In the mid-1980’s, when the reef hobby was in its infancy in North America, pioneer reefkeepers were literally bombarded with information. Trickle filters, surface overflows, high output lighting, calcium additions, etc. were all new concepts in the US, and our learning curve was a steep one indeed. As time passed, we learned from our mistakes and improved our husbandry techniques until we arrived at our degrees of success we enjoy today. Advances are, for the most part, of smaller degree and less spectacular than those of just 10 years ago. However, we still have missing pieces to the puzzle; this article details the results of experiments designed to answer one of the questions and add a small part to the overall picture. Sometimes results simply seem to confirm the anecdotal observations.
Rates of zooxanthellae photosynthesis were found to be substantially higher in corals maintained in a ‘closed’ system, as opposed to an ‘open’ system with flow-through of natural seawater (NSW). The ‘closed’ system had relatively high nutrient concentrations, and used artificial seawater (ASW).
Rates of photosynthesis with Symbiodinium species (zooxanthellae) are known to be variable as a result of environmental conditions. Certainly, hobbyists recognize that photosynthesis varies in relation to the amount of light (PAR or PPFD) falling upon the coral. However, other factors are involved, including water motion and nutrient concentrations.
The word ‘nutrients’ is usually defined as those ‘elements required for nourishment’. The ‘major’ nutrients required for plant or algae growth include carbon, nitrogen, phosphorus and potassium, and ‘micro-nutrients’ are known to be iron, copper, magnesium, zinc, and a host of others. Symbiotic dinoflagellates (zooxanthellae) have similar nutrient requirements for growth and reproduction. This article will examine the impact of relatively high nutrient concentrations that are typical of an ‘average’ aquarium (for further information, see Atkinson et. al., 1995). Reasons for the observed elevated photosynthetic rates are discussed, as well as implications on the use of artificial seawater. Suggestions on optimizing photosynthetic rates are also made.
Also, there has been some debate about the impact of certain micro-nutrients (‘trace elements’) on coral/zooxanthellae health. A concise review of literature on this subject is presented, and a circumstantial case is made about ASW ‘metal toxicity”.
Procedure
Efficiencies of photosynthesis were analyzed by use of a pulse amplitude modulated (PAM) Chlorophyll Fluorometer (“Teaching PAM”, Heinz Walz GmbH, Effeltrich, Germany). The aquarium – a ‘closed’ system, filled with artificial seawater – contained several corals that had been maintained for months. Quantum yields of Photosystem II within the corals’ zooxanthellae were measured while holding the tip of the PAM’s fiber optic cord to the surface of each coral.
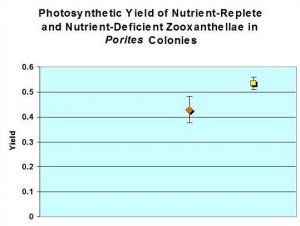
Figure 1: Photosynthetic Yields. Yield of Porites’ zooxanthellae from a ‘closed’ aquarium (yellow square) is higher than that of Porites from an ‘open’ system (orange diamond), and is probably significant (Student’s t-test: t = 7.319; p = 0.025; n=3). Error bars represent the standard deviation.
Results
Rates of photosynthesis were higher within the ‘closed system’ coral zooxanthellae. See Figure 1.
Discussion
The yields of both sets of measurements are higher than those of corals on natural reefs. Gorbunov et. al., (2000) report a variety of corals in the wild to possess an average photosynthetic yield of 0.39 ± 0.07 ( n = 350). There are several factors that could affect photosynthetic yield, including light intensity (and possible photoinhibition), nutrient availability, variations in nutrient acquisition, and possibly differences in architecture of photosynthetic units – or PSUs (Gorbunov et al., 2000). It is believed that the corals within both captive systems were exposed to an elevated nutrient concentration, and this accounts for the increased yield (see Falkowski and Kolber, 1995). This conclusion is based on the variables that could affect photosynthetic yield:
Light Intensity
These procedures were performed according to standard practice; i.e., corals were allowed to ‘dark-adapt’ for at least 20 minutes. Minimum (Fo) and Maximum (Fm) fluorescence were determined using the PAM fluorometer’s internal light source as the saturating actinic source. Since light intensity was, and is, easily standardized with the PAM, we can make the assumption that variable light intensity played no part.
The ‘light history’ of the coral likely made no difference in photosynthetic yield (see Gorbunov et al., 2000). In addition, there is limited evidence that ‘over lighting’ of reef tanks is possible even when using lower wattage lamps, and higher photosynthetic yields are possible at reduced light levels (Riddle, 2004b).
Water Motion
Before discussing nutrients and their effects on zooxanthellae photosynthesis, we should examine the impact of water motion on photosynthesis. Water movement, either laminar or turbulent, is required to lessen the boundary layer that exists around all aquatic objects. A boundary layer is simply a ‘skin’ of stagnant water surrounding a submersed object, and the boundary layer’s thickness is related to water movement. When water motion is low, the boundary layer thickens. The boundary layer decreases in thickness as water velocity increases. It is important to maintain proper water motion in aquaria. For instance, when the requirement of an element (say iron) by zooxanthellate corals exceeds the ability of the element to diffuse through the stagnant boundary layer, a nutrient deficiency will be created, and the possibility of inhibition of photosynthesis greatly increases.
Lack of water motion, or more correctly, long-term effects of deficient water motion are likely observed only in aquaria. Results of recent experiments have shown that potential effects of low water velocities around and over a relatively smooth, encrusting or mound-like coral (such as Porites ) can easily be negated with minimal water movement (Riddle, 2004a). Work is underway to establish effects of water motion on branching corals’ rates of photosynthesis.
All chlorophyll fluorescence measurements were made with ‘good’ water motion created by either powerheads or strong aeration. It is believed that water movement was sufficient to overcome diffusion-related issues.
Nutrients
Nutrients include carbon, nitrogen, phosphorus, potassium and micro-nutrients, and availability of these nutrients is associated with a number of factors, including concentration and diffusion limitations imposed by insufficient water motion. As we have just seen, we can likely rule out water motion as a negative determinate in these experiments.
Inorganic Carbon
For our purposes, ‘inorganic carbon’ is defined as carbon dioxide (CO2) and bicarbonate (HCO-3) ions. Many hobbyists suppose that carbon dioxide is the inorganic carbon species utilized in aquatic photosynthesis, and this notion is partially correct. At the pH of seawater, more than 80% of inorganic carbon exists as bicarbonate ions (Kirk, 2000). An enzyme, carbonic anhydrase, dehydrates HCO-3 to CO2 which is then used as the carbon source.
The water of the closed aquarium was tested for inorganic carbon. A ‘drip titration’ method (Lamotte) indicated the presence of Total Alkalinity = 104 ppm alkalinity as CaCO3 (or 5.8 dKH, or 2.08 meq/l, if one prefers). As a footnote, this alkalinity kit measured total alkalinity of natural seawater as 144 ppm as CaCO3 (or 8.0 dKH, or 2.86 meq/l).
Of course, coral host tissue will contain some carbon dioxide for use by zooxanthellae. Many aquaria, even those with heavy fish loadings, will have no measurable carbon dioxide when using titration methods (and this was the case with this particular aquarium – a test using a LaMotte CO2 ‘kit’ resulted in ‘zero’ free carbon dioxide).
The results of these tests indicate that the water of the aquarium was apparently deficient of inorganic carbon when compared to natural seawater. However, diffusion of inorganic carbon, even at this lower concentration, is probably not limited by low water velocity and boundary layer issues.
Increased bicarbonate concentrations have been linked to elevated growth rates of stony corals (Marubini and Thake, 1999). These researchers advanced the theory that suggested elevated ammonium (Hoegh-Guldberg and Smith, 1989) and nitrate (Marubini and Davies, 1996) will increase the resident population of zooxanthellae and photosynthetic pigment concentrations. Hence, the increased population of zooxanthellae will compete with the coral animal for inorganic carbon, usually resulting in reduced rates of calcification.
So, how high was the nitrogen level of the ‘closed’ aquarium?
Nitrogen
Nitrogen can exist in many organic or inorganic forms. The metric for nitrogen used in this procedure was nitrate. A Tetra Nitrate test kit estimated ~6 mg/l as nitrate (or about 1.4 mg/l nitrate as N). This result suggests that the aquarium water was enriched with nitrogen. Ammonium is probably the preferred source of nitrogen for corals, but nitrate uptake is possible when ammonium is limited (Atkinson et. al., 1994).
Ammonia/ammonium exists in spiked pulses in aquaria, usually, and naturally enough, a few hours after fishes are fed. The nitrate concentration is an indirect indicator of ammonia/ammonium.
With this said, some researchers believe that photosynthetic yields of zooxanthellae are low (e.g., 0.40, compared to 0.73 for macroalgae and seagrasses) due to nitrogen starvation (Kolber et al., 1988; Gorbunov et al., 2000). Zooxanthellae isolated from corals and grown in nutrient rich conditions have higher yields (0.62 to 0.66 – Kolber et al., 1988).
Phosphorus
An Aquarium Systems Phosphate test kit found the orthophosphate at ~0.2 mg/l as PO4. As with nitrogen, the aquarium water is enriched with phosphorus when compared to natural seawater. Phosphorus uptake by corals and hence zooxanthellae is proportional to water velocity (Atkinson and Bilger, 1992).
Orthophosphate is generally considered detrimental to the calcification process (Simkiss, 1964). However, Simkiss also demonstrated that high levels of bicarbonate, relative to phosphate, could overcome the inhibitory effects of phosphorus (even when calcium concentrations were relatively low).
Conclusions
Evidence suggests that elevated concentrations of two major nutrients (nitrogen and phosphorus) could be responsible for the higher photosynthetic yields within the ‘closed’ system corals. Generally, we tend to think that coral calcification and skeletal growth are linked to higher photosynthetic rates, but this is not necessarily the case. Zooxanthellae compete with the coral animal for available resources such as nitrogen and inorganic carbon.
Nitrogen and phosphorus concentrations in aquaria are generally elevated in respect to clean natural seawater, and these ‘fertilizers’ are likely responsible for increased population of, or more photosynthetically efficient, zooxanthellae within a coral’s tissues. (Increased coral growth has been noted near a Red Sea fish farm and increased nutrients are believed responsible – see Bongiorni et al., 2003).
Since alkalinity was relatively low, bicarbonate was probably not a factor in promotion of elevated rates of photosynthesis within the ASW aquarium, but higher-than-normal alkalinity seems to be very important for supplying carbon for skeletal growth. The evidence suggests that high alkalinity is a positive factor in aquaria.
Nutrient preferences and effects of nutrient enrichment/depletion on zooxanthellate corals within aquaria can be determined by further testing.
For now, these are factors seem important in maintaining high photosynthetic yields:
- Light intensity (high intensity is not necessarily better). Recent experiments have shown that rate of photosynthesis drops at higher light intensity, and ‘excess’ light energy is not used in photosynthesis and is dissipated as non-radiant heat via the xanthophyll cycle.
- Water movement is critical – it should be sufficient to completely bathe all coral polyps in order to reduce boundary layer thickness and related diffusion issues.
- Manage nutrient levels. Use efficient protein skimming and/or algal filtration for nutrient export.
- Maintain alkalinity values above that of natural seawater – 12 to 15 dKH to keep the inorganic carbon ratio more in line with the elevated nitrogen and phosphorus levels found in many aquaria. Elevated inorganic carbon levels (in the form of bicarbonate) will support photosynthesis and skeletal growth.
These factors are much along the guidelines established by anecdotal observations – and now data is lending support to these claims.
And, now, a bit of speculation:
‘Trace Elements’
‘Trace elements’ – found in abundance in artificial seawater (Atkinson and Bingman, 1999) – have been labeled as ’cause for concern’ (Shimek, 2002a; 2002b) and potentially benign (Sekha, 2003; Harker, 2003, 2004a, 2004b). These authors have presented evidence to support their claims. I will add one more opinion.
If chronic inhibition of photosynthesis can be caused by accumulation of ‘toxic’ metals, it is not apparent in the PAM measurements of the ASW corals.
Zooxanthellae, and corals, are known to concentrate heavy metals within their tissues (Bastida and Garcia, 1997). In fact, researchers have speculated that zooxanthellae may actually help regulate metals within coral tissues. A theory suggests that older, metal laden zooxanthellae are routinely expelled, thus the coral maintains only the most competent algal cells (and those with lower metals concentrations – see Harland and Brown, 1989).
Others have presented evidence that ‘excess’ metals are purged from coral tissues along with excess fixed carbon in the form of mucus (Cofforth, 1988). Mitchell and Chet (1975) demonstrated that the stony coral Platygyra survived for 10 days in the presence of 1,000 ppm (!) copper sulfate – perhaps the excessive amounts of mucus produced insulated the coral from the copper by thickening of the boundary layer. One could also speculate that mucus chelated the copper. In any case, expelled zooxanthellae and undissolved coral mucus can be removed from aquarium water by mechanical filtration or protein skimming. See Wild et al., 2004, for interesting observations on the various roles of coral mucus.
Interestingly, Shimek’s own data (2002a) could support a theory of photosynthesis limitation through lack of an important micronutrient – iron. An argument could also be made that elevated concentrations of ‘trace elements’ are required to maintain their relationship with the classical Redfield C:N:P values of 106:16:1. For further reading on iron in reef aquaria, and Redfield values, see Holmes-Farley (2002), and Klausmeier et al., (2004), respectively.
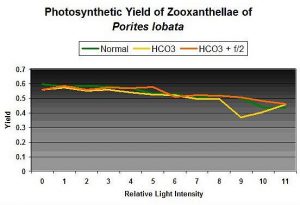
Figure 2: Photosynthetic yields of zooxanthellae of Porites lobata in matured artificial seawater, with added alkalinity, and addition of Guillard’s f/2 algal nutrient.
Post Script
I had a hypothesis that nutrient concentrations, if suddenly elevated, could stress zooxanthellae. There is some previous research that suggests certain dinoflagellate species are sensitive to pH modulations (Dason and Colman, 2004).
Curiosity got the best of me, and I conducted some quick tests. See Figure 2. Initial photosynthetic yield of a Porites lobata was tested over a range of light intensities. Alkalinity was boosted by 50% (100 to ~150 mg/l as CaCO3), and another set of yield measurements was made after approximately 20 minutes. Then a generous dose (27 milliliters each of Parts I & II to the 2,600 ml container holding the coral) of an algal nutrient mixture (Guillard’s f/2 formula) was added, and the coral was allowed in ‘soak’ in the increased nutrients for 24 hours. Then another set of yield measurements was made.
My hypothesis was not correct – there is no clear sign that rates of photosynthesis increased (or decreased, for that matter) dramatically and instantaneously as a response to elevated nutrient levels (for this particular coral under the conditions of the experiment). The goal of this experiment was not to produce results suggesting that elevated nutrients (nitrogen and phosphorus in particular) are not a concern – they, in the long term, could produce undesirable effects in a reef aquarium. These results do suggest that elevated nutrients are not an immediate concern if an aquarium is managed properly. As such, transferal of corals between aquaria with large differences in nutrient levels has been shown in this case to not cause a sudden increase in photosynthesis.
Guillard’s f/2 formula contains (based on data provided by the manufacturer): 93,300 mg/l nitrogen (sodium nitrate), 20,000 mg/l phosphorus (monosodium phosphate), iron (8,200 mg/l), manganese (340 mg/l), cobalt (20 mg/l), zinc (37 mg/l), copper (17 mg/l), molybdate (9 mg/l) and B vitamins. These components are chelated and soluble (“bio-available”) to zooxanthellae. There’s enough information to calculate theoretical nutrient concentrations – I’ll leave the calculations for someone else. Suffice it to say that the nutrient concentrations were elevated well above that of the ocean and probably most aquaria. (Note: Even with ‘strong’ aeration, there was some precipitate in the bottom of the container that appeared to be mostly iron.)
These results suggest that elevated nutrients and certain elements are not immediate stressors to this coral and zooxanthellae species. Although pH modulation is suggested to be a stressor to some dinoflagellate species, apparently short-term exposure to elevated concentrations of elements required for photosynthesis is not, at least in this case. Obviously, this particular experiment’s protocol needs some work, but there is little doubt that elements were elevated. For practical purposes, this result suggests that at least some coral species and their zooxanthellae will not immediately bleach if exposed to modulating nutrient concentrations (such as transferal from one aquarium to another). Exposure to elevated macro- and micro-nutrients, short-term and long-term, will be investigated under more rigid and controlled conditions. More later.
References
- Atkinson, M. and C. Bingman, 1999. The composition of several synthetic seawater mixes. Aquarium Frontiers Online. March, 7 pp.
- Atkinson, M.J., E. Kolter and P. Newton, 1994. Effects of water velocity on respiration, calcification and ammonium uptake of a Porites compressa community. Pac. Sci., 48(3):296-303.
- Atkinson, M.J., B. Carlson and G.L. Crow, 1995. Coral growth in high nutrient, low-pH seawater: a case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs.
- Atkinson, M.J. and R.W. Bilger, 1992. Effects of water velocity on phosphate uptake in coral reef-flat communities. Limnol. Oceanogr., 37(2):273-279.
- Bastidas, C. and E. Garcia, 1997. Metal concentration in tissue and skeleton of the coral Montastrea annularis at a Venezuelan reef. Proc. 8th Int. Coral Reef Symp., Panama. 2:1847-1851.
- Bongiorni, L., Shafir, S., Angel, D. and B. Rinkevich, 2003. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Marine Ecology Progress Series 253:137-144.
- Cofforth, M., The function and fate of mucous sheets produced by reef coelenterates. Proc. 6th Int. Coral Reef Symp., 2: 15-20.
- Dason, J. and B. Colman, 2004. Inhibition of growth in two dinoflagellates by rapid changes in pH. Can. J. Bot./Rev. Can. Bot. 82(4): 515-520.
- Falkowski, P. and Z. Kolber, 1995. Variations in chlorophyll fluorescence yields in phytoplankton in the world’s oceans. Aust. J. Plant Physiol., 22: 341-355.
- Gorbunov, M., P. Falkowski and Z. Kolber, 2000. Measurement of photosynthetic parameters in benthic organisms in situ using a SCUBA-based fast repetition fluorometer. Limnol. Oceanogr., 45(1), 242-245.
- Harker, R., 2003. Is it really in the water? A critical reexamination of toxic metals in reef tanks. Advanced Aquarist Online. II, 12.
- Harker, R., 2004a. Is it really in the water? A critical reexamination of toxic metals in reef tanks, Part 2. Advanced Aquarist Online. II, 12.
- Harker, R., 2004b. Is it really in the water? A critical reexamination of toxic metals in reef tanks, Part 3. Advanced Aquarist Online. II, 12.
- Harland, A. and B. Brown, 1997. Metal tolerance in the scleractinian coral Porites lutea. Mar. Pull. Bull., 20:353-357.
- Holmes-Farley, R., 2002. Iron in the reef aquarium. Advanced Aquarist Online. Volume I, Issue 8.
- Klausmeier, C., E. Litchman, T. Daufresne and S. Levin, 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature, 429, 6988: 171-174.
- Marubini, F. and P. Davies, 1999. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol, 127: 319-328.
- Marubini, F. and B. Thake, 1999. Bicarbonate addition promotes coral growth. Limnol. Oceanogr., 44(3):716-720.
- Riddle, D., 2004a. PAM fluorometer experiments. Advanced Aquarist Online. III, 6.
- Riddle, D., 2004b. Too much light! Advanced Aquarist Online. III, 7.
- Sekha, H., 2003. Toxicity of trace elements: Truth or myth? Advanced Aquarist Online. II, 5.
- Shimek, R.L. 2002a. It’s (in) the water. Reefkeeping.com February 2002 http://reefkeeping.com/issues/2002-02/rs/feature/index.htm
- Shimek, R.L. 2002b. It’s still in the water. Reefkeeping.com March 2002 http://reefkeeping.com/issues/2002-03/rs/feature/index.htm
- Simkiss, K., 1964. Phosphates as crystal poisons of calcification. Biol. Rev., 39: 487-505.
- Wild, C., M. Huettel, A. Klueter, S. Kremb, M. Rasheed and B. Jergensen,
- Coral mucus functions as energy carrier and particle trap in the reef ecosystem. Nature, 428, 6978: 66-70.



0 Comments