
My interest in coral nutrition began in the late 1980’s, when the reef aquarium hobby in North America was in its nascent stages. At that point in time, the solutions offered to some of the mysteries of ‘mini-reefs’ were technological in nature and centered mostly on water quality parameters. Biological requirements such as lighting of corals and their captive algae were largely unexplored, and debates raged about whether corals actually required feeding. My attempts at keeping ‘difficult’ corals met with poor success rates – I watched several Goniopora specimens waste away over a period of 18 months or so. I was convinced poor nutrition was the problem and began research. That effort resulted in a series of early 1990’s articles in Freshwater and Marine Aquarium magazine.

A Goniopora ingests an amino acid-soaked square of paper in coral nutrition experiments conducted by the author in 1992.
Today, many problems related to coral nutrition have been resolved yet some remain, and even veteran hobbyists still report ‘mysterious’ losses among their captive corals. Is improper nutrition to blame? This series of articles greatly expands upon my works that were published over 20 years ago and provides new insights.
In Part One of this series, we examined the care and feeding of zooxanthellae, including their lighting and nutritional requirements. Part Two reviewed what corals (mostly small polyp stony corals) can ingest or absorb.
This time, we’ll look at the importance of proteins and their amino acid subunits. This article takes a decidedly, yet necessary, technical turn in order to understand coral and other marine invertebrate nutrition.
Introduction
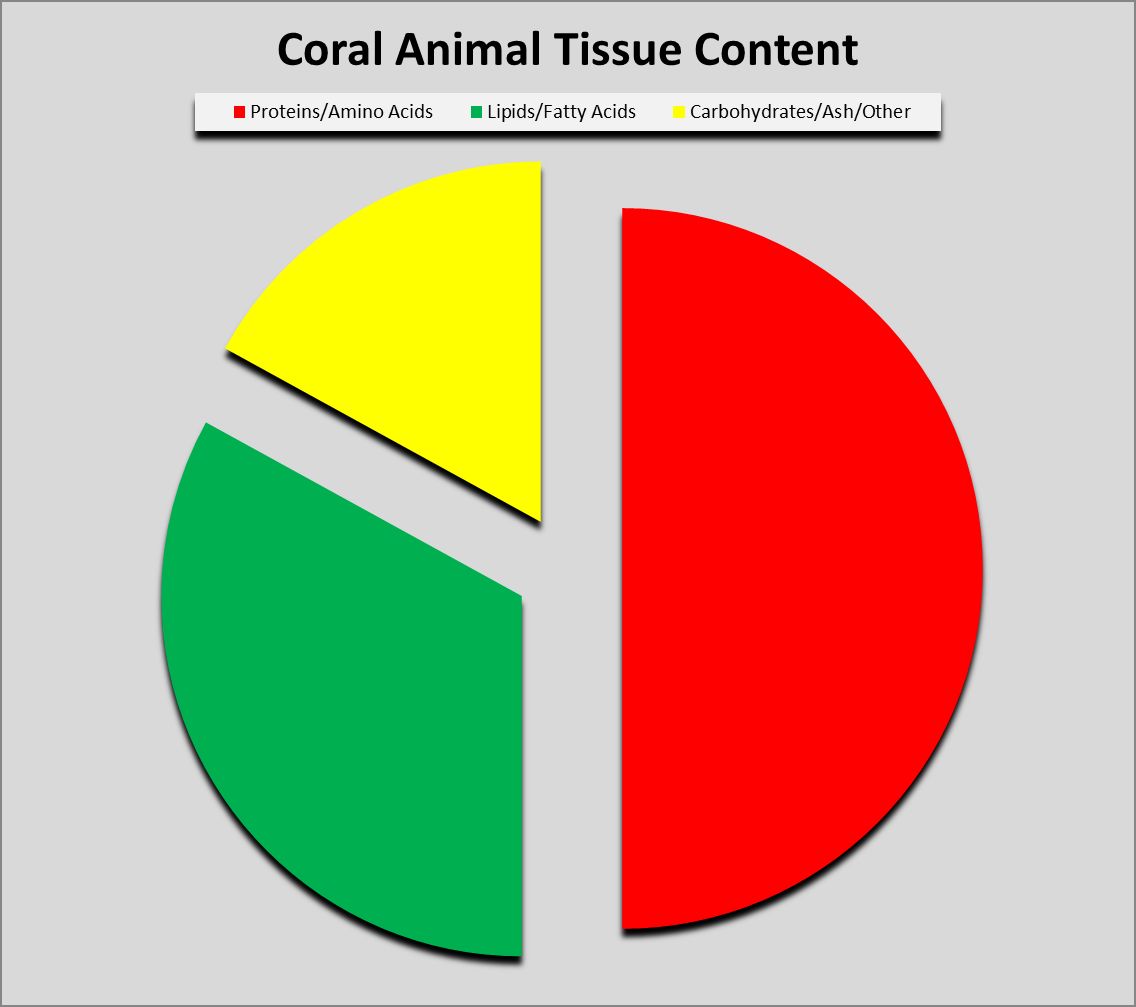
Figure 1. Proteins can compose as much as 50% or more of coral soft tissues.
Protein (from the Greek word prte, meaning ‘first’ or ‘primary’) composes a large amount of dry tissue weight (about 50%) in corals. See Figure 1. Proteins can exist in many forms and have dozens of functions. Muscle is composed of protein fibers, enzymes are specialized proteins that act as biological catalysts, some fibrous proteins act as scaffolds for cytoskeletons, fluorescent proteins (and/or non-fluorescent chromoproteins) which give corals their wondrous colorations, mycosporine-like amino acids provide protection from ultraviolet radiation, and so on. In short, proteins are essential for life.
Proteins are polymers composed of subunits called polypeptides, which in turn are made of ‘building blocks’ called amino acids. Amino acids (AAs) are organic compounds composed of an amine (-NH2) and carboxyl group (-COOH.) All proteins consist of carbon, hydrogen, oxygen, and nitrogen. Some contain phosphorus, sulfur, iron, zinc, and copper. It should be of note that proteins are usually 16% nitrogen by weight.
There are 23 proteinogenic (protein-building) amino acids in prokaryotes (bacteria, for example) and 21 in eukaryotes (animals, as an example.)
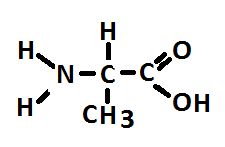
Figure 2. Alanine, an abundant amino acid made by zooxanthellae and potentially translocated to the coral animal. At least some corals make alanine as well.
An amino acid can exist in two forms that are mirror-images of each other. The orientation can be designated as ‘L’ or ‘D.’
Unlike fats or starches, the body does not store excess amino acids; hence they must be consumed regularly. If amino acid ingestion is insufficient, muscle and other tissues will be consumed for their AA content. This condition is seen in starving children – although their bellies may seem fat, just the opposite is true: They have consumed the muscle mass in their abdominal walls and their gut is no longer supported and bulges outwards. This condition is known as ‘kwashiorkor’ (one reference says it means ‘golden boy’ for the pale appearance of a starving person. Others’ definitions differ.)
“Essential” and “Non-Essential” Amino Acids
Plants, algae, bacteria, and fungi are capable of producing all their required amino acids. Animals aren’t so fortunate; hence amino acids can be classified as ‘essential’ and ‘non-essential.’ Essential amino acids are those that must be obtained through diet and in proper quantities, while non-essential are those that the animal can make, given sufficient nutrition (such as nitrogen) is available. Essential amino acids can be species specific.
It is thought that some amino acids were so abundant (and hence easily obtained) during early evolutionary stages that some animals lost their ability to synthesize these amino acids. Some, if not many, corals seem to have taken a different evolutionary path from other metazoans (animals) and have retained the ability to manufacture some amino acids considered as ‘essential’ for humans. And the plot thickens…
Essential Amino Acids Required by Humans:
- Histidine
- Isoleucine
- Leucine
- Lysine
- Methionine
- Phenylalanine
- Threonine
- Tryptophan
- Valine
Nitrogen Sources in Seawater
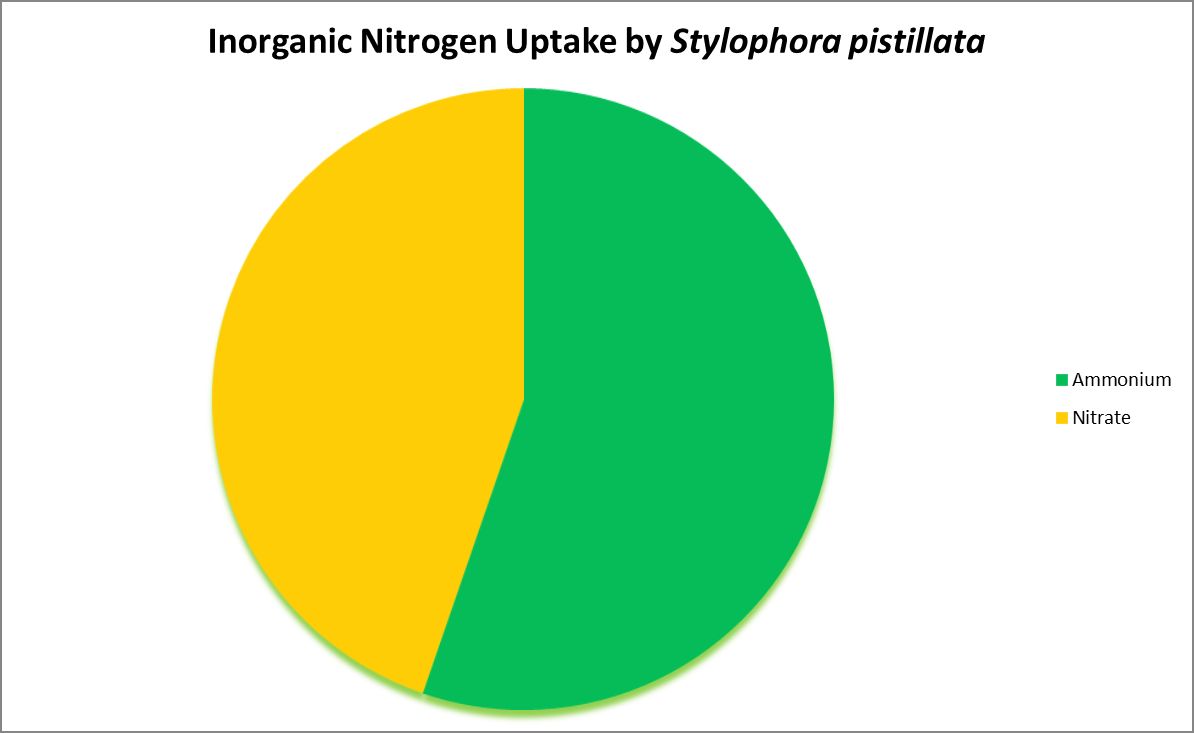
Figure 4. Ammonium is the preferred inorganic nitrogen source for this coral. In some cases, nitrogen-fixing bacteria reside within a coral’s skeleton/tissues and could provide usable nitrogen made from dissolved nitrogen gas.
Nitrogen is an essential element in the formation of amino acids and hence proteins. Usable inorganic nitrogen can exist in several common forms in water: Nitrogen gas (which must be ‘fixed’ by certain bacteria), ammonia/ammonium (species depending upon pH) and nitrate. Organic forms include urea, dissolved free and combined amino acids, and particulates.
Figures 3, 4, and 5 demonstrate uptake of various compounds that contain nitrogen.
Amino Acids: A Concise List
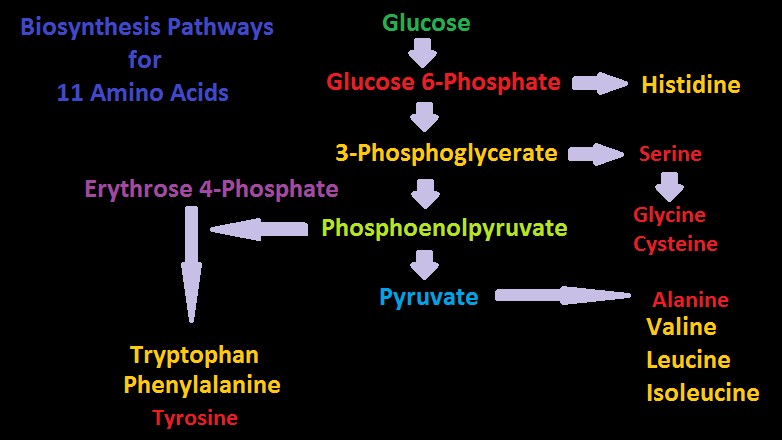
Amino acids will be listed by ‘family’ or ‘group.’ Amino acids within a Family share similar biosynthetic pathways (See Figures 10 and 11.) Those listed as ‘essential’ refers to human dietary requirements except where noted.
The -Ketoglutarate Family
Arginine, glutamic acid, glutamine, proline, and the non-proteinogenic hydroxyproline (included here as it was found in some corals) belong to the -Ketoglutarate Family.
See a greatly simplified biosynthesis pathway for these amino acids in Figure 9.
· Arginine
- Composition: (C6H14N4O2)
- Shorthand: Arg, or R
- Group: Basic
- Essential (Humans): Conditionally essential
Non-essential in humans, but can become essential in some cases (such as disease.) Detected in very small quantities in zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) Arginine is an immediate precursor of ornithine and is necessary for the formation of creatine.
· Glutamic Acid
- Composition: (C6H14N4O2)
- Shorthand: Glu, or E
- Group: Acidic
- Required for Synthesis: Vitamin B-6
- Essential (Humans): No
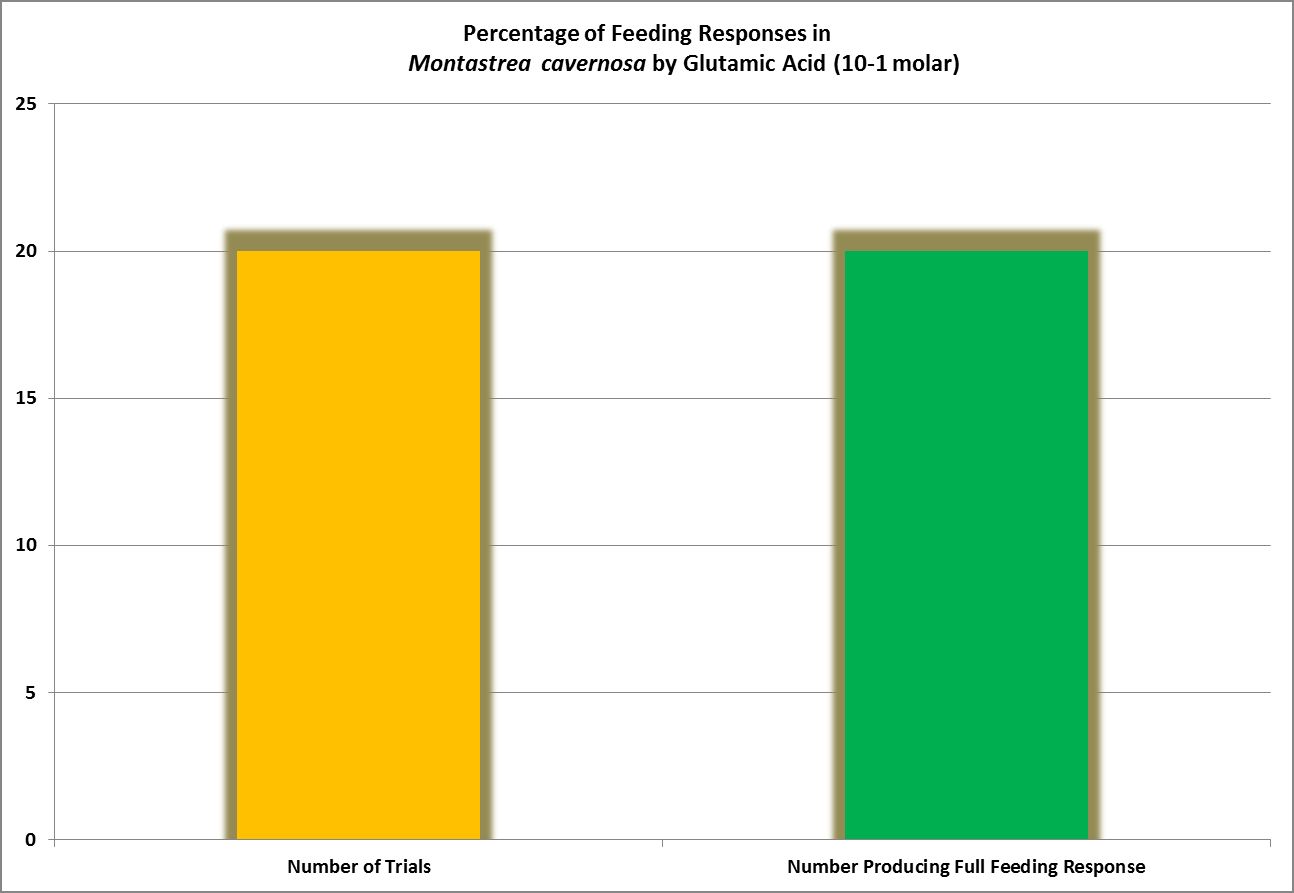
Considered non-essential, this amino acid is the most abundant AA found in the human body. The most successful feeding activator in Montastrea cavernosa (Lehman and Porter, 1973), it also acts as an antioxidant in plants, animals and some bacteria.
· Glutamine
- Composition: (C5H10N2O3)
- Shorthand: Gln, or Q
- Group: Neutral, polar
- Essential (Humans): Conditionally essential in humans. Hydrolysis converts glutamine into glutamate.
· Glx (Glutamine and Glutamate)
Glutamine and glutamate (C5H9NO4); glutamate is non-essential in humans.
Detected in zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.)
· Glutathione
Composition: (C10H17N3O6S)
An important antioxidant and a strong feeding activator in some cnidarians. It is made from the amino acids cysteine, glutamic acid, and glycine.
· Proline
- Composition: (C5H9NO2)
- Shorthand: Pro, or P
- Group: Hydrophobic, non-polar
- Required for Synthesis: Vitamin C
- Essential (Humans): No
Trace quantities detected in zooxanthellate corals Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.)
· Hydroxyproline
- Composition: (C6H9NO3)
- Shorthand: Hyp
- Required for Synthesis: Vitamin C
- Essential (Humans): No
Hydroxyproline is similar to proline, with the difference being hydroxyproline contains a hydroxyl (OH) molecule. It is thought to play a role in the incorporation of silica into diatom skeletons. Hydroxyproline is found in very small quantities in zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata. (Fitzgerald and Szmant, 1997.) However, it is considered non-proteinogenic (it is not found in the genetic code of any organism.)
· Pipecolic Acid
Composition: (C6H11NO2)
An analogue of the amino acid Proline, and used in feeding activator responses by Lehman and Porter (1973.) Interestingly, this substance has been found in meteorites.
The Pyruvate Family
The Pyruvate Group contains alanine, isoleucine, leucine, and valine.
· Alanine
- Composition: (C3H7NO2)
- Shorthand: Ala, or A
- Group: Hydrophobic, non-polar
- Essential (Humans): No
Translocated from zooxanthellae to the coral host. Precursor is glucose. Detected in zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) Non-polar. Alanine is second in abundance only to leucine. Considered non-essential in humans.
· Isoleucine
- Composition: (C6H13NO2)
- Shorthand: Ile, or I
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
Essential amino acids in humans. No feeding response at all Montastrea cavernosa (Lehman and Porter, 1973.)
· Leucine
- Composition: (C6H13NO2)
- Shorthand: Leu, or L
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
Leucine is the most abundant amino acid in nature and is considered essential in humans. Produced in small quantities by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.)
· Valine
- Composition: (C5H11NO2)
- Shorthand: Val, or V
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
Produced in small quantities by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.)
The Oxoloacetate or Aspartate Family
Amino acids found in this family include asparagine, aspartic acid (and hence aspartate), lysine, sulfur-containing methionine, and threonine.
· Asparagine
- Composition: (C4H8N2O3)
- Shorthand: Asn, or N
- Group: Uncharged, polar
- Essential (Humans): No
Non-essential for humans as it is one of the most common of the 20 proteinogenic amino acids.
· Aspartic Acid
- Composition: (C4H7NO4)
- Shorthand: Asp, or D
- Group: Acidic
- Essential (Humans): No Carboxylate and salts of Aspartic Acid is called aspartate.
· Aspartate
- Composition: (C4H7NO4)
- Shorthand: Asp, or D
- Group: Negative charge, polar
- Essential (Humans): No
Aspartate can be formed from Aspartic Acid.
· Asx (Asparagine and Aspartic Acid)
Asparagine and Aspartic Acid. Detected in zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.)
· Carnitine
Derived from lysine and methionine, and important in transporting fatty acid across the mitochondrial wall. Also found in meats.
· Lysine
- Composition: (C6H14N2O2)
- Shorthand: Lys, or K
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
· Methionine
- Composition: (C5H11NO2S)
- Shorthand: Met, or M
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
Considered essential in humans. Methionine contains sulfur. Produced in small quantities by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) Requires selenium and zinc for formation.
· Threonine
- Composition: (C4H9NO3)
- Shorthand: Thr, or T
- Group: Uncharged, polar
- Essential (Humans): Yes
Considered essential in humans. Not detectable by methods used by Fitzgerald and Szmant, 1997.
The 3-Phosphoglycerate Family
The 3-Phosphoglycerate amino acid family contains cysteine (and hence cysteine), glycine and serine.
· Cysteine
- Composition: (C3H7NO2S)
- Shorthand: Cys, or C
- Group: Uncharged or neutral, polar
- Essential (Humans): Conditionally essential
Non-essential in humans, but can become essential in some cases. Cysteine contains sulfur and requires Vitamins B6 and C for formation. Paired cysteines allow disulfide bonds to form. Susceptible to degradation in presence of carbohydrates.
· Cystine
- Composition: (C6H14N2O4S2)
- Essential (Humans): No
Formed by the oxidation of two cysteine molecules.
· Glycine
Composition: (C2H5NO2)
- Shorthand: Gly, or G
- Group: Uncharged, polar
- Essential (Humans): Conditionally essential in humans.
· Serine
- Composition: (C3H7NO3)
- Shorthand: Ser, or S
- Group: Uncharged or neutral, polar
- Essential (Humans): No
Produced by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) Non-essential in humans, but can become so in certain cases. Requires choline (a substance related to the B vitamin group) for formation.
Phosphoenolpyruvate and Erythrose 4-Phosphate, or Aromatic Families
This group contains the amino acids (or amino acid-like) phenylalanine, tryptophan, tyrosine, histidine, and ornithine.
The Shikimic Pathway is involved in the production of aromatic amino acids, including mycosporine-like amino acids (MAAs), which provide some protection from ultraviolet radiation. This pathway is not found in animals hence corals receive MAAs from their zooxanthellae, or through consumption of algae or animals that have consumed algae.
· Phenylalanine
- Composition: (C9H11NO2)
- Shorthand: Phe, or F
- Group: Neutral
- Essential (Humans): Yes
Considered essential in humans. Produced by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) No feeding response at all Montastrea cavernosa (Lehman and Porter, 1973.) Requires Vitamin B6.
· Tryptophan Composition: (C11H12N2O2)
- Shorthand: Trp, or W
- Group: Hydrophobic, non-polar
- Essential (Humans): Yes
· Tyrosine
- Composition: (C9H11NO3)
- Shorthand: Tyr, or Y
- Group: Hydrophobic, non-polar
- Essential (Humans): Conditionally essential
Produced by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) No feeding response at all Montastrea cavernosa (Lehman and Porter, 1973.) Vitamin B6 required for synthesis.
· Histidine
- Composition: (C6H9N3O2)
- Shorthand: His, or H
- Group: Basic
- Essential (Humans): Yes
Considered essential for humans. Produced by zooxanthellate corals Montastrea faveolata, Acropora cervicornis, Porites divaricata, and azooxanthellate corals Tubastrea coccinea, and Astrangia poculata (Fitzgerald and Szmant, 1997.) No feeding response at all Montastrea cavernosa (Lehman and Porter, 1973.) Histidine is somewhat of an oddity among amino acids. It is considered by some to be an ancient relic from the evolutionary transition from RNA-based to protein-based life forms.
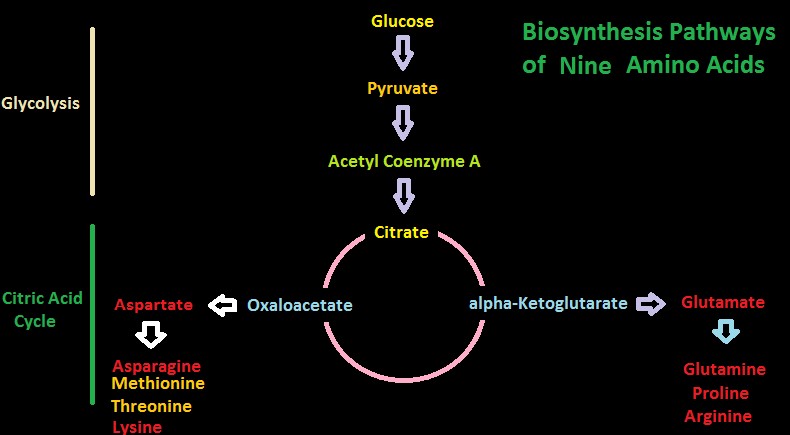
Figure 10. Two biosynthesis pathways for nine amino acids – those amino acids listed in yellow are considered essential in amino acids. Do corals possess these?
· Ornithine
- Composition: (C5H12N2O2)
- Shorthand: Orn
- Group: Basic
- Essential (Humans): No
Non-essential, but can be under some conditions. Ornithine is an important part of the Urea Cycle. It can be synthesized by some bacteria from L-glutamate.
· Taurine
- Composition: (C2H7NO3S)
- Shorthand: Tau
Taurine is a major component in animal tissues although it is not a true amino acid (it lacks a carboxyl group.) The amino acid cysteine is a precursor.
Pathways for Biosynthesis of Amino Acids
Figures 10 and 11 show greatly simplified pathways for synthesis of 20 amino acids.
Amino Acids – Which Ones Do Corals Need?
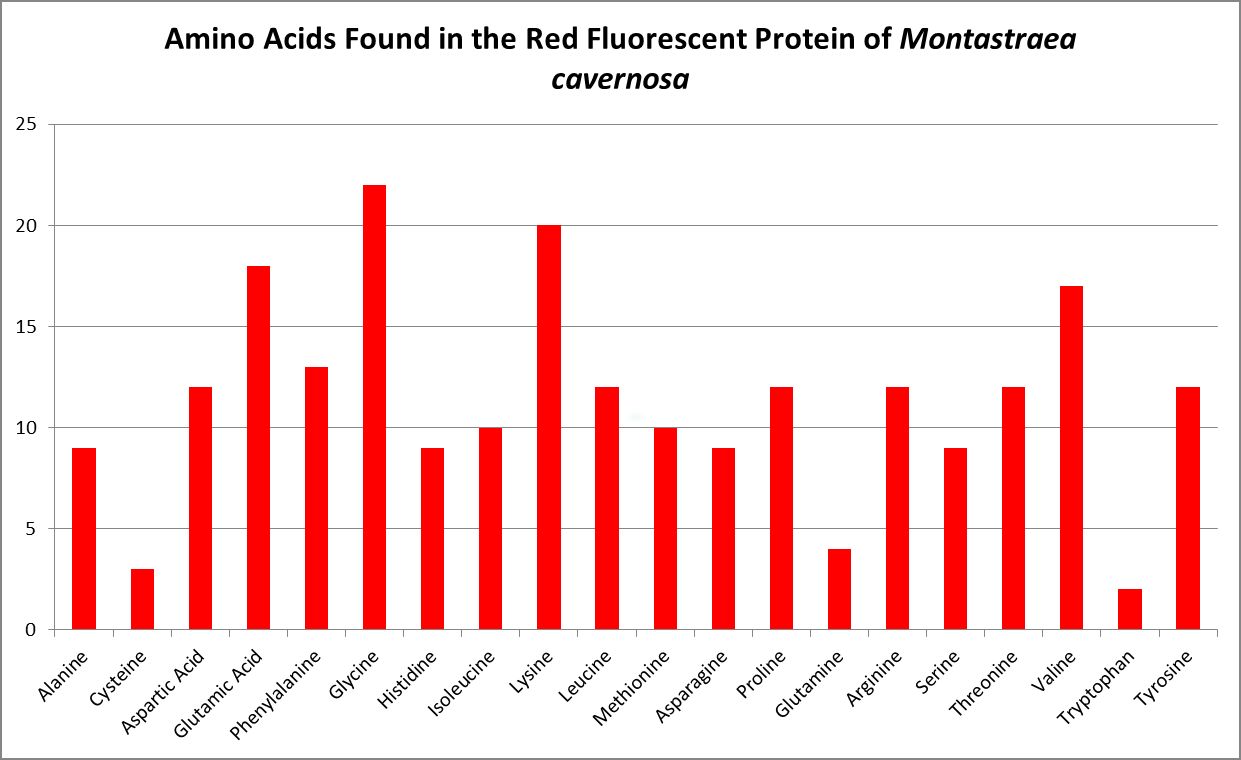
Figure 12. The genetic code of this fluorescent protein contains 20 amino acids. Chalfie and Kain, 2006.
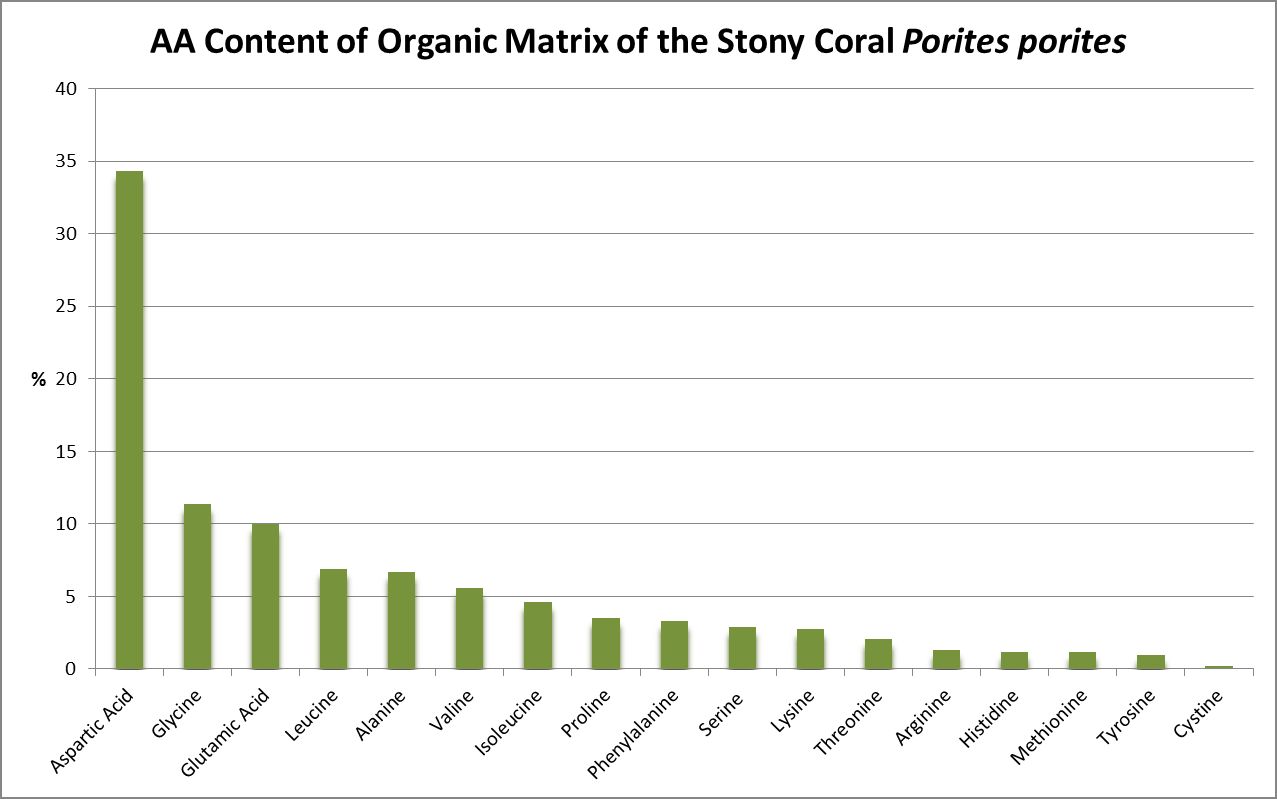
A little investigation answers this question – corals need all proteinogenic amino acids. If all are needed, then it is the proportions that become important. Figure 12 shows the amino acid content of a fluorescent protein made by the Atlantic stony coral Montastrea cavernosa. Figure 13 shows the amino acid content of the organic matrices of Caribbean stony corals Acropora cervicornis and Acropora palmata; Figure 14 is that of Porites porites, while Figure 15 shows AA contents of matrices found in a number of Atlantic marine invertebrates.
Since the coral animal produces these colorful proteins, we can safely assume corals need all amino acids required for building proteins. I’ll have much more to say about amino acids and coral coloration in a future article. But which are essential?
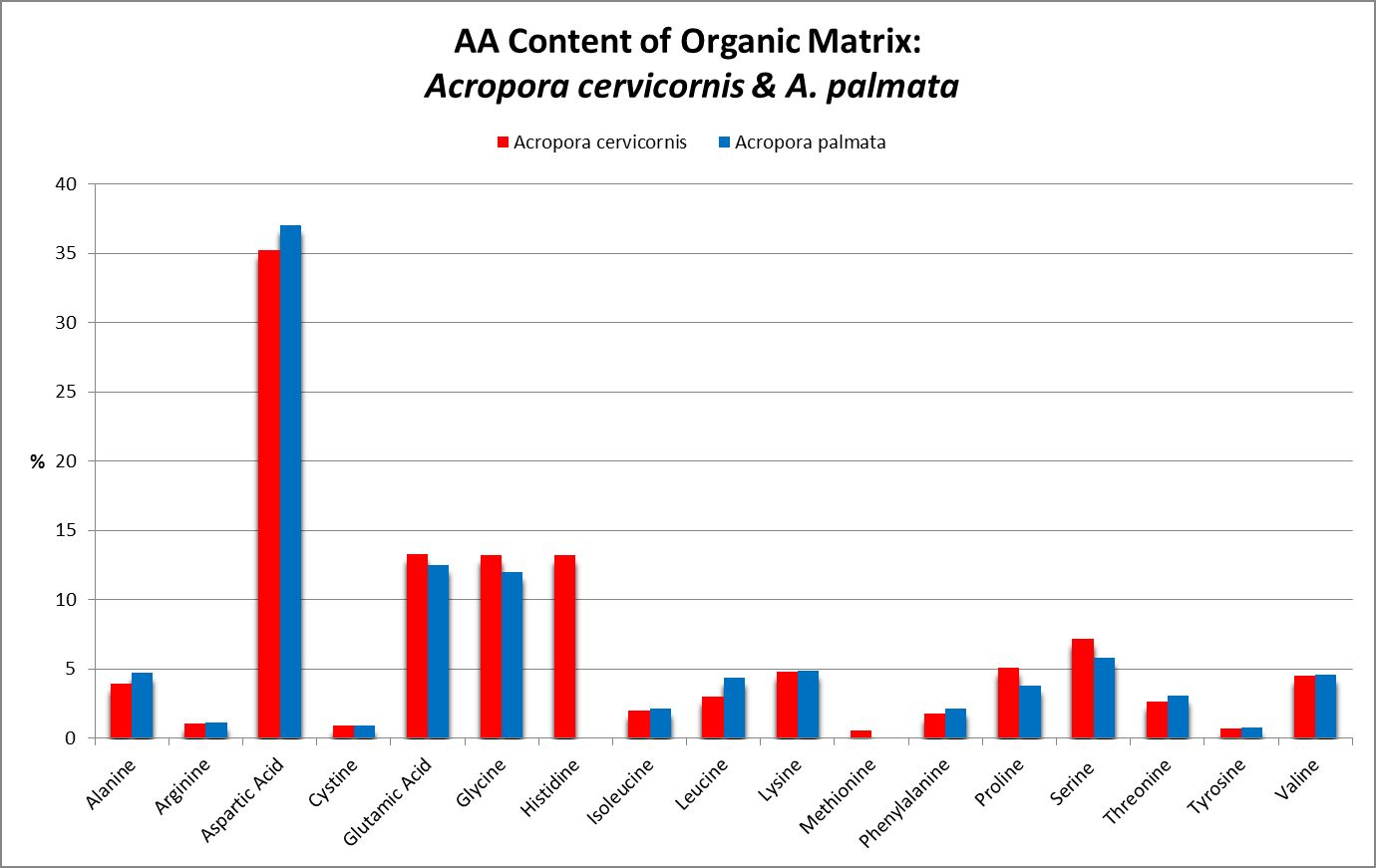
Figure 13. Note the differences in these two matrices – Acropora palmata lacks histidine and methionine.
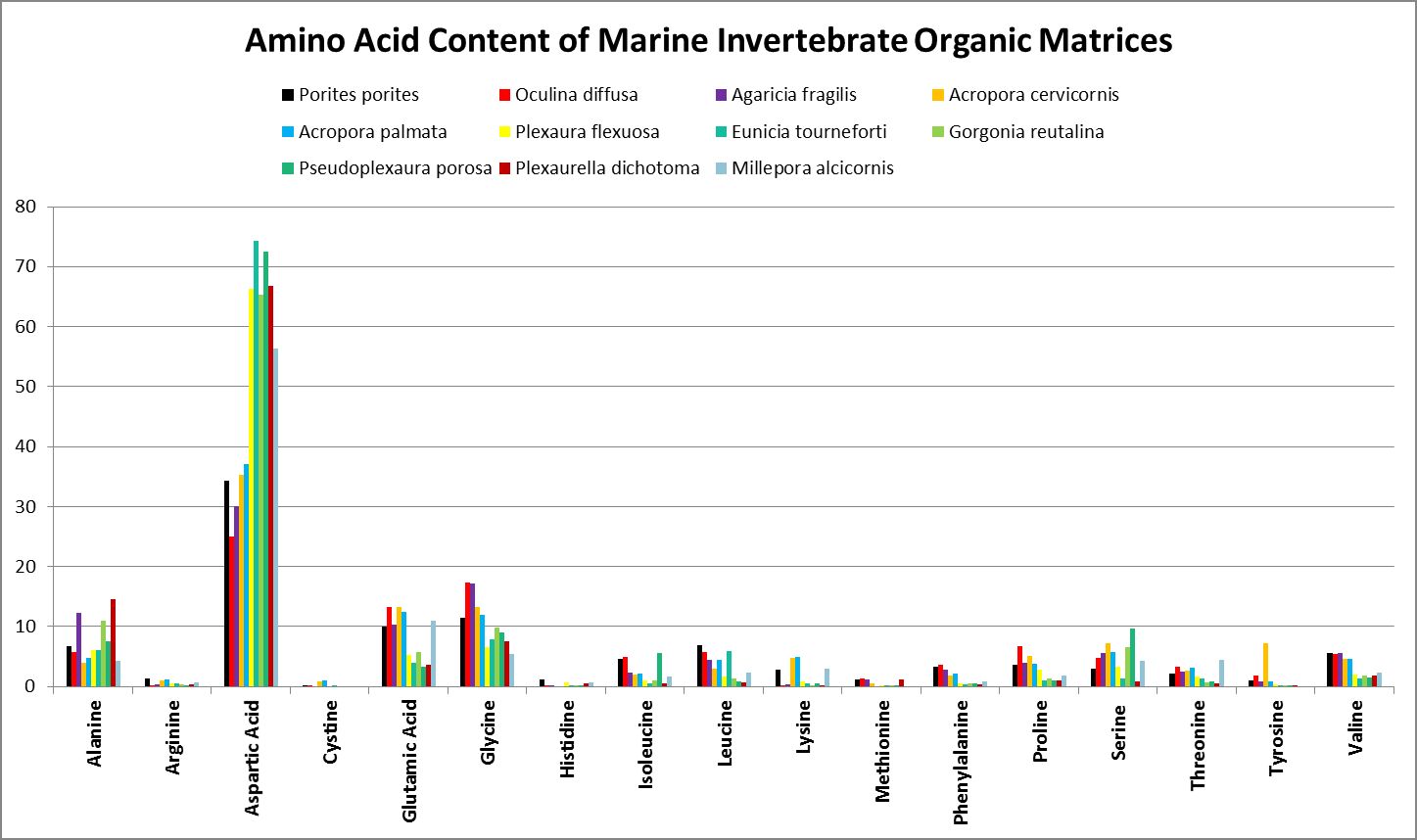
Figure 15. Aspartic acid is the number one amino acid in all organic matrices tested. It is not an essential amino acid.
A Glance at Amino Acid Requirements of Two Fishes
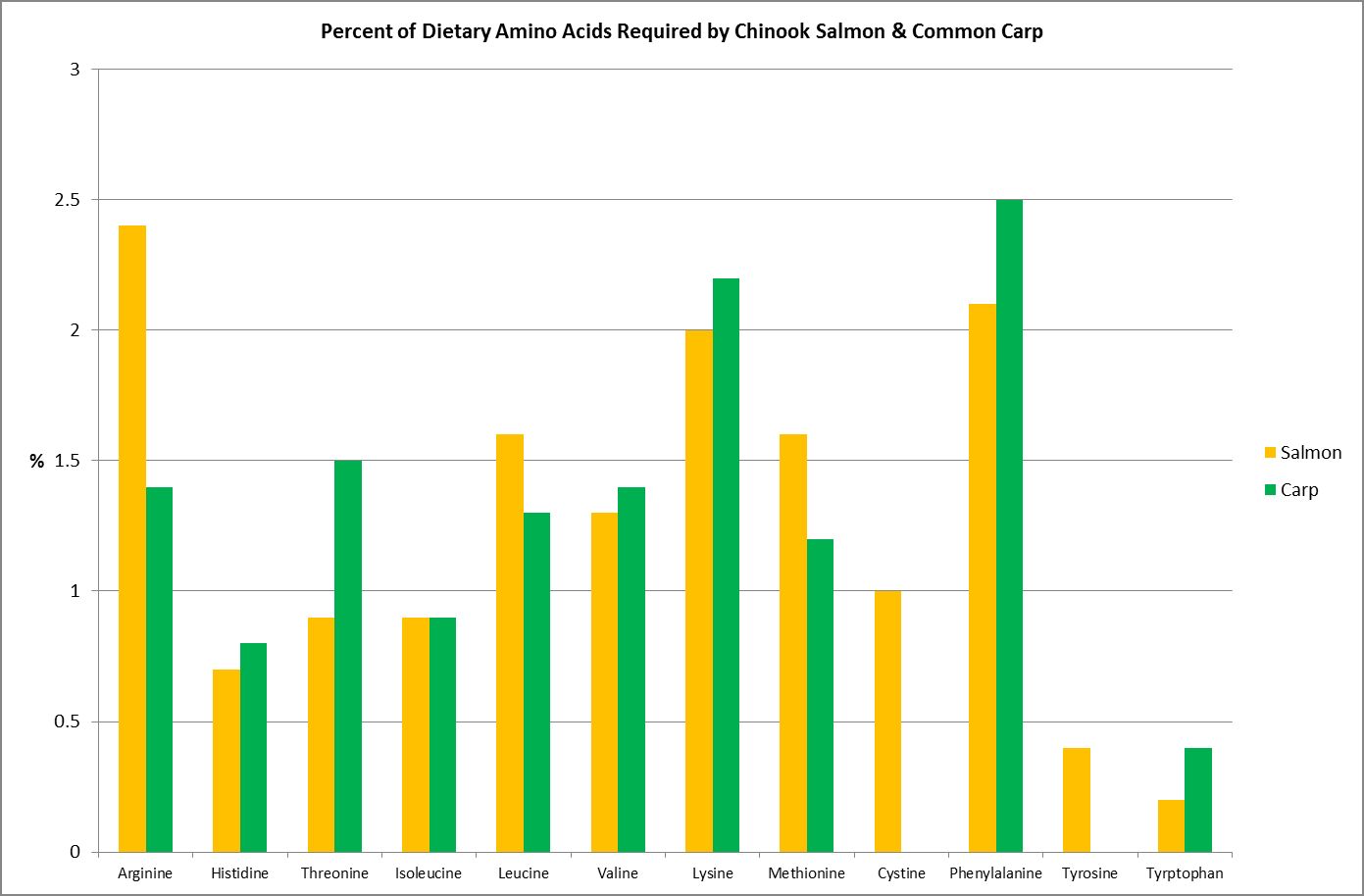
Figure 16. Both these fishes require their diet consist of ~40% protein. These are their essential amino acid requirements. From Spotte, 1992.
Although this article will attempt to mesh together pieces of the puzzle as to which amino acids are ‘essential’ to cnidarians, much research is needed before the picture is complete. It would be easy to assume those amino acids considered essential to Homo sapiens would be the same for another animal, such as, say, Acropora millepora. Unfortunately, such is not the case. Study of various research papers reveals that amino acid requirements are species specific.
As an example, Figure 16 shows the amino acid requirements of two fishes – the Chinook Salmon requires two amino acids (cystine/cysteine and tyrosine) that the Common Carp does not.
Synthesis by Zooxanthellae
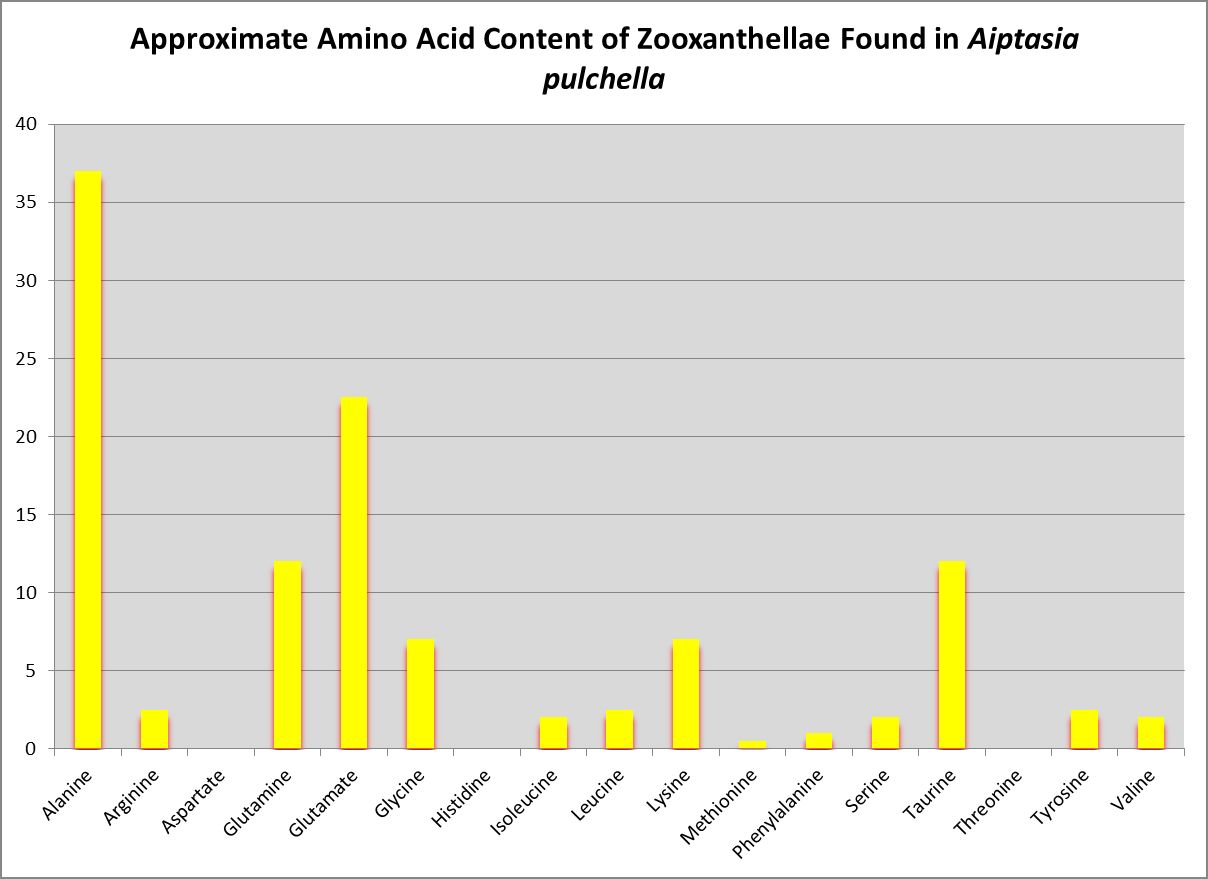
Figure 17. Little wonder zooxanthellae translocate alanine to their host. Zooxanthellae clade is likely B or B1.
Nature loves to confound us, and the symbiotic relationship between Symbiodinium species/clades and the coral animal and the nutritional implications is a good example.
Translocation of Photosynthetic Products
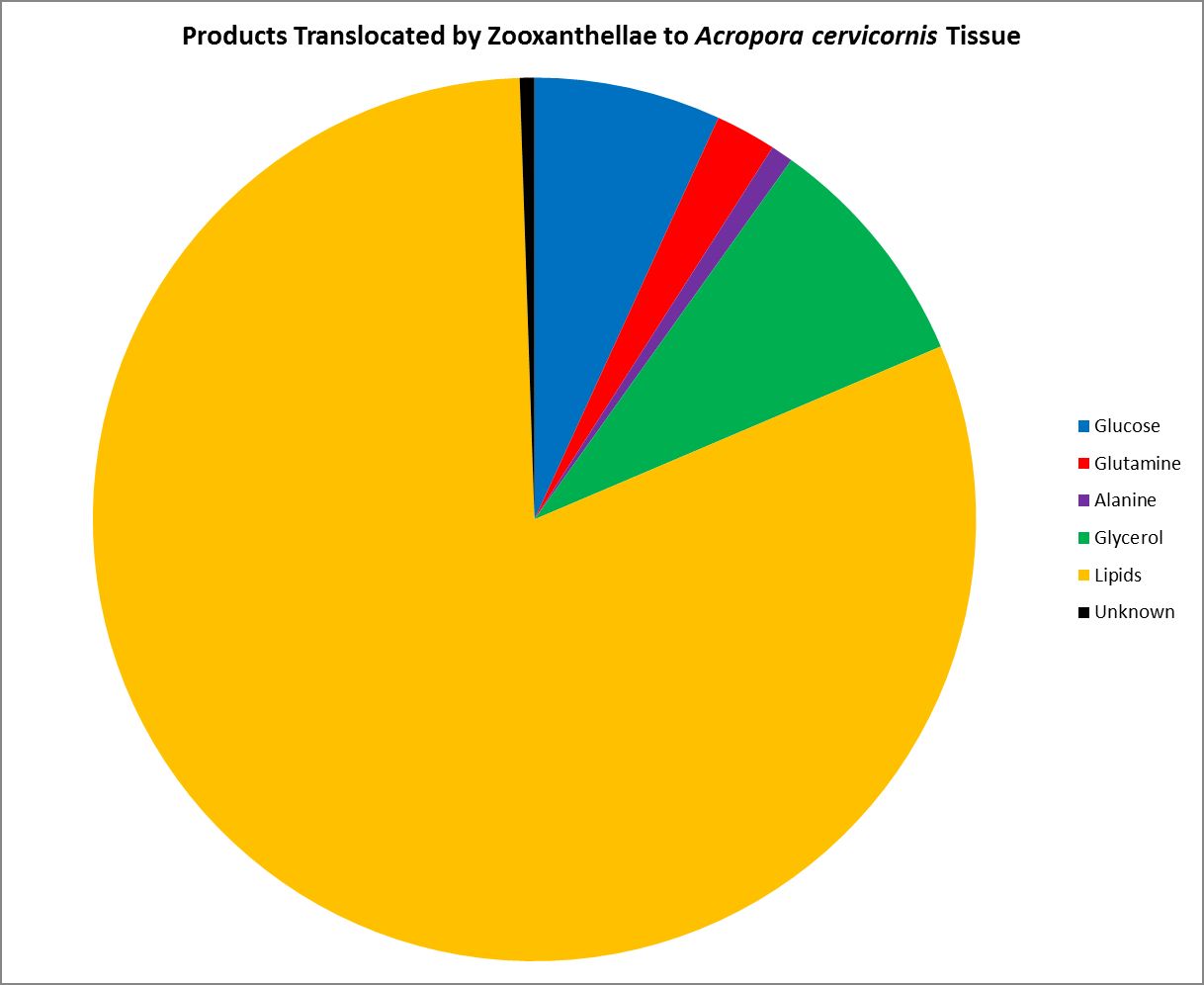
As we know, Symbiodinium (zooxanthellae) are considered primary producers, hence they can produce all their nutritional needs given proper conditions. Symbiotic zooxanthellae are known to be ‘leaky’ with some of the substances they produce. Figure 18 shows those substances shared (translocated) by zooxanthellae with its Atlantic coral host Acropora cervicornis. Most of the translocated product is lipid (81%), while two neutral amino acids (alanine and glutamine) stand at 1% and 2%, respectively.
Synthesis of Amino Acids by Cnidarians
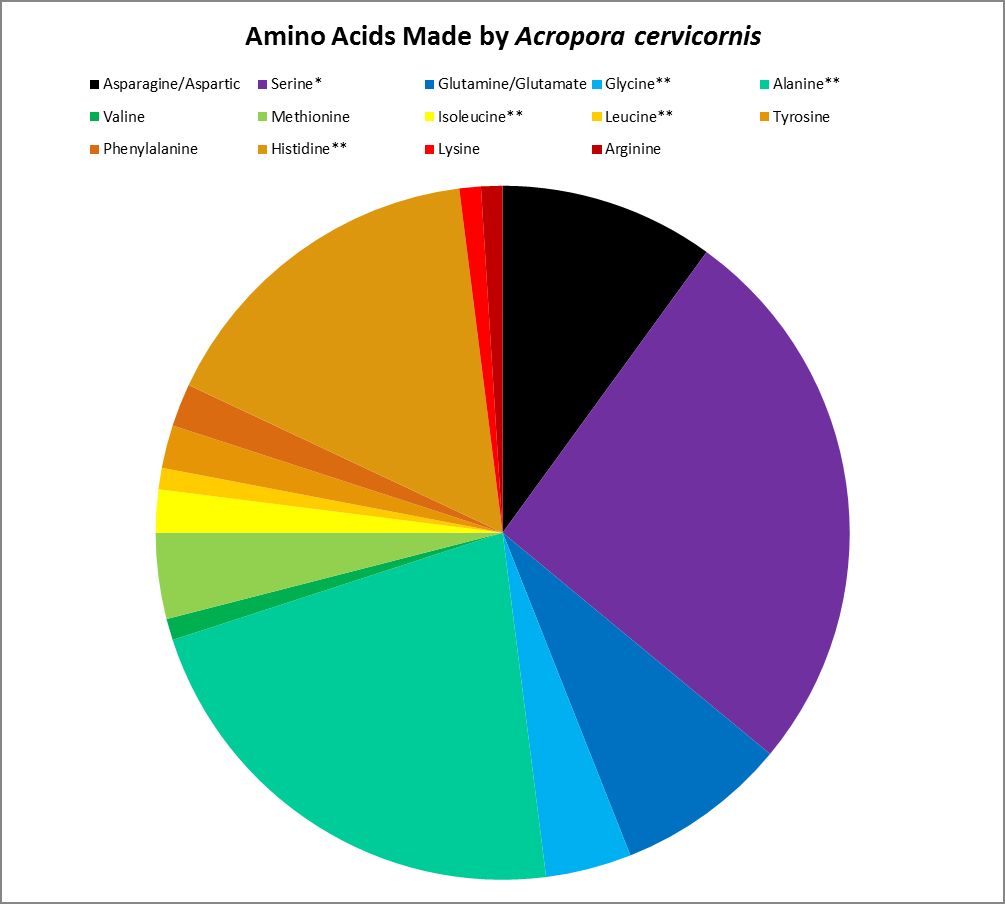
Figure 19. Proteinogenic amino acids synthesized by the Atlantic coral Acropora cervicornis. ** = Not attributed to bacterial synthesis. * = Possibly underestimated. Methodology used incapable of detecting tryptophan and cysteine. This coral undoubtedly produces more amino acids than tested for.
Before reviewing some of the works on amino acid synthesis by corals, we should remember that bacteria can produce all amino acids. While researchers have taken steps to suppress bacterial activity in or on corals using antibiotics and aggressive rinsing, some bacteria undoubtedly remain, raising the possibility that they are the producers and not the cnidarian host. Still, this would be important, as production of amino acids by bacteria and translocation to the coral host could be an important source of nutrition.
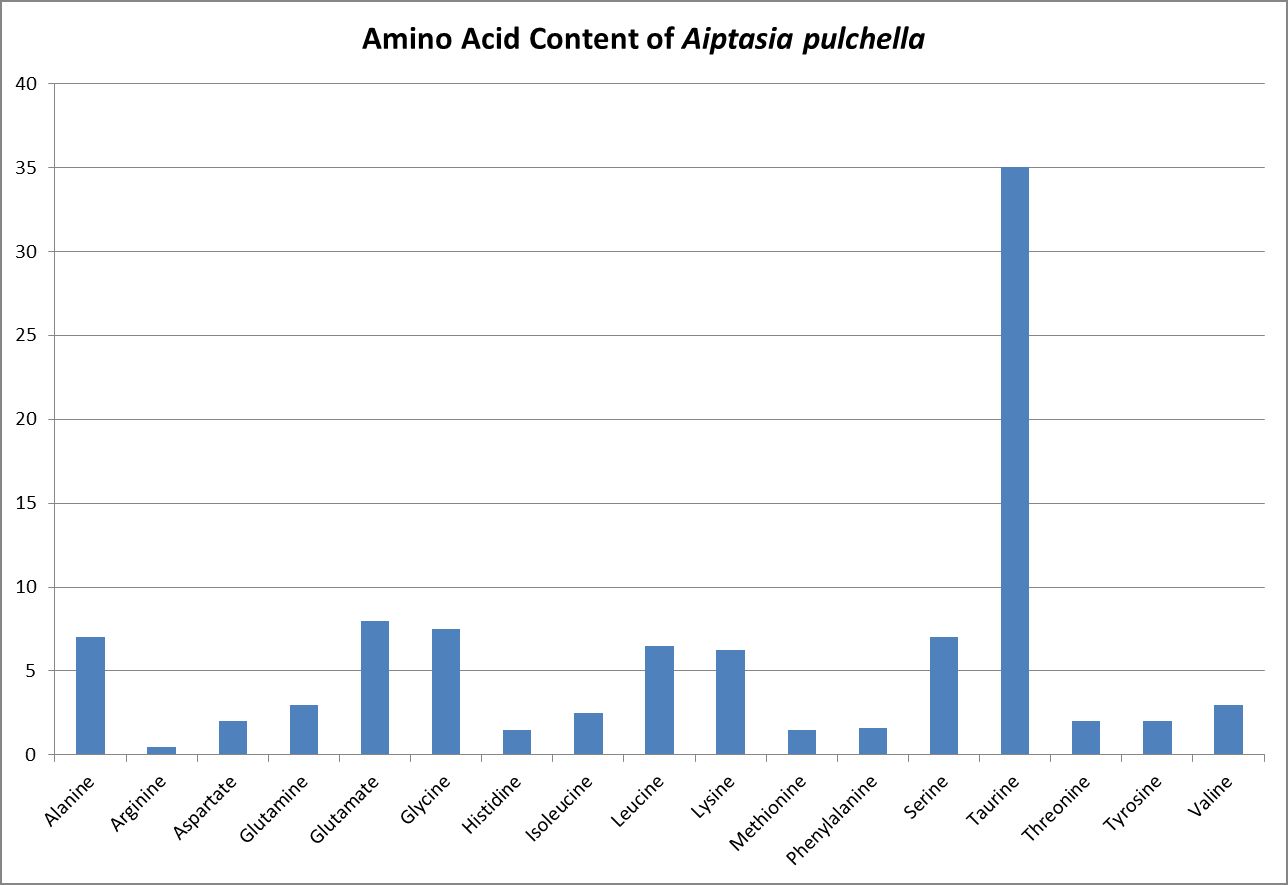
Wang and Douglas (1999) found the anemone Aiptasia pulchella produce amino acids generally considered to be essential for animals – methionine and threonine.
Uptake of Amino Acids by Marine Invertebrates
Once we have an idea of which amino acids are produced by zooxanthellae and by the host, we can look at another method marine animals obtain them – diffused and carrier mediated transport.
Dissolved Amino Acids
Generally, amino acids dissolved in water can exist in two forms: Combined and Free.
Dissolved Combined Amino Acids (DCAA)
Amino acids can be adsorbed to humic and fulvic acids (major substances of soil resulting from decaying organic matter), clay particles and other substances. These are known as dissolved combined amino acids.
Dissolved Free Amino Acids (DFAA)
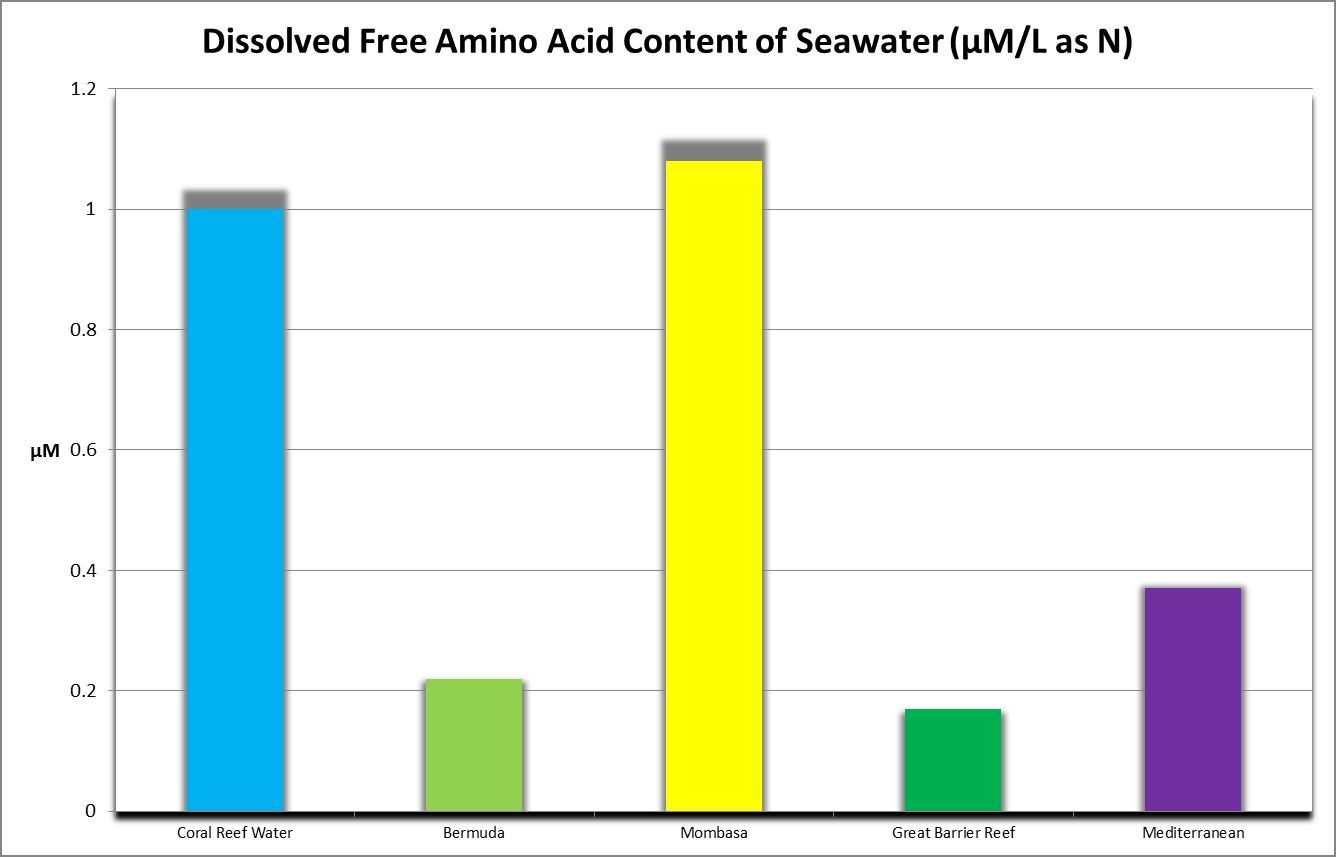
Discrete amino acids (those not adsorbed to other substances) that are dissolved in the water column are known as free amino acids. Grover et al. (2008) report oligotrophic Mediterranean seawater contains 0.11 to 0.37 µMol/L DFAA as N, with an average of 0.24 µMol/L, or about 0.003 mg/L as Nitrogen. Figure 20 shows DFAA concentrations from various locations.
Importance of Water Motion
Astute readers will recognize a common thread throughout these articles – that of water velocity. Water velocity controls the thickness of the boundary layer (the higher the water flow across the coral, the thinner the layer of stagnant water – the boundary layer – surrounding the coral.) This is importance as diffusion of substances (such as ammonia/ammonium and dissolved amino acids – good sources of nitrogen so the coral can build proteins) is hampered by poor water flow and a thick boundary layer. Perhaps more importantly, corals can sense water velocity and hence control expansion. If water velocity is too low, the coral will retract it polyps since the energy required to keep the polyp expanded is greater than the energy supplied by food capture. Not surprisingly, food capture rates are finely tuned to water flow rates (see Riddle, 2014 for details.) On the other hand, polyps are retracted when water velocity is too great and they are in danger of being damaged. Water velocity is sufficient when coral polyps look fields of grain rippling in a gentle breeze.
Uptake of Amino Acids – Active Transport or Diffusional Transport?
Do corals selectively transport dissolved amino acids as opposed to simple diffusion transport? The answer is yes, at least in the case of the stony coral Galaxea fascicularis (Al-Moghrabi et al., 1992.) This coral expends energy to selectively consume neutral amino acids.
Neutral Amino Acids – Some Corals Uptake These Preferentially:
- Alanine
- Glycine
- Isoleucine
- Leucine
- Methionine
- Phenylalanine
- Proline
- Tryptophan
- Valine
We can remember these using this rather amusing mnemonic: GAV LIMP TP, or a guy named GAVin LIMPs because he has Toilet Paper on his shoe.
How Does Selective Amino Acid Uptake Work?
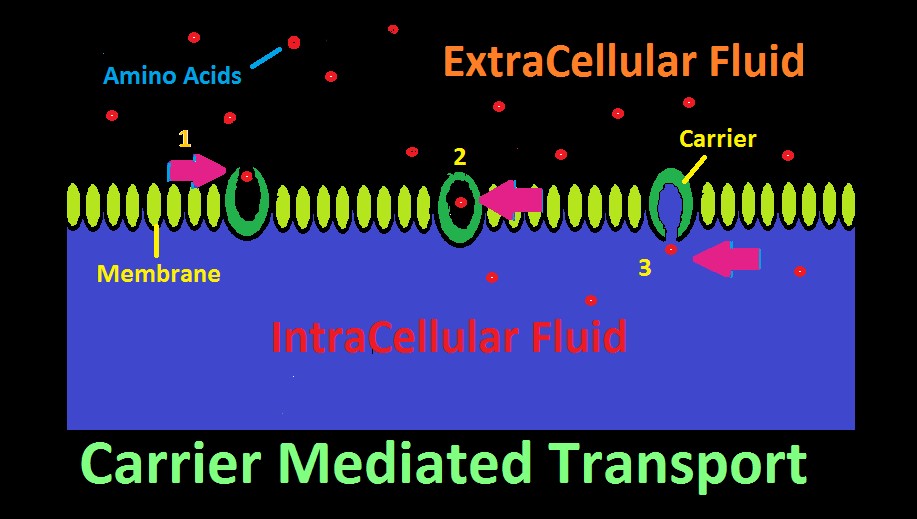
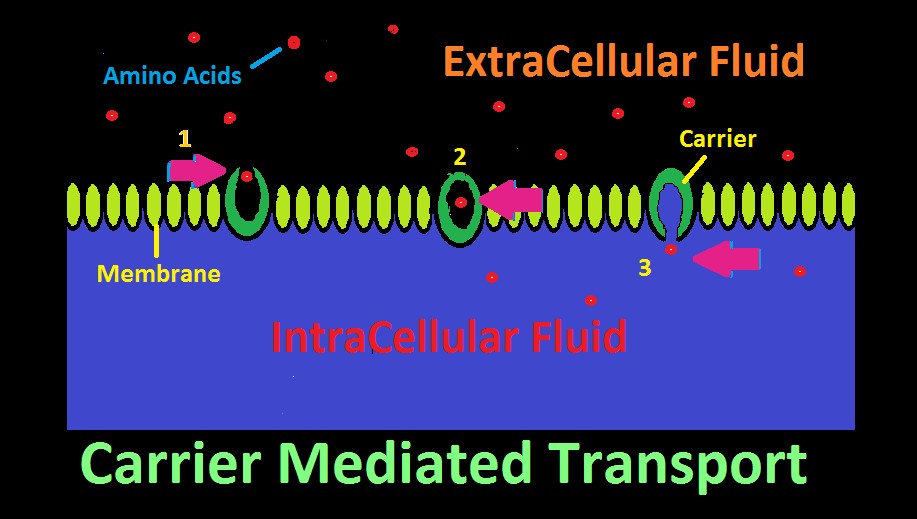
Figure 22. Carrier Mediated Transport. In this cartoon, amino acids are selectively transported across a membrane.
Many aquatic animals selectively consume dissolved substances via a process called Carrier Mediated Transport. This mechanism allows consumption of larger molecular substances, as opposed to smaller molecules such as oxygen, carbon dioxide, etc. which can pass through a membrane with relative ease.
The ‘carrier’ is a protein with an affinity for a certain substance, and has two openings, although only one might be open at a time. The carrier acts as a gateway for a substance to cross a membrane. See Figure 22 represents how an amino acid moves from extracellular fluid to inside a cell. To the right in the diagram, the carrier’s ‘door’ to extracellular fluid is open, and a specific amino acid (or type of amino acid) is attracted and enters. Once inside, the carrier’s door closes. In step 3, the door to the inside of the cell has opened, and the amino acid has successfully migrated across the cell’s membrane.
Uptake of Free Amino Acids
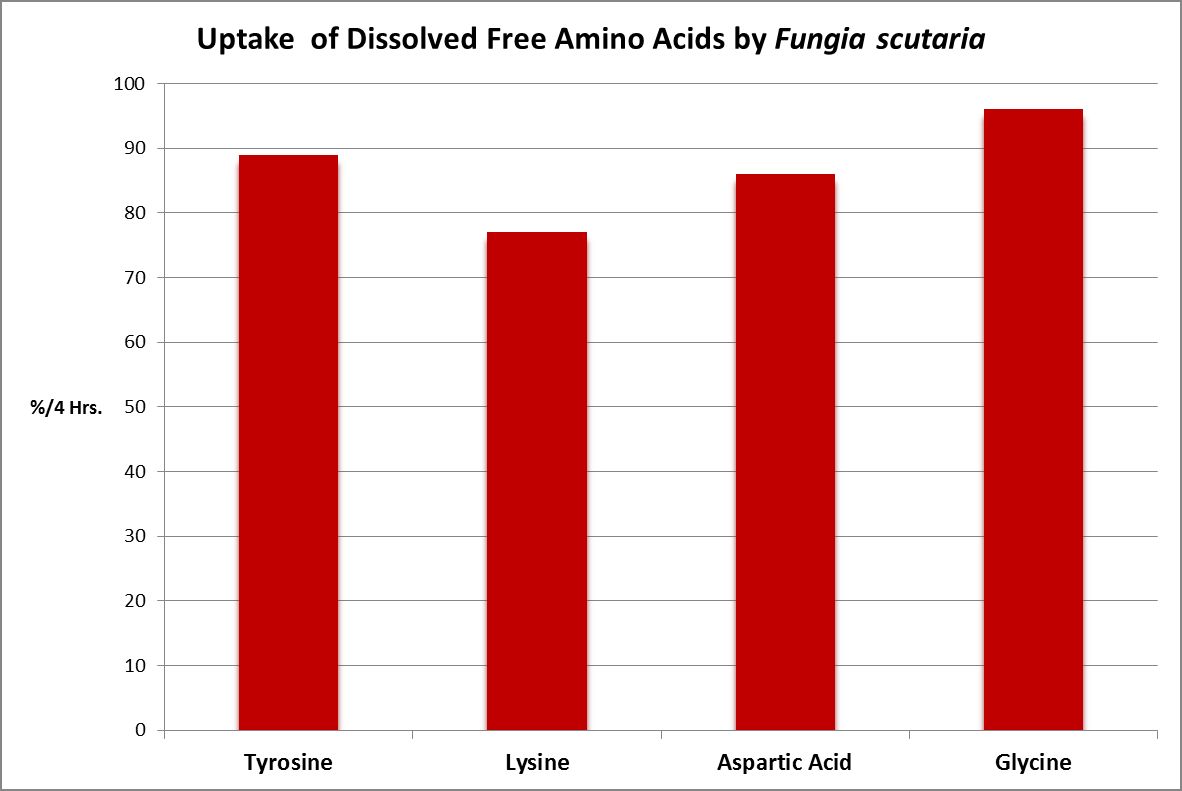
Figure 23. The stony coral Fungia scutaria is capable of absorbing amino acids from the water column. Tyrosine, lysine, and glycine are neutral amino acids, and aspartic acid is acidic. From Stephens, 1962.
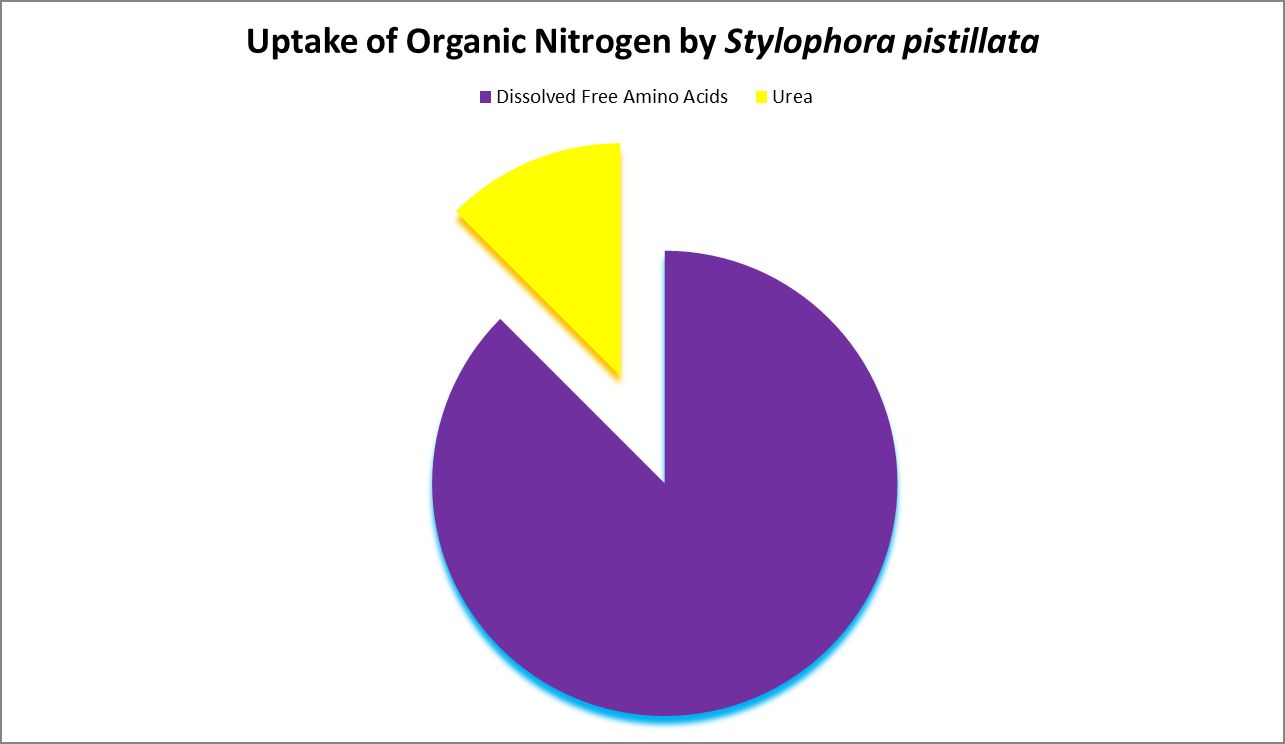
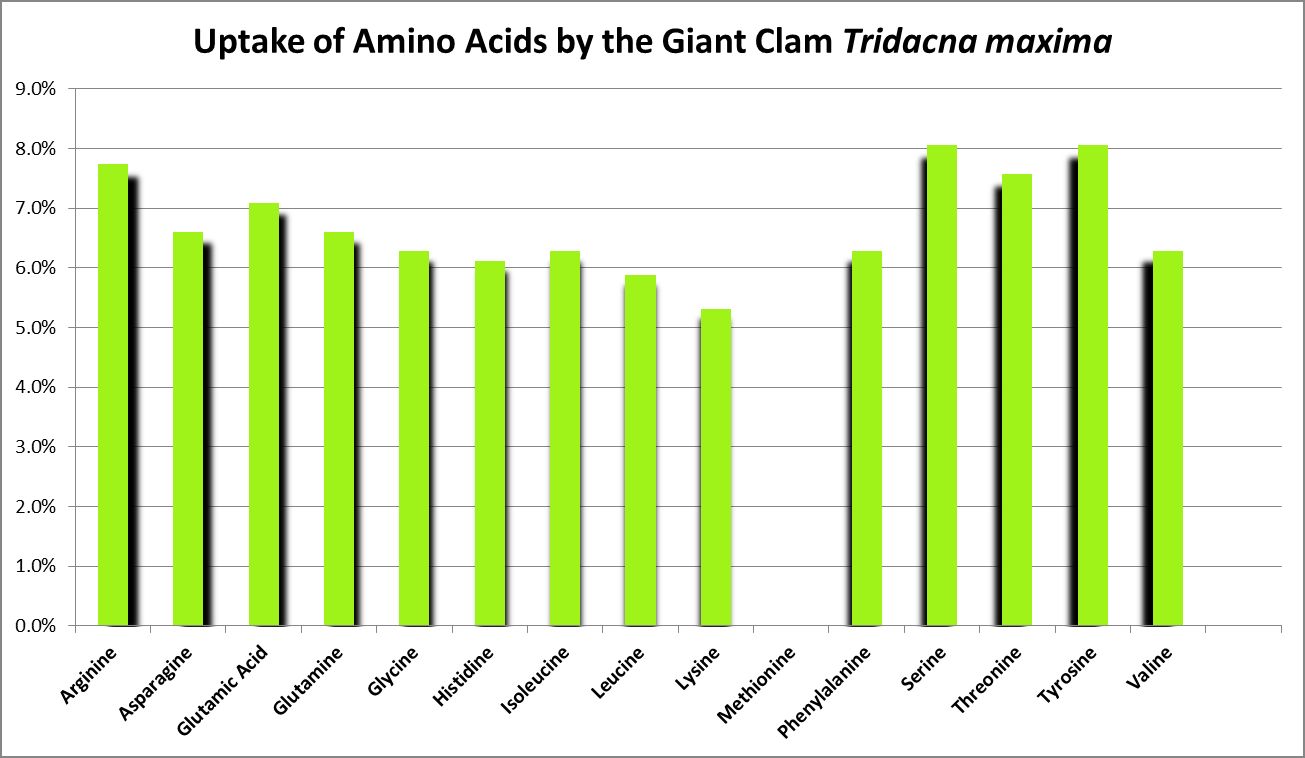
A number of studies have examined the uptake of free amino acids by corals. This is potentially a decent source of nitrogen scarce in ogliotrophic environments for some corals (Figures 23 and 24), but not for Tridacna clams (Figure 25.) By extension, we might consider free AA’s a poor source of nitrogen in fleshy stony corals.
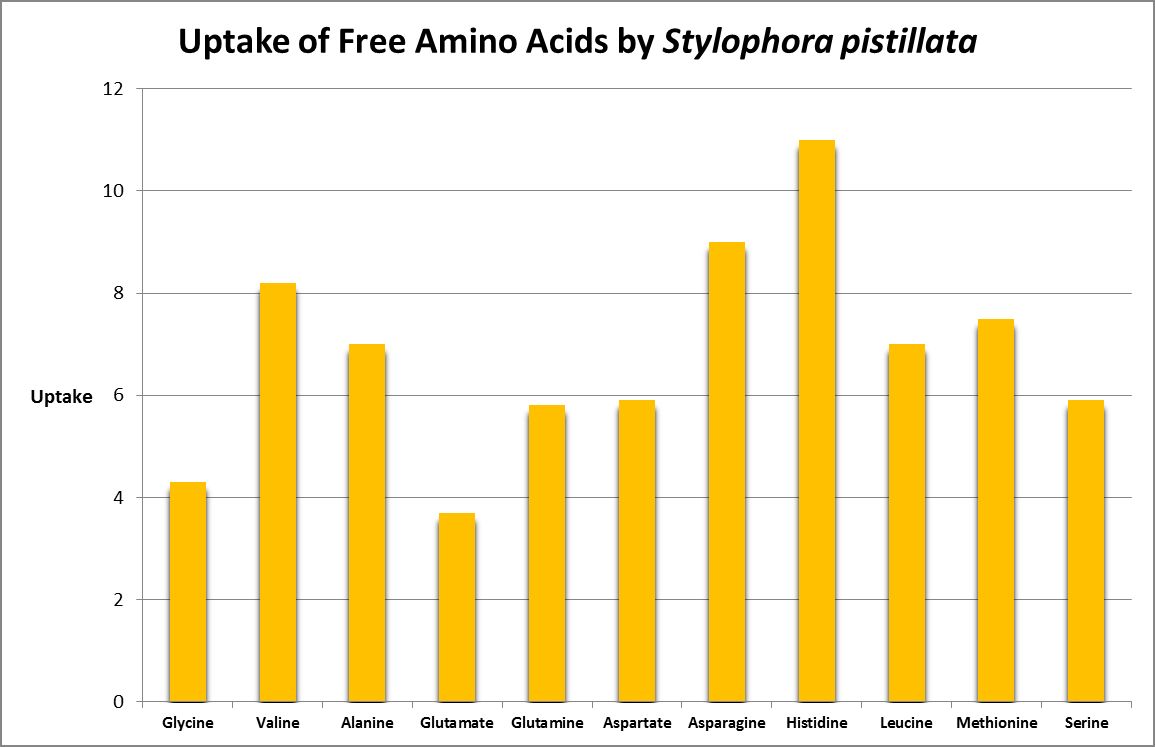
Figure 24. Histidine is most strongly absorbed by this stony coral. From Grover et al., 2008.
Effects of Light Intensity on Uptake of Free Amino Acids
Light intensities influence DFAA uptake in the stony corals Heliofungia actiniformis (Baker, 1994) and Pocillopora damicornis (Moreno & Hoegh-Guldberg, in Ambariyanto and Hoegh-Guldberg, 1999.) Lower uptake in light thought to be due to relatively high concentrations of amino acids translocated from zooxanthellae to the host.
Ambariyanto and Hoegh-Guldberg (1999) found that the giant clam Tridacna maxima aborbs 16 amino acids. Interesting, a chart in this work states there is no uptake of methionine in high light conditions (1,000 – 1,300 µmolm²sec), but there is during periods of darkness. The highest uptake across the 4 conditions of these tests was arginine, while the lowest was histidine. Initial amino acid concentrations were 0.4 µM. (I think the chart in this work may be incorrect due to the absence of uptake of methionine, but approximate concentrations from that work are shown in Figure 25.) The researchers concluded that the uptake of AAs by this clam is not a significant source of nitrogen.
Feeding Activators
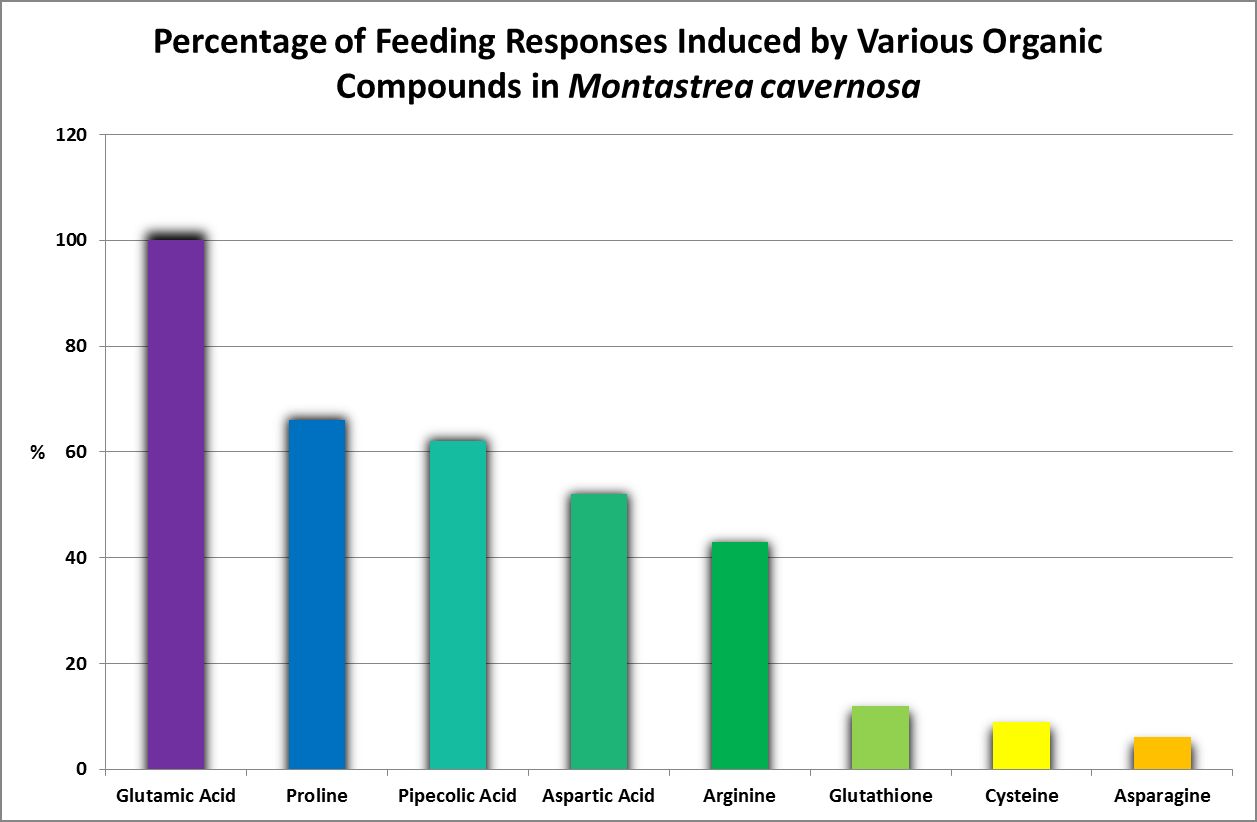
Figure 26. Oddly, none of these amino acids are considered essential for humans. This work was done years before production of amino acids by corals was examined by other researchers.
Feeding activators are those substances that produce a feeding response (ingestion.) These experiments are easily performed. Small squares of Whatman filter paper are soaked in dilute solutions of amino acids and placed on the oral disk or near the mouth(s) of corals (a photo of a Goniopora specimen ingesting such a small bit of AA-soaked lab paper is at the introduction of this article). Feeding activators are shown in Figures 26 and 27.
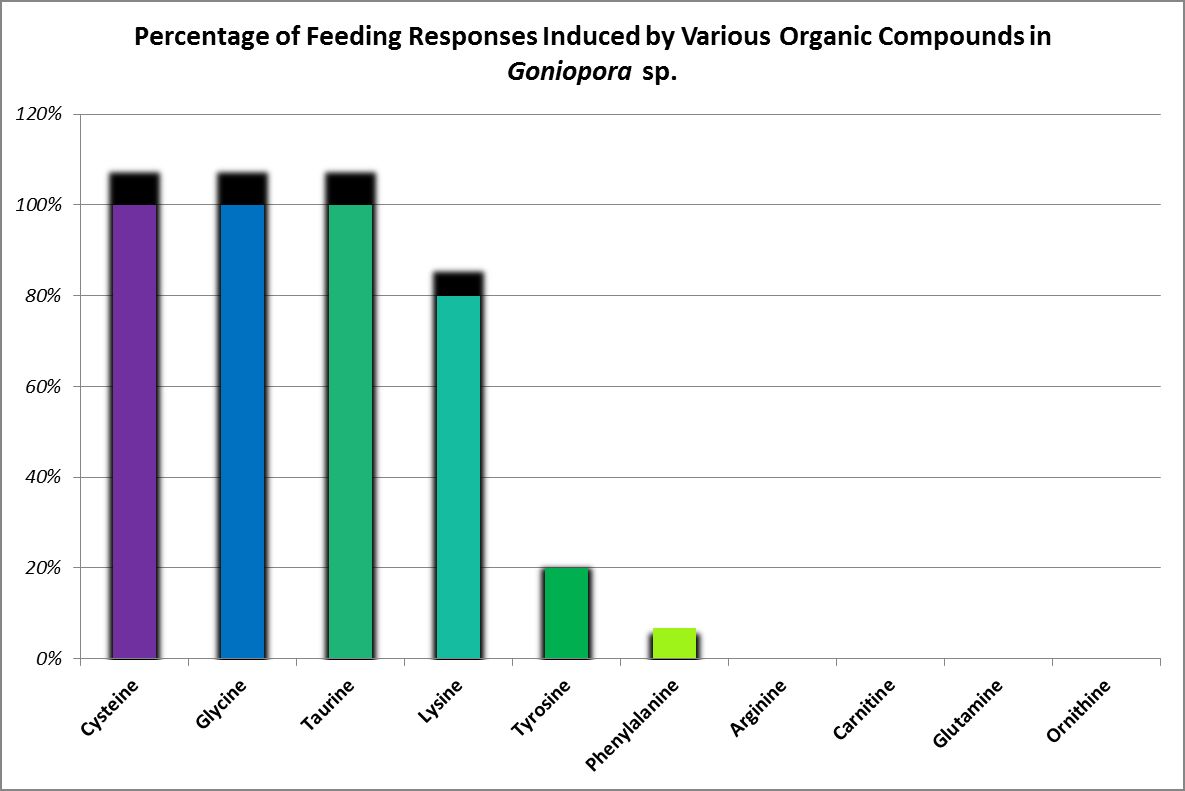
Figure 27. A Goniopora specimen’s feeding responses. A Catalaphyllia (Elegance coral) had similar reactions to glycine, lysine, and taurine. See below. From Riddle, 1994.
Feeding Activators in Catalaphyllia jardinei

Figure 28. The Catalaphyllia specimen used in feeding experiments in the early 1990’s. Not all responses were positive.
Glycine, lysine, and taurine produced a feeding response in this coral (ingestion of paper squares soaked in various amino acids.) Although cysteine-soaked paper was ingested by Goniopora, it was not so with Catalaphyllia. Arginine, carnitine, cysteine, glutamine, ornithine, phenylalanine, tyrosine produced no feeding response. Ingestion of an amino acid is not necessarily a positive thing. Paper squares soaked in glycine were discharged from this coral’s mouths along with hundreds of small, dark particles. These were not examined, but it is possible these were coalesced zooxanthellae.
Other experiments have used different substances. For example, sugars (glucose, fructose, and sucrose) produced no response in Montastrea cavernosa.
Loss of Amino Acids
Amino acid/protein loss is inevitable. Figure 29 shows the composition of coral mucus. Alanine and glutamine are relatively small fractions of the total.
Some critics of coral amino acid uptake studies believe that simultaneous losses to the water column should be included to show the complete picture.
Commercial Amino Acid Supplements
Amino acid supplements are relatively new offerings in the pet industry. Some promise better coral coloration while others advertise their product to mimic amino acid content of natural foods for fishes with narrow dietary requirements.
Two amino acid products were obtained through commercial channels and analyzed for pH, Total Nitrogen, ammonia, nitrite, nitrate, Biochemical Oxygen Demand and effects on alkalinity.
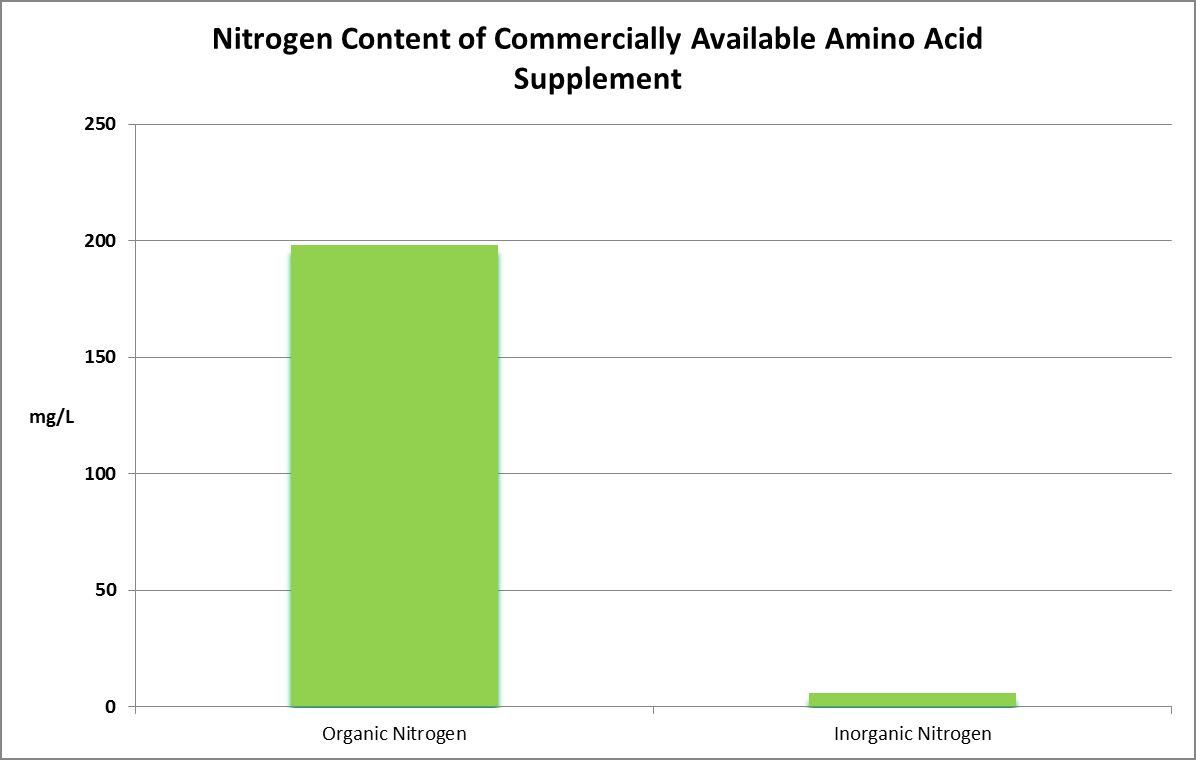
Figure 30. Organic faction is assumed to be amino acid content while the inorganic faction is the sum of ammonium, nitrite, and nitrate.
Total Nitrogen
Two commercially available supplements were analyzed for total nitrogen. One was found to contain 204 mg/L total nitrogen. The other contained over 2,000 mg/L, or 0.2%.
Testing Protocol for Total Nitrogen
Hach’s method of determining Total Nitrogen was used. The sample, along with potassium persulfate is added to a vial containing acid, and digested at 105°C for 30 minutes. Seawater samples have to be diluted in order to avoid interference from chloride.
Biochemical Oxygen Demand (BOD) of Amino Acid Supplements
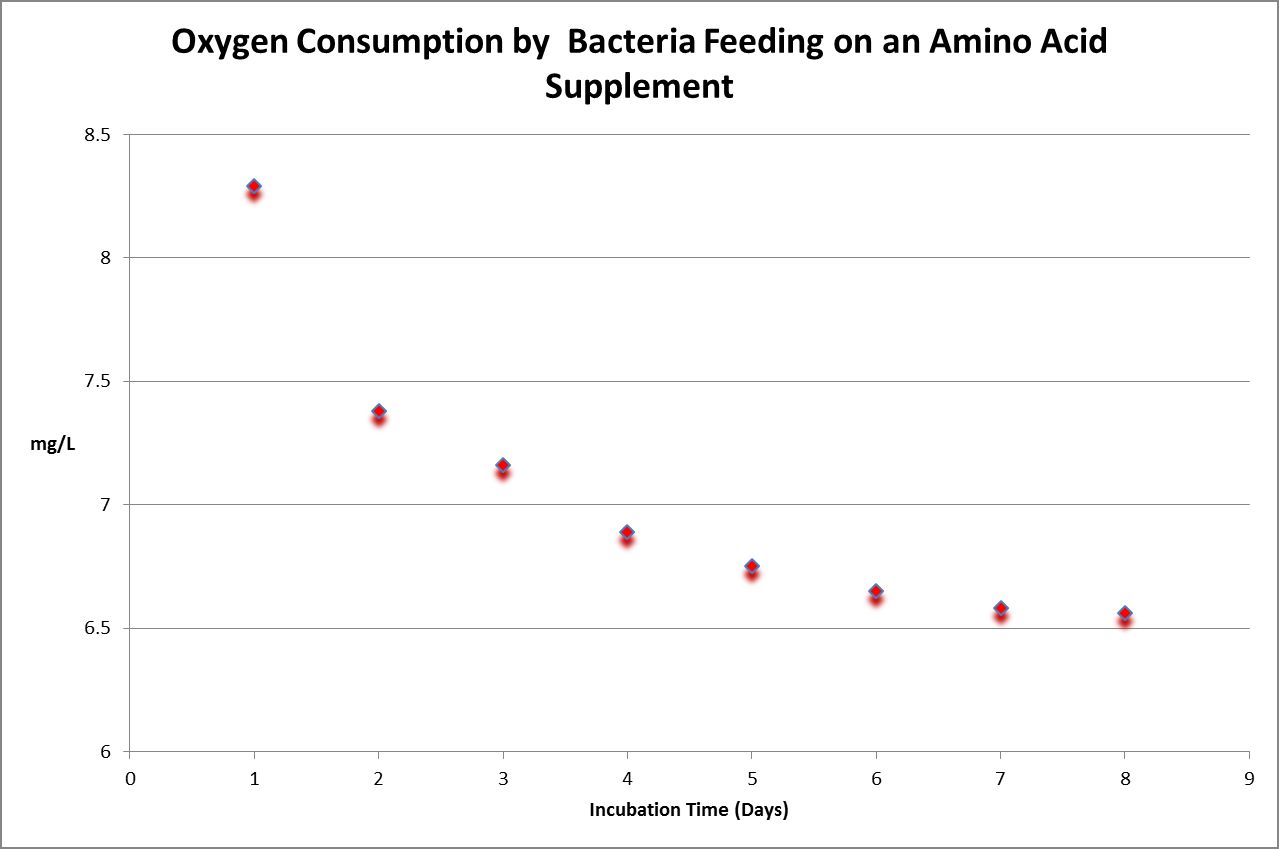
Figure 31. Time-course oxygen consumption by bacteria in a diluted sample of a commercially available amino acid supplement for aquaria. BOD is calculated by subtracting final dissolved oxygen from the initial reading, and then multiplying that by the dilution factor.
Biochemical Oxygen Demand (BOD) is a five-day test conducted under controlled conditions. It is sometimes incorrectly called ‘biological oxygen demand.’ The hypothesis for this experiment was as follows: Amino acids would be rapidly consumed by bacteria. The experiment was conducted using standard protocols (incubation of bacteria-inoculated samples in 300 milliliter bottles which were held for at least 5 days in darkness at 20°C.) Simply stated, BOD is the amount of oxygen consumed while bacteria are ‘eating’ a food source. Since some samples do not contain any (or enough) bacteria, it is imperative that bacteria are added to the sample.
In order that the test is concluded successfully, it is important that oxygen content is not depleted (depletion is defined as dissolved oxygen concentration of less than 1 mg/L at the end of the 5-day test.) In many cases, this requires dilution of the sample. In Figure 30, we see bacteria have consumed available substances by Day 7 and 8, as evidenced by stable dissolved oxygen readings.
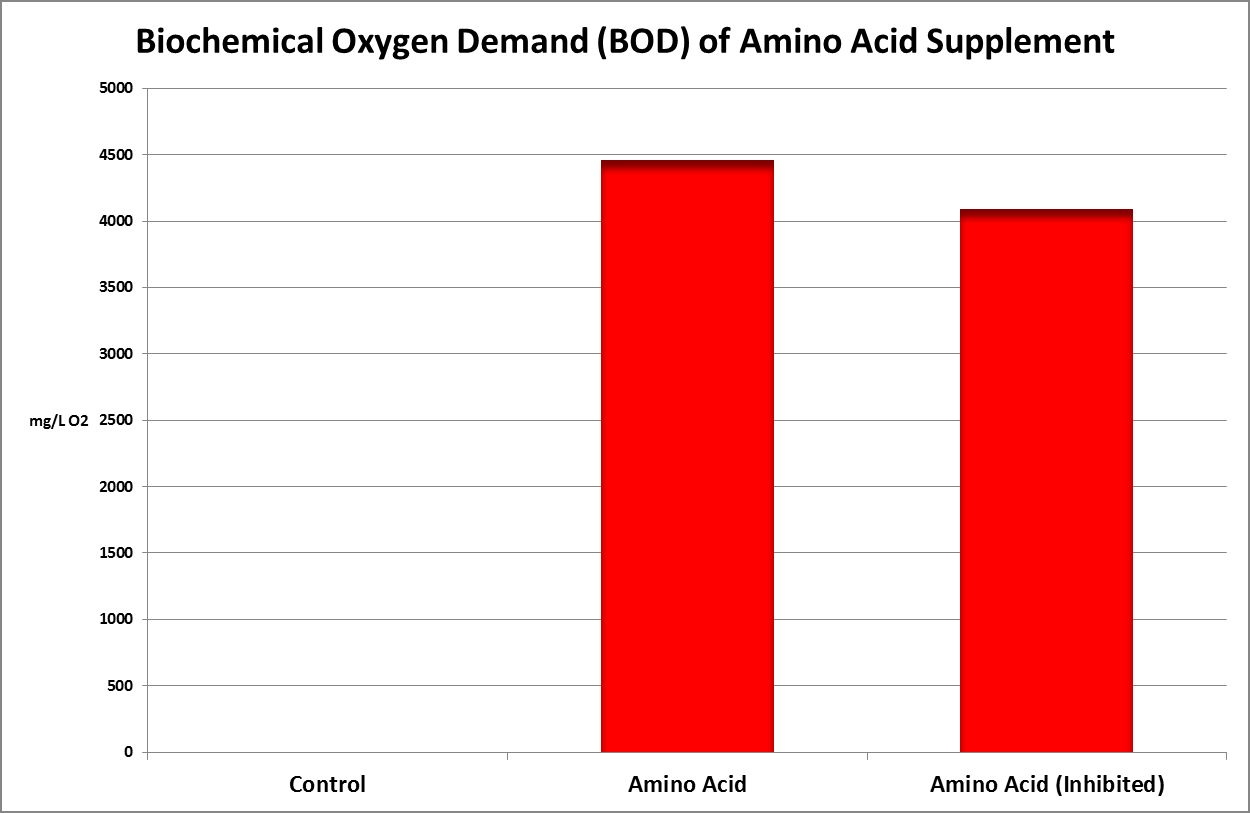
Figure 32. This Biochemical Oxygen Demand (BOD) test demonstrates this amino acid supplement is subject to rapid degradation by bacteria.
In these cases, bacteria ‘seeded’ diluted samples, and oxygen concentrations were determined with an oxygen meter and luminescent dissolved oxygen sensor with stirring rod (Hach Corporation, Loveland, Colorado, USA.)
As mentioned, the BOD test is one lasting 5 days, and is determined using this formula:
Initial Dissolved Oxygen Content – 5-day Dissolved Oxygen Content x Dilution Factor
Figure 32 shows the result – the BOD is almost 4,500 mg/L. To put this in perspective, raw domestic sewage has a BOD of 250-300 mg/L. Treated wastewater discharges are generally regulated to 30 mg/L or less. Oceanic seawater has a BOD of ~0.2 mg/L.
A couple of comments are in order. Recall this oxygen consumption is over the course of 5 days. The nitrogen in the amino acids does not disappear – their uptake by bacteria may become incorporated into new bacteria, or it may be converted into ammonia (evidenced by the 10% reduction of BOD in the diluted sample spiked with a substance known to inhibit nitrification (2-chloro-6-(trichloromethylpyridine.)
Discussion
Amino acids/proteins are an important part of corals’ diets, although amino acid requirements are species specific.
Zooxanthellae are primary producers and produce all their required amino acids, with at least two being translocated to the coral host (alanine and glutamine.)
Corals can make many amino acids considered to be essential to humans – they haven’t lost the ability to synthesize them. However, some amino acids are produced by the coral animal in small amounts and may not be sufficient for tissue maintenance. Amino acids produced by Acropora cervicornis in small amounts (<5% of total) are: Glycine, isoleucine, leucine, lysine, methionine, phenylalanine, tyrosine, and valine.
Tryptophan is only sometimes described as present in marine invertebrate tissues. Is this caused by the testing method used, or is tryptophan truly seldom seen in coral tissues?
Vitamins B6 and C are important in the synthesis of some amino acids, as is choline. Metals (such as zinc and selenium) are important as well.
There is little doubt that marine invertebrates (including corals) can uptake dissolved amino acids. However, there is some evidence suggesting that uptake will be higher at night when amino acids are not produced (or produced in lesser amounts) by the coral or translocated to corals from zooxanthellae. Researchers have shown that neutral amino acids are preferentially ‘eaten’ by at least one coral species (which is not particularly surprising since these AAs are the majority of those considered proteinogenic.)
Amino acid supplements are highly acidic and vary in their amino acid content. Testing showed AA content can vary on the order of at least a magnitude. Biochemical Oxygen Demand tests showed supplements’ amino acids to be degraded quickly (starting immediately, and consumed almost totally in days) by bacteria. Severe overdosing could result in lowered oxygen concentrations due to bacterial blooms as well as reduced alkalinity and pH. The benefit of AA supplementation might not be the amino acid content, but the resulting bacterial bloom (in itself a food source for many corals.) This is strictly speculative, but deserves further attention.
Feeding activators also vary by species. Surprisingly, most studies have used those amino acids considered to be non-essential to humans.
This concludes our short examination of the importance of amino acids in coral nutrition. Next time, we’ll look at something a little more difficult – fatty acids, requirements, and what some, acting as indicators, tell us.
Appendix 1
| Feeding Activators | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | Molar Solution | Montastrea cavernosa | Montastrea cavernosa | Montastrea cavernosa | Montastrea cavernosa | Cysphastrea ocellina | Cysphastrea ocellina | Manicina areolata | Eumilia fastigata | Isophyllia sinuosa | Mussa angulosa | Scolymia lacera |
| Alanine | n/a | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | Yes | Yes | Yes | Yes | Yes |
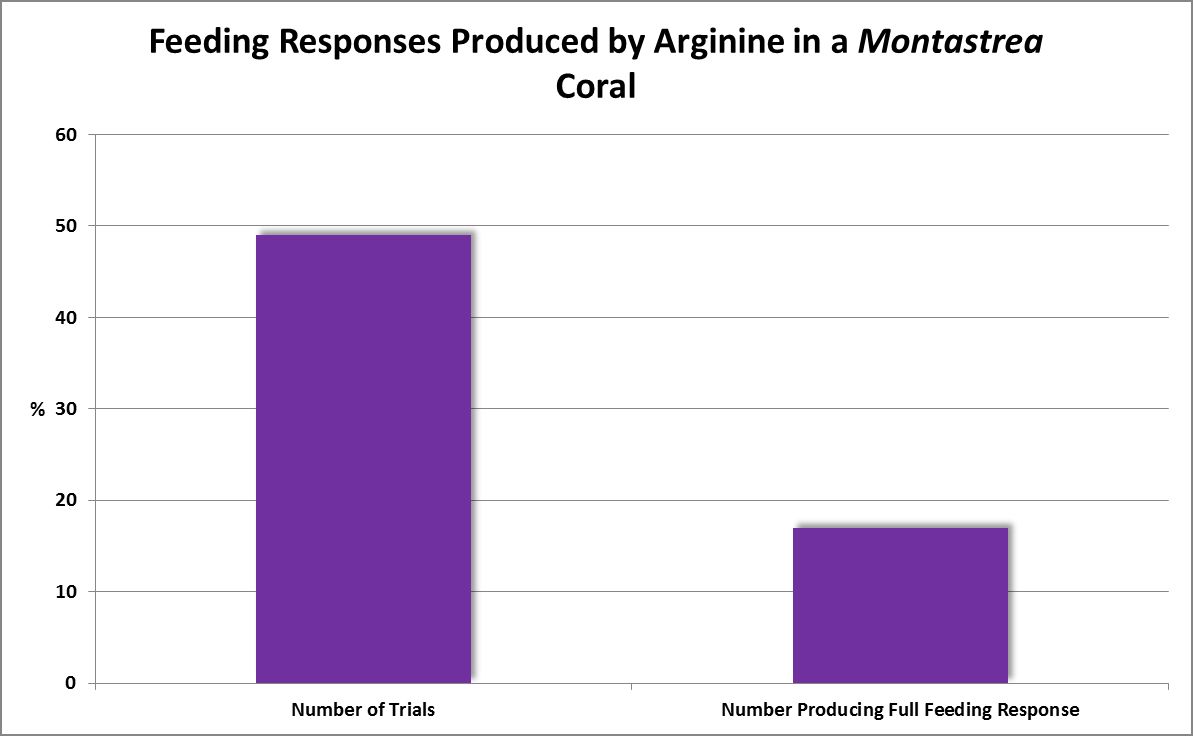
| Arginine | 10.-1 | 0.55 | 0.43 | 0.39 | 0.35 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Arginine | 10.-3 | 0.1 | 0.6 | 0 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Asparagine | 10.-1 | 0.06 | 0.06 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Asparagine | 10.-3 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
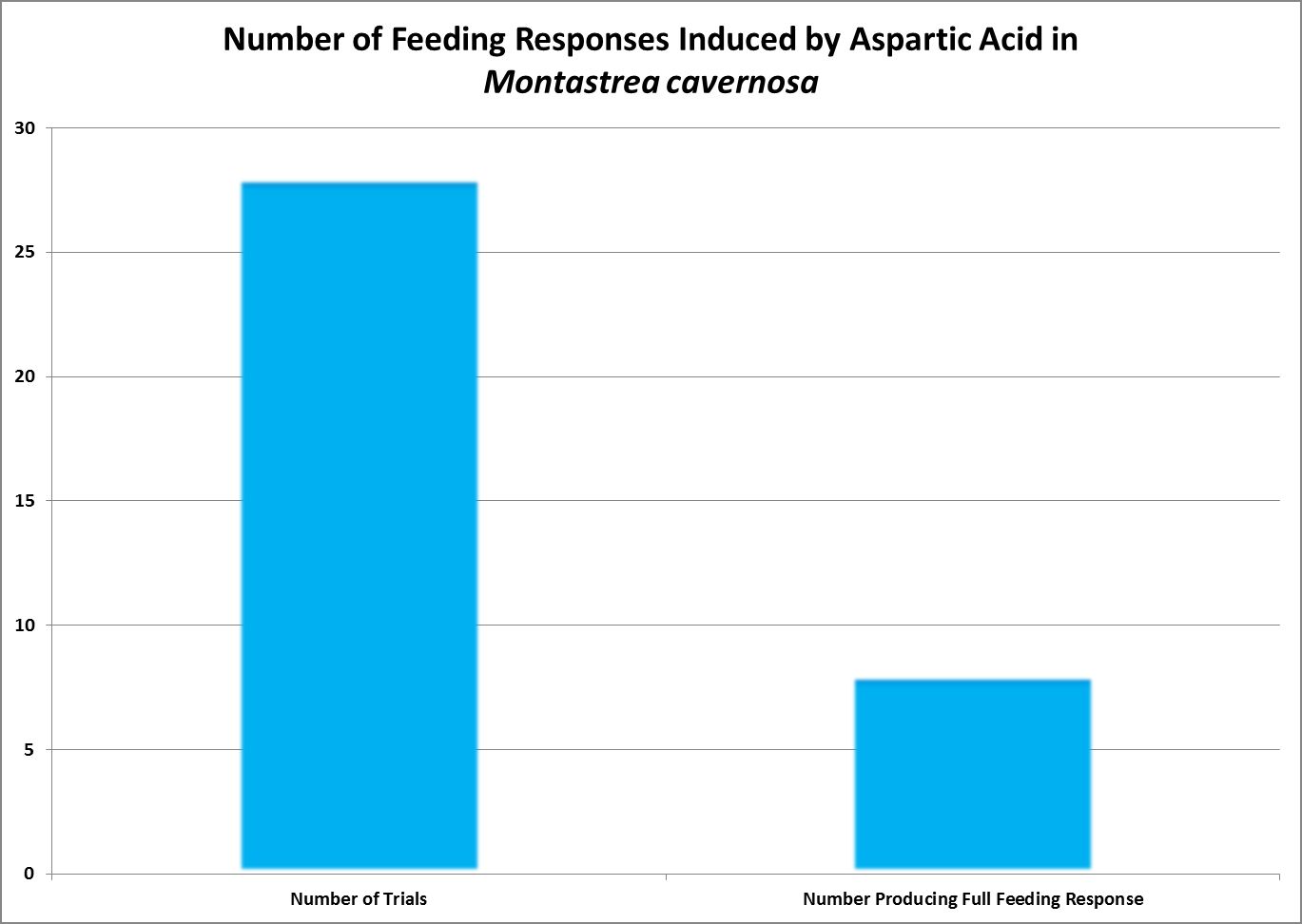
| Aspartic Acid | 10.-1 | 0.76 | 0.52 | 0.32 | 0.32 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Aspartic Acid | 10.-3 | 0 | 0 | 0 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Cysteine | 10.-1 | 0.18 | 0.09 | 0 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Cysteine | 10.-3 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutamic Acid | 10.-1 | 1 | 1 | 1 | 1 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutamic Acid | 10.-2 | 0.6 | 0.33 | 0.13 | 0.13 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutamic Acid | 10.-3 | 0.17 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutathione | 10.-1 | 0.75 | 0.12 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutathione | 10.-3 | 0 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glutathione | n/a | – – – – | – – – – | – – – – | – – – – | Yes | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Glycine | n/a | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | Yes | Yes | Yes | Yes | Yes |
| Leucine | n/a | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | Yes | Yes | Yes | Yes | Yes |
| Phenylalanine | n/a | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | Yes | Yes | Yes | Yes | Yes |
| Pipecolic Acid | 10.-1 | 0.79 | 0.62 | 0.52 | 0.38 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Pipecolic Acid | 10.-3 | 0.23 | 0.15 | 0.08 | – – – – | – – – – | – – – – | Yes | – – – – | – – – – | – – – – | – – – – |
| Pipecolic Acid | n/a | – – – – | – – – – | – – – – | – – – – | Yes | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
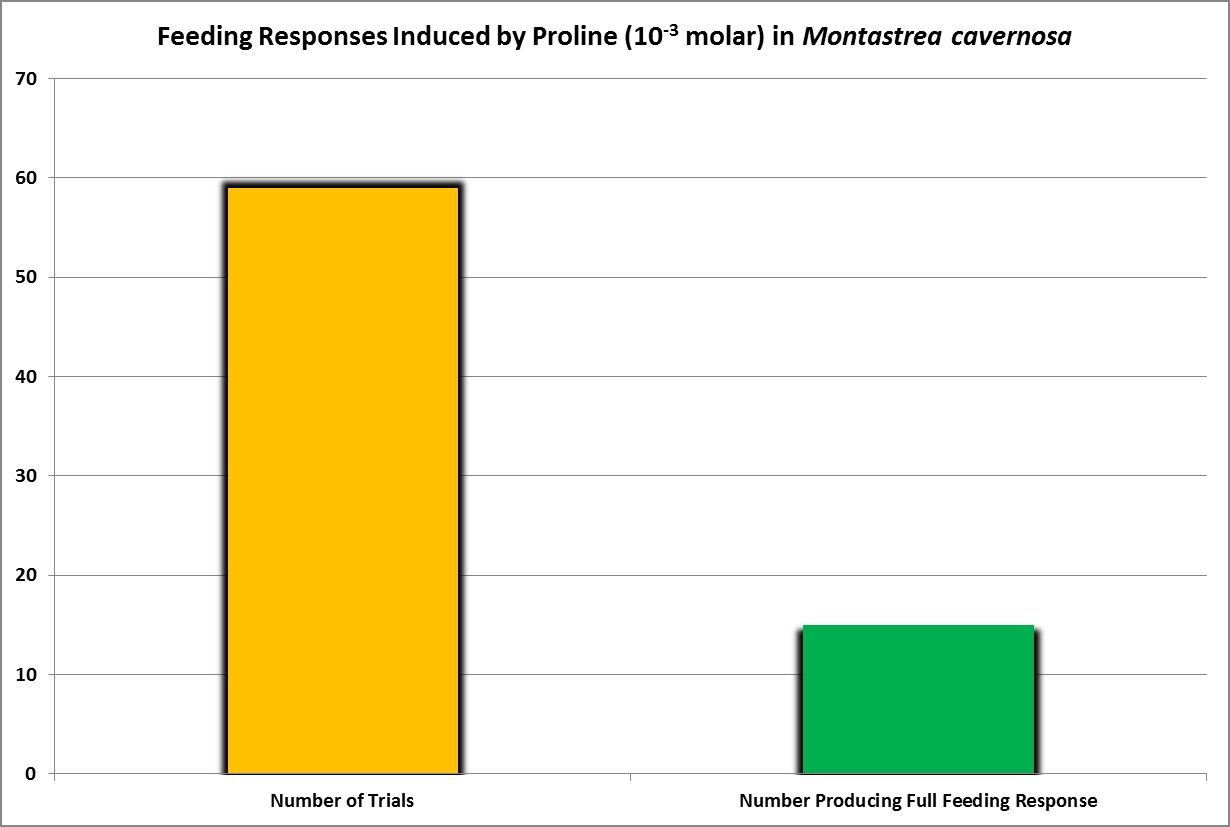
| Proline | 10.-1 | 0.79 | 0.66 | 0.59 | 0.52 | – – – – | – – – – | – – – – | 0.59 | – – – – | – – – – | – – – – |
| Proline | 10.-2 | 0.8 | 0.2 | 0.2 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Proline | 10.-3 | 0.2 | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
| Proline | n/a | – – – – | – – – – | – – – – | – – – – | Yes | – – – – | – – – – | – – – – | – – – – | – – – – | – – – – |
Appendix 2
| Porites porites | Oculina diffusa | Agaricia fragilis | Acropora cervicornis | Acropora palmata | Plexaura lexuosa | Eunicia tourneforti | Gorgonia reutalina | Pseudoplexaura porosa | Plexaurella dichotoma | Millepora alcicornis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspartic Acid | 34.3 | 25 | 30 | 35.2 | 37 | 66.3 | 74.3 | 65.3 | 72.5 | 66.8 | 56.4 |
| Glycine | 11.4 | 17.4 | 17.1 | 13.2 | 12 | 6.49 | 7.91 | 9.8 | 9.07 | 7.53 | 5.42 |
| Glutamic Acid | 10 | 13.3 | 10.3 | 13.3 | 12.5 | 5.31 | 3.86 | 5.78 | 3.25 | 3.68 | 11 |
| Leucine | 6.87 | 5.81 | 4.5 | 2.98 | 4.36 | 1.6 | 5.9 | 1.35 | 0.83 | 0.74 | 2.23 |
| Alanine | 6.68 | 5.81 | 12.2 | 3.92 | 4.73 | 6.13 | 6.02 | 11 | 7.57 | 14.6 | 4.31 |
| Valine | 5.58 | 5.35 | 5.56 | 4.53 | 4.57 | 1.95 | 1.36 | 1.74 | 1.41 | 1.77 | 2.3 |
| Isoleucine | 4.6 | 4.95 | 2.38 | 1.98 | 2.18 | 1.04 | 0.51 | 0.94 | 5.5 | 0.54 | 1.67 |
| Proline | 3.54 | 6.77 | 3.96 | 5.1 | 3.83 | 2.75 | 1.05 | 1.26 | 1.02 | 0.98 | 1.88 |
| Phenylalanine | 3.33 | 3.67 | 2.79 | 1.76 | 2.13 | 0.46 | 0.33 | 0.55 | 0.47 | 0.38 | 0.8 |
| Serine | 2.92 | 4.79 | 5.57 | 7.19 | 5.8 | 3.24 | 1.31 | 6.5 | 9.6 | 0.87 | 4.3 |
| Lysine | 2.73 | 0.19 | 0.42 | 4.82 | 4.89 | 0.76 | 0.46 | 0.13 | 0.55 | 0.19 | 2.95 |
| Threonine | 2.09 | 3.27 | 2.54 | 2.62 | 3.09 | 1.7 | 1.29 | 0.63 | 0.85 | 0.57 | 4.41 |
| Arginine | 1.32 | 0.18 | 0.38 | 1.04 | 1.17 | 0.55 | 0.43 | 0.3 | 0.26 | 0.29 | 0.64 |
| Histidine | 1.19 | 0.14 | 0.21 | 0 | 0 | 0.63 | 0.14 | 0.16 | 0.21 | 0.43 | 0.71 |
| Methionine | 1.14 | 1.28 | 1.21 | 0.58 | 0 | 0.25 | 0.1 | 0.17 | 0.15 | 1.2 | 0 |
| Tyrosine | 0.99 | 1.81 | 0.78 | 7.2 | 0.8 | 0.37 | 0.12 | 0.12 | 0.25 | 0.21 | 0 |
| Cystine | 0.19 | 0.18 | 0 | 0.9 | 0.96 | 0 | 0.11 | 0 | 0 | 0 | 0 |
| Amino Acid Content in Percent | |||||||||||
References and Further Reading
- Al-Moghrabi, S., D. Allemand and J. Jaubert, 1992. Neutral amino acid uptake by the symbiotic coral Galaxea fascicularis: effect of light and feeding. Proc. 7th Int. Coral Reef Symp., Guam. 1: 379.
- Ambariyanto, and O. Hoegh-Guldberg, 1999. Net uptake of dissolved free amino acids by the giant clam, Tridacna maxima: alternate sources of energy and nitrogen? Coral Reefs 18: 1, 91-96.
- Antla, N. J., AND C.Y. Lee. The determination of “free” amino sugars
- Baker, A., 1994. Nutritional sources and sinks of carbon and nitrogen in the solitary coral Heliofungia actiniformis. Hounors Thesis. University of Sydney.
- Bester, C., F. Lipschultz, R. Ream, D. Tomasino, and H.G. Trapido-Rosenthal, 1997. Effects of exposure to exogenous amino acids on the cellular physiology of cultured zooxanthellae. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1287-1290.
- Chalfie, M. and S. Kain, 2006. Green Fluorescent Protein: Properties, Applications, and Protocols. John Wiley and Sons, Hoboken, New Jersey. 443 pp.
- Fitzgerald, L. and A. Szmant, 1997. Biosynthesis of ‘essential’ amino acids by scleractinian corals. Biochem. J. 15, 322(1): 213-231.
- Fitzgerald, L.M. and A.M. Szmant, 1988. Amino acid metabolism: adaptations to low light conditions? Proc. 6th Int. Coral Reef Symp., Australia. 3:5-9.
- Grover, R., J-F. Maguer, D. Allemand, and C. Ferrier-Pages, 2008. Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J. Exp. Biol., 22:860-865.
- Hoegh-Guldberg, O., S.G. Dove, and D. Siggaard, 1997. Dissolved free amino acid (DFAA) concentrations in Great Barrier Reef waters: the implications for the role of DFAA transport by Acanthaster planci. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1237-1243.
- Johannes, R.E. and K.L. Webb, 1965. Release of dissolved amino acids by marine zooplankton. Science, 150:76-77.
- Johannes, R.E. and W.J. Wiebe, 1970. Method for determination of coral tissue biomass and composition.
- Johannes, R.E., S.L. Coles and N.T. Kuenzel. The role of zooplankton in the nutrition of some scleractinian corals.
- Lehman, J. and J. Porter, 1973. Chemical activation of feeding in the Caribbean coral Montastrea cavernosa. Biol. Bull., 145: 140-149.
- Goreau, T., N. Goreau, and C. Yonge, 1973. On the utilization of photosynthetic products from zooxanthellae and of a dissolved amino acid in Tridacna maxima f. enlongata (Mollusca: Bivalvia). J. Zool. London 169: 147-154.
- Mitterer, R.M., 1978. Amino acid composition and metal binding capability of the skeletal protein of corals. Bull. Mar. Sci., 28(1):173-180.
- Riddle, D., 1994. Coral Nutrition. Part II, Proteins. Freshwater and Marine Aquarium, 17(5): 80-91.
- Spotte, S., 1992. Captive Seawater Fishes: Science and Technology. Wiley Interscience.
- Stephens, G. C. and R.A. Schinske. Uptake of amino acids by marine invertebrates.
- Stephens, G. Uptake of organic material by aquatic invertebrates. 1. Uptake of glucose by the solitary coral, Fungia scutaria.
- Swanson, R. and O. Hoegh-Guldberg, 1998. Amino acid synthesis in the symbiotic sea anemone Aiptasia pulchella. Mar. Biol., 131(1):83-93.
- Wijgerde, T., 2014. Aquarium corals: Amino acids and corals: Sources, roles and supplementation. http://www.advancedaquarist.com/2014/3/corals
- Yanushevic, Y., M. Bulina, N. Gurskaya, A. Savitskii and K. Lukyanov, 2002. Key amino acid residues responsible for the color of green and yellow fluorescent proteins from the coral polyp Zoanthus sp. Russian Journal of Bioorganic Chemistry, 28(4): 274-277.



0 Comments