Lighting is an extremely important consideration for reef aquarium hobbyists, yet there are still many unanswered questions concerning artificial light sources and their effects on captive corals. This article will examine the results of experiments designed to investigate the effects of light spectra on coral coloration, some of which were unexpected.
Many benthic invertebrates inhabiting coral reefs contain fluorescent and reflective pigments, and it has long been speculated that these are produced as a protective response to ‘strong sunlight’ (suggested by Kawaguti in 1944 and re-examined by Salih et al., 2000). Further, researchers have examined excitation and emission spectra of fluorescent coral pigments (Mazel, 1995; 1997) and the instantaneous effects of altered spectral quality on apparent coloration (Fux and Mazel, 1999, and unpublished). However, very little information exists on influences of artificial light sources (particularly those producing narrow bandwidths) on coral host and zooxanthellae pigmentation.
Reef aquarists often report drastic shifts in apparent and true coloration of captive corals, especially when altering the spectral quality of primary artificial light sources (especially metal halide lamps). Some of these ‘changes’ are due simply to the reflective nature of the coral host tissue and zooxanthellae. Occasionally, reflected light cannot explain coloration shifts, and intriguing reports state that certain coral colorations can be maintained only under certain (usually ‘high’) Kelvin lamps are sometimes heard.
A simple experiment was devised to test the hypothesis that relatively narrow spectral bandwidths can play a role in inducing colorful coral pigments. Initial experiments involved a submersible lighting system consisting of four LED lamps (blue, green, yellow and red), which illuminated portions of genetically identical coral fragments for 6 weeks. Replicate trials followed a few months later with the same outcome. Results strongly suggest spectral quality can have profound effects on host and algal pigmentation.
Procedure – Round One
The coral chosen for this experiment was the ‘Rose Coral’ ( Pocillopora meandrina ) – the common name for this coral is well chosen, as coloration in some colonies is red to hot pink. A small branch (approximately 13 cm long) was obtained from an aquarium within the Natural Energy Laboratory Hawaii (NELHA) complex in Kailua-Kona, Hawaii. The branch was subdivided into 4 fragments, which were then glued to acrylic pedestals.
The branches were transferred to an open-system aquarium at NELHA’s display area. This aquarium (122 cm X 46 cm X 46 cm, holding approximately 257 liters) had been prepared in advance to meet the requirements of this wave-washed P. meandrina specimen. A Carlson Surge Device (CSD – approximately13 L capacity) situated about 1 meter above the aquarium provided periodic wave surges similar to those found in the coral’s natural environment. Although the average water detention time within the aquarium was only 30 minutes, adjustments of ‘warm’ and ‘cold’ water streams to the CSD were necessary to control temperature within the tank. (The NELHA complex has available seawater pumped from two depths – 25 and 615 meters, with temperatures of 27° and 5°C, respectively.) Aquaculture and other firms can dial in a temperature through simple feed water valve adjustments. Minimum and maximum temperatures observed within the aquarium during the experiments were 23.3° and 27.7° C.
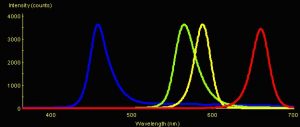
Figure 1: Spectral Signatures of LEDs. Blue = Nichia Blue (peak emission @ 457 nm); Green = Radio Shack Green LED (peak emission @ 564 nm); Yellow = Radio Shack Yellow LED (peak emission @ 587 nm); Red = Radio Shack Red LED (peak emission @ 659 nm).
Natural sunlight provided light to the aquarium, but was attenuated to a maximum of ~10% intensity (200 – 215 μmol·m2·sec) by two layers of shade cloth. This light intensity was well below the maximum of ~1,100 μmol·m2·sec experienced by most shallow-water corals in the area at noon. Previous experiments suggested the intense pink coloration would be lost below a ‘coloration threshold’ of about 200 – 250 μmol·m2·sec. All light measurements were taken with a Li-Cor Model 189 quantum meter and 2 pi submersible sensor. A sheet of Lexan clear glazing material attenuated ultraviolet radiation to levels of 1 μw·cm2 UV-A and <1 μw·cm2 UV-B (as measured with an Ultraviolet Products radiometer and UV-A and UV-B sensors). An Ocean Optics USB-2000FL spectrometer determined the effective wavelength cutoff of this material was 390 nm.
Five Light-Emitting Diodes (LEDs) were used in these experiments. Nichia America manufactures the Ultraviolet-A (UV-A) and blue LEDs; the green, yellow and red LEDs were obtained at a local Radio Shack. (See Figure 1. Spectral signature of the UV-A LED is not shown. The maximum output of the red LED is dead on the absorption peak of chlorophyll a.)
The amplitudes in Figure 1 represent spectral quality and not absolute intensity (since measurements were taken by positioning the LEDs to deliver ~3,500 counts to the spectrometer’s sensor). When measurements were made at a standardized distance (~6 mm) from the tip of each LED with a Li-Cor quantum meter, the intensities were:
- Nichia Blue LED = 400 μmol·m2·sec
- Radio Shack Green LED = 15 μmol·m2·sec
- Radio Shack Yellow LED = 50 μmol·m2·sec
- Radio Shack Red LED = 323 μmol·m2·sec
Appropriate resistors and wiring were housed within clear vinyl tubing, and the LEDs were friction-fitted into the end of the tubing and glued in place with silicon cement. U-shaped acrylic jigs, each drilled to accept vinyl tubing and an individual LED were attached to a black acrylic platform with nylon bolts and wing nuts. PVC spacers under each jig allowed for vertical positioning of the LEDs, while the nylon bolts acted as the pivot point for horizontal movement (See Figure 2.)
The LEDs were pointed toward shaded areas of the coral fragments, where maximum ambient light intensity (with the LEDs off) was ~20 μmol·m2·sec. A mechanical timer switched the LEDs on and off and lamp photoperiod was incrementally increased from an initial 2 hours to 12 hours over a 30-day period.
Results – Round One
The reduced light level within the aquarium apparently caused loss of pink coral coloration within 2 weeks after transfer, and the animal turned to a ‘zooxanthellae brown’ color. The fragment’s soft tissues in heavily shaded areas (those not exposed to LED illumination) died and slowly receded.

Figure 3 and 4: On the left, loss of zooxanthellae (within black circle) apparently caused by red light on Day 22 of the experiment. On the right: Day 31. Expression of the pink coloration is noted (within the circle to the right of the bleached area) in area illuminated by the blue LED.
On Day 14 of the experiment, the yellow LED failed, due to a leak in the silicon seal between the LED and vinyl tubing.
On Day 22, loss of brown coloration in the area illuminated by the red LED was noted (see Figure 3). Examination of this area with Underwater Kinetics Light Canon dive light fitted with NightSea excitation and barrier filters revealed no chlorophyll red fluorescence, indicating the coral had ‘bleached.’ However, the coral tissue did not necrotize. (Note: Red fluorescence of chlorophyll should not be confused with the reflective pink/red pigments found in the coral host tissue. These are two entirely different pigments!)
On Day 28, coral fragments were again examined under the light of the dive light and filters. The area illuminated by the blue LED had strong chlorophyll red fluorescence. Normal, but not elevated, chlorophyll fluorescence was noted on the fragment illuminated by the green LED. The bleached area illuminated by the red LED remained, as it did on Day 22, apparently free of zooxanthellae and no chlorophyll fluorescence was noted.
On Day 31, a spot of pink coloration (~5 mm in diameter) was noted at the area illuminated by the blue LED (see Figure 4). Since that day, the spot has intensified in coloration but not size.

Figure 5: A close-up of the bleached and pink pigmented spots (circled). Notice that these areas are recessed and were shaded from direct sunlight during the experiment.
These results were briefly discussed during my 2002 MACNA presentation in Fort Worth, Texas and this article was submitted to Advanced Aquarist On-Line shortly thereafter. During the peer-review process, it was suggested that the experiments be replicated; the ‘new’ experiments should standardize the light intensities falling upon the corals’ surfaces. I agreed, but had no idea at the time that circumstances and time constraints would delay initiation of the project for almost half a year.
Procedure – Round Two
It was decided that the second round of the experiment would utilize only the blue and red LEDs, plus an LED producing ultraviolet radiation. Charlie Mazel of NightSea, Inc. was kind enough to provide several LEDs producing UV-A with a peak output at 370 nm. This peak wavelength is very close to the UV-A output of metal halide and other lamps containing the element mercury – these lamps have a ‘spike’ at 365 nm. What effect would UV-A radiation have on corals’ zooxanthellae and pigmentation?
The underwater cable and LED holder would have to be re-designed to prevent leakage and failure (especially a concern since the UV LEDs would be quite expensive to replace). The new underwater LED system was built and tested, and passed with flying colors (perhaps floating colors would be more appropriate).
Round Two would also involved fragments from the Rose Coral ( Pocillopora meandrina ). These fragments were collected (under a scientific collection permit from the Hawaii Department of Aquatic Resources) from a coral specimen damaged during a particularly strong early-Spring southern swell. The coral fragments were glued to acrylic pedestals and allowed to recover in the 257-liter aquarium at the Natural Energy Laboratory. As in Round One, natural sunlight intensity was attenuated with shade cloth and acrylic, and the coral fragments faded from hot pink to brown in a couple of weeks’ time.
Blue and red LED light intensities falling upon the coral fragments’ surfaces were standardized to 215 μmol·m2·sec. The UV-A LED was positioned to deliver approximately 300 microwatts per square centimeter (approximately 3X that measured in Walter Bobe’s mainland aquarium containing magnificently colored Acropora specimens). The photoperiod was set for 3 hours per night, and the experiment began. Photoperiod was incrementally increased every few days. There was some concern that the blue and red lamp intensities would not be sufficient to induce either color or bleaching. However, some lightening of the area illuminated by the red LED was noted on Day 23 of the experiment, with the photoperiod at 11 hours. No apparent changes were noted under the UV and blue LEDs. No further visual changes were observed through Day 50. A trip to the mainland prevented observations between Days 51 through 70. On Day 71, it was found that the coral areas under the blue LED had gained pink coloration, and the area illuminated by the red LED had lost more zooxanthellae – it had bleached (See Figures 6 and 7). No visible changes were observed within the area irradiated by the UV LED.
Discussion
A macro shot (below) of the Pocillopora meandrina and the area exposed to the blue LED. The pink pigmentation is not as intense or pervasive as seen in the initial experiments. Note: This is the same coral as in Figure 1 – a classic case of ‘_Pocillopora_ polymorphism’ in response to a change in environmental conditions (perhaps water motion).

Figure 6: The results of this experiment suggest that narrow bandwidths of essentially ‘pure’ red and blue wavelengths have profoundly different effects on zooxanthellae health and host tissue pigmentation.
It appears that red light induced bleaching in the two experiments. It is also worthy to note that bleaching was noticed on Day 22 and Day 23 of the first and second set of experiments, respectively, even when red LED lamp intensity differed by ~20%. What would explain this? In the 1940’s, Emerson et al demonstrated that monochromatic red light (at 680 nm) is about 36% more efficient in the promotion of photosynthesis than monochromatic blue light at 460 nm (reported in Hall and Rao, 1999). This is possibly due to the direct absorption of red wavelengths by chlorophylls and Pigment 680 (P-680) found in specialized chlorophyll molecules within the reaction centers of Photosystem II. The relative inefficiency of monochromatic blue light (as opposed to monochromatic red light) to promote photosynthesis might be caused by the less than perfect transfer of light energy collected by chlorophylls a and c, as well as some accessory antennae pigments- the major accessory carotenoid peridinin has been shown to transfer harvested light energy with >85% efficiency (Schofield et al., 1996). The energy collected by these pigments is channeled to the specialized P-680 chlorophyll molecules, and the convoluted process of photosynthesis is begun.
Red light of different wavelengths has an ability to promote photosynthesis in a phenomenon called the Emerson Enhancement Effect. Researchers determined that the total amount of photosynthesis promoted in the presence of a mixture of red (~650 nm) and far-red (>685 nm) light is greater than the sum of the amount of photosynthesis observed during separate experiments with individual beams of red and far-red light. These experiments’ results led to the discovery of two different types of chlorophyll a – one produces an oxidant and the other a reductant – and the realization that there are two distinct photosystems (I and II) acting in photosynthetic concert. In effect, far-red light prevents a traffic jam of electrons from occurring on the road connecting Photosystem II to Photosystem I (see Hall and Rao, 1999). This is quite interesting since corals often live in environments with depleted amounts of red light. To overcome this, zooxanthellae of corals living in shaded areas and deeper water alter the ratios of their photopigments and become more efficient in absorption of wavelengths above 680 nm (Titlyanov et al, 1980).
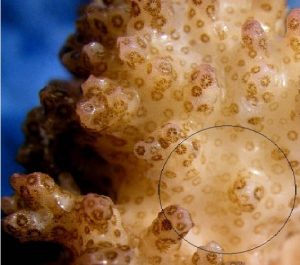
Figure 7: Another macro shot of P. meandrina. The area within the circle is the general area illuminated by the red LED. Note the reduced zooxanthellae within the coral polyps – the coral has bleached, though not as intensely as in the initial experiment where red light intensity was greater.
In order for photosynthesis to proceed smoothly, there must be a balanced energy distribution between Photosystem I and Photosystem II. Photosystem II produces an oxidant and PS I a reductant – which is important in maintaining the electron flow between the photosystems. This redox balance might be achieved through control and/or alteration of photosynthetic pigment content of zooxanthellae (Kinzie et al, 1984). In other words, zooxanthellae can custom tailor (within limitations) their photopigments to maximize use of available light energy. For instance, the major accessory pigment peridinin (which harvests light in the green portion of the spectrum, up to ~550 nm) transfers harvested light energy to chlorophyll a, and hence the reaction centers of PS II. On the other hand, the carotenoid beta-carotene transfers its harvested energy to chlorophyll a and PS I. Increased absorption of light energy above 680 nm is associated with aggregated forms of chlorophyll a and PS I (Titlyanov et al., 1980).
Since a water column rapidly attenuates red light, many, if not most, corals found on natural reefs are exposed to only a fraction of red light energy found at the water’s surface. Hence, chromatic adaptations by corals are a reversal of ‘sun’ and ‘shade’ terrestrial plants: Corals found at shallow depths (‘sun’ corals, if you will) are potentially adapted to, among other wavelengths, moderate amounts of radiation in the red portion of the spectrum, while deeper corals (‘shade’ corals) are likely adapted to an environment where green/blue wavelengths predominate and red light is greatly reduced. It is interesting to note that those pigments involved in photoprotective dynamic photoinhibition (i.e. xanthophylls diadinoxanthin and diatoxanthin) absorb blue wavelengths and not red wavelengths. Hence, coral zooxanthellae do not possess an ability to rapidly deal with red light and might bleach when suddenly exposed to increased amounts of red radiation.
Kinzie et al (1984, 1987) reported effects of different spectra (including blue, white, green, blue-green and red) on two Hawaiian corals ( Pocillopora damicornis and Montipora verrucosa – now M. capitata ). The results of these experiments suggest that red light promoted poor coral growth and zooxanthellae growth/reproduction. Interpreted by some to mean that red light is inefficient in the promotion of photosynthesis, it could be that exactly the opposite is true – that bleaching (either loss of algal cells or reduction in pigmentation) was caused by an exposure to elevated levels of more photosynthetically efficient red light. Consider that red light is attenuated by ~40% in the first meter of the clearest seawater – Type 1 Oceanic (Jerlov, 1976) – and much more in all other optical classifications of seawater.
The results of Kinzie et al. experiments, and those in these procedures, suggest that red light might play a role in regulating zooxanthellae pigmentation and density.
What are the possible effects on zooxanthellae of sudden exposure to altered spectral quality? This question is not easily answered; however, Iglesias- Prieto (1997) offers some interesting insights. Although this paper discusses thermal effects on zooxanthellae photosystems, some parallels are drawn between destruction of photosynthetic ability by heat and photosynthetic photon flux density. In essence, loss of re-oxidation capacity by Photosystem II creates an ‘excitation pressure’ (through generation of oxygen radicals) and can result in irreversible damage, possibly resulting in bleaching or photosynthetic pigment loss. It is possible that the red LED produced insufficient radiation to drive PS I, resulting in destructive pressure on PS II.
With that said, this discussion of bleaching and pigment loss will end – the primary purpose of my experiments was to observe the response of the coral host pigmentation to narrow bandwidth light sources.
The reasons for corals’ production of a pink pigment under blue light are not as easily explained – a theory could be advanced that some corals (likely only those genetically predisposed – see Takabayashi and Hoegh-Guldberg, 1995) react to blue light by the manufacture of reflective/fluorescent pigments. Could intensity of blue light be the environmental factor triggering the production of pink/red pigment(s) to protect zooxanthellae from the more photosynthetically efficient long-wave (red) visible light? It is interesting to note the time scale of pink pigmentation production under the differing blue light intensities used in the two experiments. Expression of the pink pigment at a light intensity of ~400 μmol·m2·sec was observed on Day 31 of the first experiment. In the second experiment with the red light intensity at 215 μmol·m2·sec, the pink pigment was weakly expressed between Days 50 and 70. These results suggest that the expression of the pink pigment is a response to blue light intensity. The concentration of the pigment (as judged visually) and the time frame required for expression of the pigment seems also to be linked to the intensity of blue light.
The blue and red LEDs produce essentially no ultraviolet radiation, strongly suggesting that UV played no part in either promotion of the bleaching episodes or the expression of the pink pigment. Near-infrared and infrared radiation (IR, which we perceive as heat) production by all these LEDs is extremely low, hence the energy transfer of near-IR and IR from water-cooled LEDs makes any harmful effect of thermal stress unlikely.
The UV-A LED produced no visual response on coral host coloration and zooxanthellae. This result suggests that UV-A radiation plays little or no part in inducing reflective/fluorescent pigments, at least in the case of the pink/red pigment in these P. meandrina specimens under the conditions of this experiment. (Fluorescent coral pigmentation in low UV environments has been previously reported – see Riddle and Amussen, 1998). Interestingly, the coral did not bleach when irradiated with 300 microwatts per square centimeter of UV-A energy (sunlight in Hawaii at noon on a clear day delivers approximately 2,100 microwatts of UV-A energy). Experiments are planned to examine the effects of ultraviolet energy from artificial light sources on captive corals. These experiments will measure the quantum yield of zooxanthellae through use of a pulsed amplitude modulation (PAM) fluorometer.
Lack of apparent response to the output of the green LED is more easily explained. First, the output intensity is very low at only 15 μmol·m2·sec and, second, the peak emission at 564 nm is just outside of the absorption bandwidth normally associated with the antenna pigment peridinin. For the second set of experiments an attempt to increase green light intensity was made by bundling multiple green LEDs. This was not successful – bundling did not significantly increase light intensity – and insight on the effects of monochromatic green light on coral host pigmentation remains elusive.
From a practical standpoint for hobbyists, the results suggest that narrow bandwidth blue light produced by the Nichia LED is sufficient to not only maintain zooxanthellae health (at least short-term) but can apparently promote colorful host tissue pigmentation, if light intensity is high enough. Of more importance are perhaps the observations of bleaching, and the realization of potential effects of light quality from artificial light sources on captive corals. The possibility certainly exists that red light content of artificial light sources is the environmental trigger for control of pigment content and/or zooxanthellae density within captive corals.
Many questions remain unanswered. What are the results on coral/zooxanthellae pigments when red and blue LEDs are bundled and the coral is subjected to a reasonably balanced spectrum? Are the results of these experiments applicable to common aquaria lamps such as fluorescent and metal halide lamps? Results of recent experiments seem to confirm that broadband spectral qualities make little difference to photoacclimated Fungia corals (within the context of rates of photosynthesis and the conditions of the experiment – Riddle, article in preparation). However, the effects of spectral quality on corals with the ability to alter apparent coloration through expression of reflective and fluorescent pigments seem to be a different story.
Acknowledgements
I wish to thank Sara Peck of Sea Grant Hawaii for her unwavering support, and to Charlie Mazel of NightSea, Inc. (www.nightsea.com) for providing the Nichia LEDs and encouragement. Many thanks also go to the Friends of NELHA for all those little things that added up to a lot.
References
- Fux, E. and C. Mazel, Unpublished. An experimental method to separate the fluorescence and reflectance components of the spectral signatures of corals.
- Fux, E. and C. Mazel, 1999. Unmixing coral fluorescence emission spectra and predicting new spectra under different excitation conditions. Applied Optics. 38, 3: 486-494.
- Hall, D. and K. Rao, 1999. Photosynthesis: Studies in Biology. Cambridge University Press, Cambridge, UK. 214 pp.
- Iglesias-Prieto, R., 1997. Temperature dependant inactivation of photosystem II in symbiotic dinoflagellates. Proc. 8th Int. Coral Reef Symp., Panama. 2:1313-1318.
- Jerlov, N., 1976. Marine Optics. Elsevier Oceanography Series, Elsevier Sci. Publ. Co., New York. 231 pp.
- Kawaguti, S., 1944. On the physiology of reef corals VI. Study on the pigments. Palao Trop. Biol. Sta. Study, 2:617-674.
- Kinzie, R.A., P.L. Jokiel and R. York, 1984. Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar. Biol., 78:239-248.
- Kinzie, R.A. and T. Hunter, 1987. Effect of light quality on photosynthesis of the reef coral Montipora verrucosa. Mar. Biol., 94: 95-109.
- Mazel, C.H., 1997. Coral fluorescence characteristics: excitation – emission spectra, fluorescence efficiencies, and contribution to apparent reflectance. Ocean Optics XIII. 240-245.
- Mazel, C.H., 1995. Spectral measurements of fluorescence emission in Caribbean cnidarians. Mar. Ecol. Prog. Ser., 120:185-191.
- Riddle, D. and A. Amussen, 1998. Coral Pigments. www.reefs.org/library/talklog/d-riddle-042698.html
- Salih, A., A. Larkum, G. Cox, M. Kuhl and O. Hoegh-Guldberg, 2000. Fluorescent pigments in corals are photoprotective. Nature, 408: 850-856.
- Schofield, O., B. Prezelin and G. Johnsen, 1996. Wavelength dependency of the maximum quantum yield of carbon fixation for two red tide dinoflagellates, Heterocapsa pygmaea and Prorocentrum minimum ( Pyrrophyta ): Implications for measuring photosynthetic rates. J. Phycol., 32, 574-583.
- Takabayashi, M. and O. Hoegh-Guldberg, 1995. Ecological and physiological differences between two colour morphs of the coral Pocillopora damicornis. Mar. Biol., 123: 705-714.
- Titlyanov, E.A., M.G. Shaposhnikova and V.I. Zvalinskii, 1980. Photosynthesis and adaptation of corals to irradiance. I. Contents and native state of photosynthetic pigments in symbiotic microalga. Photosynthetica 14 (3): 413-421.




0 Comments