are available for testing for iron. This colorimeter has generated results favorable with more expensive spectrometers.
- Atomic Weight: 55.874
- Concentration in seawater: 0.002 – 0.02 mg/kg
- As: Fe(OH)2; Fe(OH)4
Iron is a familiar element, and is considered essential for life processes in plants and animals, but is indeed a ‘trace’ element in aquatic environments. Since it is an important factor in photosynthetic enzymes, it can be a growth-limiting factor to plants and phytoplankton. At a concentration of 0.002 mg/kg of seawater, one pound of iron is found in about in 60,000,000 gallons. A ‘trace’ element indeed! Interestingly, iron is actively incorporated into many coral skeletons. See Figures 25 and 26.
Rasmussen et al. (1992) state that, due to structural incompatibility, iron is only weakly incorporated into the aragonite skeleton. However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
The U.S. EPA has a secondary (suggested) standard for iron content of drinking water. Iron concentrations over 0.3 mg/l can impart a bitter taste as well as stain clothing.
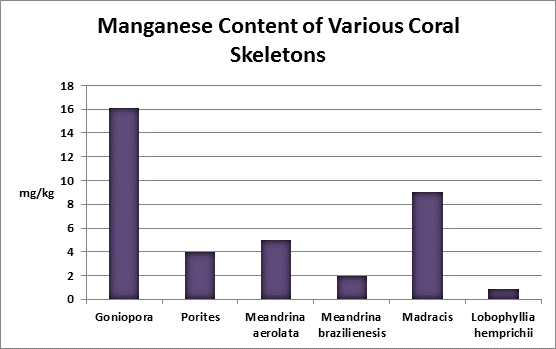
Manganese (Mn):
- Atomic Weight: 54.938
- Concentration in seawater: 0.001 – 0.01 mg/kg
- As: Mn++, MnCl-
Manganese is considered an essential element for photosynthesis and is also an essential trace element for animals. Manganese deficiencies have been linked to reduced rates of photosynthesis.
Manganese is a grayish white, brittle, yet hard metal. It stains laundry when found in concentrations exceeding 1 mg/l so water utilities take measures to remove much of it from potable water supplies. It is found in seawater at a concentration of 0.002 mg/L (1 pound in 60,000,000 gallons.) A test kit is available but is useful only in potable water testing as its detection limit is far too high to be of use in testing normal seawater concentrations.
Rasmussen et al. (1992) state that, due to structural incompatibility, manganese is only weakly incorporated into the aragonite skeleton (relative to the incorporation of calcium.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
Mills (1999) found that low manganese levels in tobacco plants can lessen the activities of important enzymes (dismutases that reduce oxygen radical content generated by photosynthesis. He found that these superoxide enzymes were affected: Manganese SOD, Iron SOD, Copper/Zinc SOD, APX or Ascorbate Peroxidase.)
Mercury (Hg):
- Atomic Weight: 200.59
- Concentration in seawater: 3 x 10-10
- As: HgCl24-, HgCl2
Mercury is a well-known cumulative poison. Non-polluted seawater contains very little and this is reflected by its small presence in coral tissues.
Molybdenum (Mo):
- Atomic Weight: 95.94
- Concentration in seawater: 0.0005 mg/kg
- As: MoO24–
Molybdenum is an essential element for plants and animals (as well as nitrogen-fixing bacteria.) It is an important cofactor in many enzymes, such as nitrate reductase and nitrogenase. Interestingly, it is thought to help prevent tooth decay in humans.
In the early days of reef-keeping in the United States, it was touted as an essential supplement in reef aquaria. This fad, as many others have, seems to have passed and its popularity as a supplement has decreased, if not disappeared altogether. Mo is found in natural seawater at about 1 pound in 240 million gallons.
An atomic absorption spectrometer (with a detection limit of ~2 mg/kg) failed to find any molybdenum in a Goniopora skeleton.
Nickel (Ni):
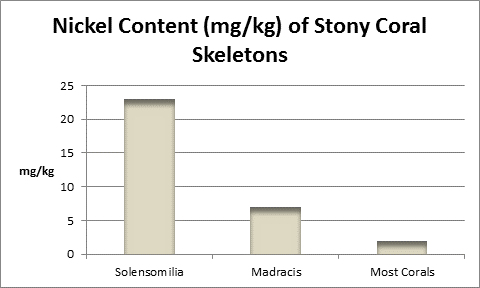
Figure 29. Nickel varies among coral skeletons. In addition to those above, I found 0.43 mg/kg in a Goniopora skeleton.
- Atomic Weight: 58.69
- Concentration in seawater: 0.0001 mg/kg
- As: Ni++
One pound of the transition metal nickel can be found in about 1,000,000,000 gallons of seawater (or 0.0001 mg/kg). It is suspected of being an essential element for life in plants and animals (Standard Methods for the Examination of Water and Wastewater).
Rasmussen et al. (1992) state that, due to structural incompatibility, nickel is only weakly incorporated into the aragonite skeleton (relative to calcium concentrations.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc.
Silver (Ag):
- Atomic Weight: 107.87
- Concentration in seawater: 0.0003 mg/kg
- As: AgCl2-
An atomic absorption spectrometer (with a detection limit of ~2 mg/kg) failed to find any silver in a Goniopora skeleton.
Thorium (Th):
- Atomic Weight: 232.038
- Concentration in seawater: ?
- As: Th(OH)4
Thorium was found in trace amounts in a Lobophyllia hemprichii skeleton by Ohde and Kitano (1981.)
Titanium (Ti):
- Atomic Weight: 47.90
- Concentration in seawater: 1 µg/l
- As: Ti(OH)4
Titanium is in coral skeletons has been reported to be below the detection limit of the instrument.
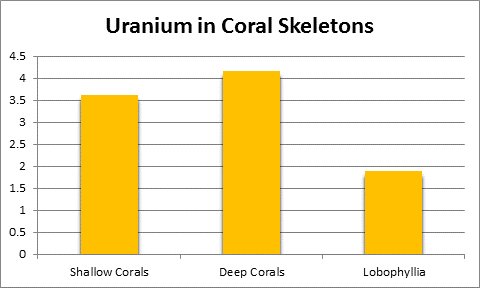
Uranium (U):
- Atomic Weight: 238.07
- Concentration in seawater: 3.3 µg/l
- As: U02(CO3)
Uranium is present in natural seawater at 3.3 µg/l (or 1 pound in 36,000,000 gallons) but values differ among references. It is not essential for life yet is found in coral skeletons and is perhaps an artifact of atomic testing. See Figure 30.
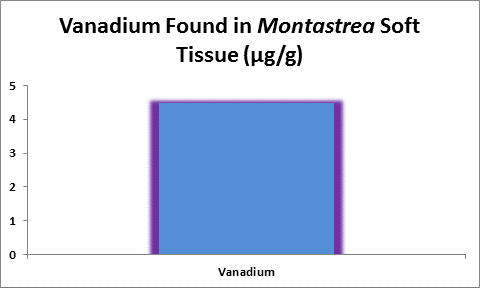
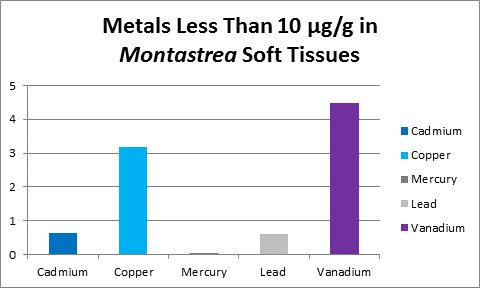
Figure 31. Vanadium concentration in soft tissue of the stony coral Montastrea.
Vanadium (V):
- Atomic Weight: 50.942
- Concentration in seawater: 0.0003 mg/kg
- As: H2VO4- and HVO24-
Vanadium is not essential for higher plants and animals but is necessary for some green unicellular algal species (such as common Scenedesmus species) and some microorganisms. Vanadium deficiencies have been linked to reduced rates of photosynthesis.
Bastidas and Garcia (1996) examined metal content of Montastrea annularis from Venezuelan reefs with ‘high’ and ‘low’ sedimentation rates and found vanadium in its soft tissues.
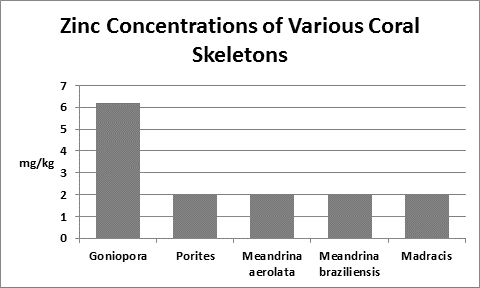
Zinc (Zn):
- Atomic Weight: 65.37
- Concentration in seawater: 0.005 mg/l
- As: ZnOH, Zn++, ZnCO3
Zinc is an essential element for plants and animals. Zinc is found in natural seawater at a concentration of 0.005 mg/l, or 1 pound in 23,980,000 gallons although it can be much higher (0.14 mg/l) in coastal waters. Zinc is a micronutrient important for photosynthesis as well as playing a part as a cofactor in the enzyme carbonic anhydrase which splits carbonates into carbon dioxide for use in the calcification process. Zinc compounds add strength to the jaws of polychaete worms.
Mills (1999) found that zinc deficiency in tobacco plants caused a significant decline in these important enzymes: Manganese SOD (superoxide dismutase), Iron SOD, Copper/Zinc SOD, and APX or Ascorbate Peroxidase.
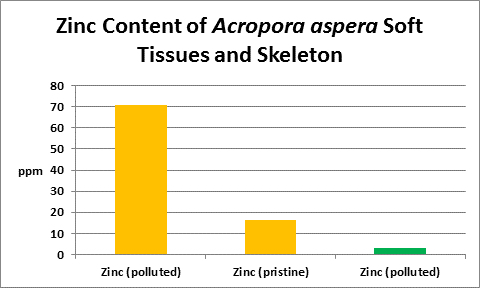
Figure 33. From McConchie and Harriott (1992.) Orange columns are zinc contents of soft tissues; green is of the skeleton.
Rasmussen et al. (1992) state that, due to structural incompatibility, zinc is only weakly incorporated into the aragonite skeleton (relative to calcium concentrations.) However, its content in the skeleton is influenced by element concentration, water temperature, salinity, and species specific factors such as microenvironment (such as coastal or oceanic water or those under the influence of ground or surface fresh water), feeding habits, etc. McConchie and Harriott (1992) found zinc concentrations up to 10 to 18 times higher in coral soft tissues than in skeletons.
Most sea foods are rich in zinc hence using them as feeds could be an important input. In addition, zinc might enter tap water from deterioration of household galvanized iron plumbing. The EPA has a secondary (suggested) standard of 5 mg/l in drinking water.
Interestingly, Goh and Chou (1992) found zinc concentrations of 0.1 and 1 mg/l to significantly inhibit zooxanthellae cell growth. These were grown in seawater enriched with the f/2 formula as well as the zinc. Figure 33 shows the result of tissue analyses of McConchie and Harriott (1992) – zinc concentrations in coral tissues were as high as 70 ppm. We do not know how zinc is partitioned in their samples – was it concentrated in zooxanthellae or coral tissue? We do not know why zinc is high in tissue and low in the skeleton. What is for sure is that corals concentrate zinc in soft tissues and it is not toxic. Do exudates from zooxanthellae chelate this?
Next time we’ll continue our examination of metals and corals, specifically Alkali Metals, Metalloids, and Other Metals.
A complete reference list will be presented at the end of our discussion of metals.




0 Comments