Pocillopora meandrina is one of the most common corals of the Hawaiian archipelago. It is also widely distributed across the Indo-Pacific and eastward to the west coast of Central and South America. However, this abundant coral species has some unusual spawning habits. It is one of the few stony corals known to spawn during daytime hours (just after sunrise) and does so during spring months (here in Hawai’i in April and/or May just after the full moon). Surprisingly, this is about all we knew about the breeding habits of this animal.

Figure 1. Spawning Pocillopora meandrina colonies. Billions of coral gametes make the water appear ‘smoky’. Photo courtesy of Denise Ulrich.
When preparing for observations of the spawning event, I checked with several marine biologists and got different opinions on what we should expect to see. Some thought we would see male colonies ejecting sperm to fertilize eggs held within a female colony, where they are ‘brooded’, hatched and ultimately released as relatively large sized planula larvae. On the other hand, we might expect to see gonochoric broadcast spawning (where an individual coral colony is either male or female, and its gametes are released to the water column where eggs and sperm mix for fertilization). Timing of egg and sperm release was in doubt and it was suggested that male and female gamete release could be separated by as much as an hour. In short, we didn’t really know what to expect and we would be in for somewhat of a surprise if we were fortunate enough to witness spawning.
Kahalu’u Beach Park is a popular recreational area located near the town of Kailua-Kona on the west side of the Big Island of Hawai’i. Kahalu’u is sometimes called ‘Children’s Beach’ due to the mysterious ‘Menehune Wall,’ which partially shelters the beach area from extreme wave action (see Figure 2).

Figure 2. The reef flat at Kahalu’u, western side of the island of Hawai’i, one of the sites where Pocillopora meandrina colonies spawnings were reported. Note the submerged light sensors (PAR and UV) and their cords in the lower left hand corner.
(Legend has it that the exposed rock wall (top of picture) was in existence when the first Polynesians arrived on the island. Its existence is attributed to efforts of a race of mischievous ‘small people’ – the Menehunes.)
Kahalu’u also has an expansive reef flat filled with ‘pot holes’ which are home to green sea turtles, reef fishes, and, of course, various invertebrates including soft and stony corals.
Early in 2008, I began working with the University of Hawaii Sea Grant College Program Extension Agent and the ReefWatcher volunteers, and began planning to observe the seasonal Pocillopora meandrina spawning events. I was placed in charge of selecting a site suitable for a number of observers, where each volunteer took a task, such a photography, data acquisition, and sample collection. I chose Kahalu’u as one of the observation sites. It is usually sheltered from strong wave action, water depth would be shallow at the predicted time of spawning, has readily available public parking early in the morning, water entry is not very difficult and would allow our ‘floating laboratory’ to be launched with relative ease. It also happens to be about 1 mile from my laboratory – it would be a perfect spot to observe the spawning of the stony coral Pocillopora meandrina (commonly known as the Cauliflower coral (Figure 3), or the Rose coral when it contains the pink pigment called ‘pocilloporan’ – Figure 4). This article will report results of coordinated observations made in April, May and June 2008 along the west coast of the Big Island of Hawai’i.

Figure 3. The Cauliflower coral (Pocillopora meandrina). The pinkish dots are gall crab openings. Photo by the author.

Figure 4. A pink color morph of Pocillopora meandrina – little wonder this coral is sometimes called the ‘rose coral”. Photo by the author.
A Floating Laboratory

Figure 5. The author makes last minute preparations and adjustments to the floating laboratory during the pre-dawn hours of April 22, 2008. Photo courtesy Dave Kearnes ([email protected]).
Many reports list long term environmental factors thought to promote and trigger coral spawnings. We approached this differently and decided to investigate the effects (if any) spawning corals might have on their environment, as well as other environmental factors that may act as the final trigger for reproduction, such as light and ultraviolet radiation intensity. To this end, a floating laboratory housing was designed and built with data loggers to record pH, salinity, air and water temperature, plus ‘air’ and ‘water’ photosynthetically active radiation (PAR) and ultraviolet radiation (UVR – ultraviolet radiation data is not discussed further in this article). (See Figure 5). Oxidation-reduction potential (ORP) was monitored by a battery-operated meter and sensor, and these measurements were recorded manually.
Field Observations
The following is a compilation of reports from 22 observers during the P. meandrina spawning events of April, May and June 2008.

Figure 6. Sites and times of P. meandrina spawnings in April 2008. Sunrise time was approximately 6:07 am. The most distal observation points (red dots) are ~35 miles apart.
Timing of Spawnings
Pocillopora meandrina colonies were observed spawning for two days each in April and May, and once in June 2008. Interestingly, the spawnings occurred earlier in the morning during the May spawning events (by as much as 23 minutes earlier) and earlier still in June. See Figures 6, 7, and 8.
There are many environmental factors influencing chemical and physical aspects of aquatic environments. Since pH, salinity and temperature measurements remained flat slightly before and during the morning of spawning, we will not discuss those further. However other conditions were in state of flux, and include:

Figure 7. Times of May spawnings. Note that spawning occurred about 10 to 23 minutes earlier – ‘official’ sunrise time is 16 minutes earlier than in April, but light intensity can influenced by shadows and clouds.

Figure 8. A minor spawning event occurred in Kealakekua Bay at 6:45 am on June 20, 2008. Note that the spawning was observed earlier than those in April or May. Is the time of sunrise (or light intensity) a trigger for P. meandrina spawnings?
Sunlight
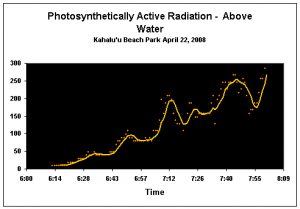
Figure 9. Surface PAR (Photosynthetically Active Radiation; reported in micromole per square meter per second). Light intensity at the surface of the water at the time of spawning was about 225 µmol·m²·sec (we estimate ~40 µmol·m²·sec underwater). The yellow line is a five-minute running average.
Sunlight, more than any other single factor we observed, seems to play a role in triggering spawning in P. meandrina. Unfortunately, this is anecdotal at this time since the data logger recording the underwater PAR and UVR failed during the morning of the observed spawning. However, the other PAR and UVR data logger (recording readings from above the water) functioned flawlessly (see Figure 9). Based on other data sets, we estimate the Pocillopora meandrina corals in Kahalu’u Bay spawned when light intensity reached about 40 µmol·m²·sec (April spawning). Interestingly, observers, in some cases, noted that coral colonies in deeper water spawned later than their shallow water counterparts. This deserves further investigation, but our focus changed to gamete collection during the May spawnings and the floating laboratory was not deployed.
Moonlight
Moonlight, according to some sources, is a fine-tuning mechanism for initiating synchronous coral spawnings, and indeed, moon phase is a convenient way to predict approximate timing of coral reproduction (to within a day or so). Positioning of the moon, in relation to the Earth and Sun, also determines tidal cycles. It is not within the scope of this article to discuss coral photoreceptors and moonlight or the influence of celestial bodies on tides (these will be discussed in detail later in this series). For now, we’ll present moon phase as a convenient predictor of P. meandrina spawnings (See Figure 10).
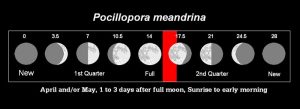
Figure 10. Spawning period (as lunar days – those numbers above the moon drawings) for Pocillopora meandrina. Some believe the spawning event is only coincidental to moon phase, and that lunar periodicity plays no functional role. Others disagree. We will examine the evidence for and against the role of moonlight later in this series.
Figure 11 shows spawning dates and the hours of potential available moonlight in hours.
Tide

Figure 12. The P. meandrina colonies spawned on a low, but still falling, tide. Spawning times were 7:36 and 7:16 on April 22 and May 22, respectively (at Kahalu’u). The vertical blue line is time of spawning in April; the green line is for May.
Though moon phase and its possible impact on coral spawning is a matter of debate, there can be little doubt about the importance of tidal action on successful fertilization. Pocillopora meandrina colonies spawn on the Big Island during a falling tide, approaching ebb tide, when decreased water depth minimizes dilution of gametes (especially in their preferred environments of high water motion which are generally reef crest and flats).
P. meandrina spawns on a falling tide, but it is not at the lowest or ebb tide for the day and is not the lowest low tide for either month (that occurs around the new moon phase). See Figure 12.
There is approximately 1†difference in water depths between the April and May spawnings.
Results from the Floating Laboratory

Figure 13. Oxidation-reduction potential rose sharply during the time of spawning (this was not observed the previous day when no spawning was observed). Spawn time was 7:36 am (Kahalu’u reef flat).
Of the information we gathered during the April spawning, one parameter showed a surprising trend – that of the reduction-oxidation potential (ORP, or Redox – See Figure 13). We assumed that possible release of hormones from corals could be a signal for mass spawning and had reason to believe that this might be measured using a redox probe. We expected a lowered redox value; instead we saw a sharp rise. This was not a response to probe conditioning and slow response. We are uncertain of why this occurred, and feel it deserves further investigation.
This concludes our very brief look at the natural environment. We’ll now turn our attention to the artificial conditions within a small wet laboratory.
Laboratory Observations
Even the largest P. meandrina eggs we observed were tiny. We used an eyepiece reticle calibrated to a stage micrometer to estimate their diameters (about 100 micrometers, hereafter called microns). The ‘average’ egg of other coral species is much larger at 400-500 microns. Minimal water currents probably keep the P. meandrina eggs in suspension, but when held in laboratory beakers, they were found to be negatively buoyant – they sink. Since lipid droplets within many coral species’ eggs cause them to float, it seems reasonable to assume that Pocillopora meandrina eggs contain have less lipid content than many other coral eggs.
Technique Used for Egg Fertilization
In theory, the plan to launch a floating laboratory and collect samples seems like a day at the beach. In reality, it is a lot of work even when the workload is divided among 6 volunteers. Our tasks were made more difficult simply because we did not know exactly what to expect.
In my view the most difficult assignment was that of collecting the spawn in the shallows of Kahalu’u Bay. We used two methods – small plankton net (80 micron mesh) was pulled through the water column when spawnings were observed. We also collected gametes with a kitchen baster at the moment of release from the coral. These samples were transferred to Ziploc bags. Aliquots were drawn and mixed with the contents of other bags to aid in ‘crossing’ eggs and sperm from different spawning colonies. These mixed samples were placed in a mesh net bag suspended from the floating laboratory. Other than the gentle rocking motion from wave action, no stirring or mixing occurred after crossing of the samples.
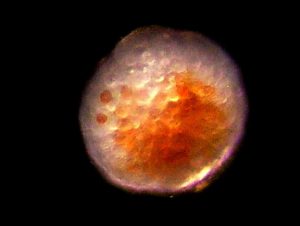
Figure 15. A Pocillopora meandrina egg. Note zooxanthellae cells within the egg. This egg is the diameter of a human hair. Photomicrograph by the author.
These bagged samples were transported within the mesh bag to the laboratory (~1 mile away). We arrived there about 30 minutes after the spawning event.
April Spawning: Every egg from the very first sample drawn for microscopic examination showed signs of fertilization. The beakers were full of planula larvae about 30 hours after collection, but had disappeared by hour 54. We missed an excellent opportunity to learn much more than we did.
Experience is the best teacher, and we would be more prepared in May for what we realized as an event akin to an aquatic fire drill.
May Spawning: Samples were obtained from Joan Prater and John Lynch (as well as those I collected). Planula larvae were kept alive at ~150 hours of age but they did not settle on the walls or bottoms of the beakers nor did they attach to a substrate covered with a calcareous algae believed to be a Mesophyllum species.

Figure 16. P. meandrina eggs were collected with a kitchen baster at the moment of release. Some eggs did not seem mature (as evidenced by their size), and not all contained zooxanthellae. Photomicrograph by the author.
June Spawning: Only a few eggs were collected during this weak spawning, and no effort was made to culture them.
Summary
A synopsis of our observations for Pocillopora meandrina spawnings along the west coast of the Big Island of Hawaii is:
- Pocillopora meandrina colonies are hermaphroditic and simultaneously broadcast eggs and sperm
- Spawning is seasonal and is extended over the course of at least 3 months (1 more month than previously described)
- Egg cleavage can begin several hours after the spawning event
- Sperm are still alive after ~10 hours (but viability is likely reduced)
- Eggs contain zooxanthellae (symbiont transmission is vertical)
- Eggs are negatively buoyant
- Eggs are of various sizes, but all are 100 microns or less
- Planula larvae are apparent as late as 24 hours after eggs are fertilized
- Planula will collect at the surface in very calm water
- Planula larvae are small (200-250 microns)
In Closing
Witnessing a coral spawning, especially in the daytime, is an unusual and memorable event. We have much to learn about coral reproduction (a comment was made that we know more about moon rocks than we know about the reproductive habits of corals, some living in only a foot or two of water).
Countless hours of research and preparation preceded our observations, yet this barely readied us for the all too brief spawning activity and the hours of laboratory observations and experiments that followed (I will never again sit in an air-conditioned laboratory for 6 hours in a very wet and cold wet suit!).
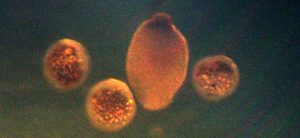
Figure 18. Eggs and a planula larva of Pocillopora meandrina. Since planula larvae take time to develop, this photo suggests a previous, unobserved spawning had occurred. Photomicrograph by the author.
This article closes now but it is only the first of a series entitled ‘Coral Reproduction.’ Next time, we’ll look at asexual coral reproduction in aquaria and lay a foundation for future articles in this series. We’ll examine what we’re doing right, and what improvements could be made. More importantly, information will be presented in order to improve our chances of successful sexual coral spawnings.
Figure 19. P. meandrina sperm are barely visible in this photomicrograph negative image. Photo by Barbara Kuehner, University of Hawaii Center, West Hawaii.

Figure 20. A copepod seems huge next to a P. meandrina egg. A blue light was used to check for green fluorescence within the egg – it was not found, but the light source made objects appear bluish. Photomicrograph by the author.
Acknowledgements
My hat is off to all the observers along the coast of west Hawaii who, instead of staying in bed, braved pre-dawn darkness, a south swell and strong surf to find their ways into tide pools, reef flats and deeper reefs (often traversing lava rocks still wet and slippery from a falling tide). Volunteers snorkeled and SCUBA dived in the sub-tropical Hawaiian ocean that is still cool enough at this time of year to wake you up in a hurry. In particular, Sara Peck with the University of Hawaii Sea Grant program toiled tirelessly to coordinate volunteers’ efforts. David Kearnes ([email protected]; http://www.pacificwatercolors.com) donated his time, talent and underwater photography gear to document the spawning at Kahalu’u Bay (see Figures 5 & 14). Barbara Kuehner with the University of Hawaii Center, West Hawaii, collected gamete samples and provided several photomicrographs (Figure 19 is one result of her efforts). Denise Ulrich was kind enough to share her photographs (one is published as the introductory photo). John Lynch, Bruce Brooks, Kevin O’Connor, Bob Ruhsak, Joan Prater, Matt Binder, Dallas Schaffer, Catherine Wynn, Ambika Rose, Janet Christmann, Robin Bourdon, Suzie Weaver, Pam Higgins, Sammie Stanbro, Bo Pardau, Bob Teytaud, Jennifer Rabalais and Peggy Spelman also added their observations from up and down the west coast of the Big Island. My sincere apologies to anyone I have inadvertently omitted.
Dr. Fenny Cox (Hawaiian Institute of Marine Biology) was kind enough to share her thoughts and describe methods for maintaining planula larvae in vitro. Amy Apprill (University of Hawaii, Manoa) and Heather Marlow also took time from their busy schedules to relay their experiences with Pocillopora meandrina.
Reference
- Cox, E., 2006. Connectivity of Pocillopora meandrina populations: Genetic and oceanic approaches. Hawaii Institute of Marine Biology and Joint Institute of Marine and Atmospheric Research, Grant Number NA04NOS4260172.






0 Comments