We often hear that stability of the physical and chemical environments are extremely important in the successful maintenance of a reef aquarium, and of course this is true. Significant deviations of any of the major parameters (such as temperature, pH, salinity and many others) from the accepted norm can cause a collapse of the ecosystem with dire consequences for the captive animals. But how does the stability of an aquarium compare to that of a natural reef? I recall reading in a peer-reviewed research article that aquaria conditions were ‘stressful’ to their inhabitants – is this necessarily so?

Low tide at Kahalu’u (Keauhou), Big Island of Hawai’i, site of many observations discussed in this article. In the foreground are tide pools. Above and to the left of the tide pools is the reef crest (where the wave is breaking). The relatively calm water to the right of the reef crest is the reef flat.
Before continuing, we must realize that no single definition adequately describes what a ‘natural reef’ actually is. Corals grow where environmental conditions can be tolerated, including distinct biotopes such as tide pool, reef flat, and reef slope.
In this article, we’ll compare some physical parameters of various types of reef biotopes to those observed in a small aquarium. How does the stability of this 140 gallon system stack up to various natural environments? What are the long term effects of the varying conditions seen in an aquarium?
This report is not meant to be an exhaustive analysis of aquarium conditions, but I have attempted to include the impacts that several factors could have. Some of this information allows us to make generalized statements regarding different situations.
Reef Biotopes
We generally think of a coral reef as a community consisting of a staggering number of animals found in shallow, wave-washed, tropical waters. Although true in many cases, a reef can actually be a grouping of non-photosynthetic corals living in dark, frigid water.
For the purposes of this article, we’ll divide a reef into 3 different biotopes, including tide pool, reef flat, reef crest, reef slope and rubble zone and we’ll examine data from 3 different locations (tide pool, reef flat, and reef slope). These are the definitions:
- Tide Pool
- A relatively small body of water isolated from the ocean by a falling tide. A rising tide reconnects the two bodies of water.
- Reef Flat
- The portion of a reef between the beach and the reef crest. The reef crest usually absorbs most of the wave energy, thus the reef flat is often semi-protected.
- Reef Slope
- A transition from shallow to deeper water on the ocean side of the reef crest.
See the introduction photo for an image of these biotopes.
Description of the Aquarium System
Aquaria data were collected from a system totaling about 140 gallons in total volume. The display aquarium holds approximately 55 gallons and contains marine algae and a number of photosynthetic invertebrates. This tank is illuminated for about 12 hours with six 39-watt T5 fluorescent lamps. An overflow allows water to gravitate to a 100 gallon Rubbermaid™ tub (holding about 85 gallons of water). This sump contains marine algae and is illuminated with a fluorescent lamp at night, when the display aquarium lights are off. An ETS™ model 800 protein skimmer is driven with a MagDrive 900™ pump and operates continuously. An Ehiem™ 1250 water pump provides water circulation between sump and display tanks.
We’ll begin our discussion of various physical parameters with temperature.
Temperature
Temperature is an important physical parameter since it is a major factor in biological activities. For instance, if the temperature is too warm or too cool, life-supporting enzymes might fail to function resulting in the death of the exposed organism. It is generally accepted that biological activities double with every increase of temperature in increments of 5.5°C (~10°F) – within the limitations thermal tolerances of course!
Generally, ‘tropical’ reefs grow where the water surface temperature does not drop below 20° C (68° F). Upper temperature limits depend upon the adaptive nature of zooxanthella ‘types’ and their tolerance of warm water, but, for aquaria, the generally recommended upper limit is ~27° C (~80° F).

Figure 1. Water temperature of a reef flat in Hawai’i. The range is approximately 2°C (3.6°F) over the course of the data set. Lower observed temperature was ~25.5° C (78°F) to 27.5°C (81.5°F).
Temperature was measured with a WatchDog™ data logger and submersible temperature probe manufactured by Spectrum Technologies, Inc., Plainfield, Illinois.
Temperature of a Reef Flat
This reef flat is situated on the west coast of the Big Island of Hawai’i in Keauhou and is referred to as the Kahalu’u Beach Park. A man-made stone wall offers the flat some protection from the surf but does not prevent good water exchange and mixing. Water depth varied with the tide and was just a few inches in the morning hours and about 1 meter in depth during the afternoon high tide.
Temperature was monitored from about 6 am to 5 pm, when retrieval of the datalogger became necessary due to rough surf.
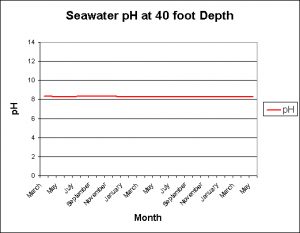
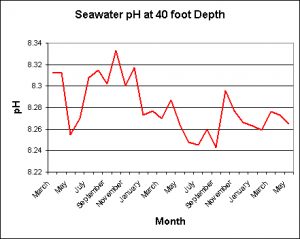
Temperature of a Reef Slope
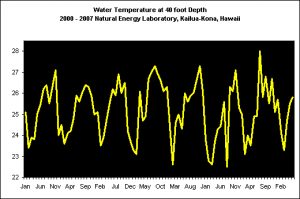
The following data were provided by the Natural Energy Laboratory (NELHA) on the Big Island of Hawaii. These measurements were taken on their shallow-water intake located at a depth of ~40 feet. Taken monthly, Figure X demonstrates temperature modulations seen over the course of 2000-2007. The range is approximately 22.5° C (72.5° F) to ~28.5° C (83.3° F).
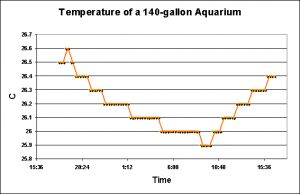
Temperature in an Aquarium
As would be expected, temperature of the aquarium water rose when the lights (6 T5 lamps) were on. The delta temperature observed was 0.7°C (1.26°F).
pH
pH is a logarithmic scale expressing the acidic or basic characteristics of a solution. As we know, natural seawater is often slightly basic in nature with a pH of about 8.0-8.5. However, extraneous influences (such as fresh water intrusion or stagnation combined with modulations in CO2 concentrations and temperature) can create conditions where pH values are outside of a traditionally-accepted range.
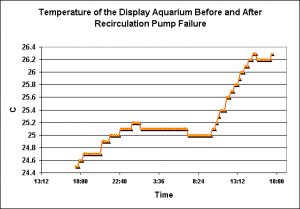
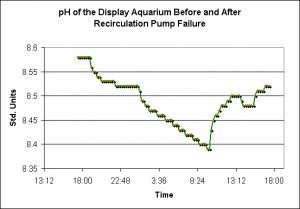
Figure 4. Size matters! Larger bodies of water will tend to be more stable than smaller bodies. Note the rapid increase in temperature when the recirculation pump failed at about 10 am.
A great number of chemical and biochemical reactions depend upon the pH of the surrounding water. In the case of symbiotic invertebrates, pH is also influenced by the photosynthetic activity of the symbiont and respiration of the host coral.
pH values of the west Hawaii tide pool, reef flat and aquarium (reported below) were determined through use of a data logging device (a HQ40D multi-meter manufactured by Hach, Loveland, CO, USA) and a standard pH probe. pH values from the intake at the Natural Energy Laboratories Hawaii Authority (NELHA) were obtained from their laboratory. Samples were grabs and were analyzed at their onsite lab.
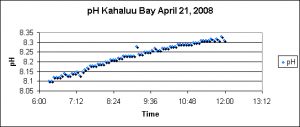
pH – Reef Flat
pH measurements were made using a Hach HQ40d multi-meter and pH probe during one of the seasonal spawnings of Pocillopora meandrina.
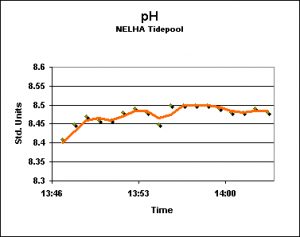
pH: Tide Pool
The entrance road to the Natural Energy Laboratories in Kailua-Kona, Hawaii borders the Pacific Ocean and many tide pools can be found to the west of the highway. The Hach HQ40d multi-meter with pH and dissolved oxygen probes was deployed for a few minutes during low tide. Only occasionally did a wave break with enough force to push fresh ocean into the semi-isolated tide pool. It was my thought that this environment would display extreme environmental conditions, but this was not the case during the few minutes of data logging. See Figure 6.
pH: Reef Slope
Again, many thanks to the Natural Energy Labs staff for providing the following information.
pH: Aquarium
An aquarium is subject to a number of variables that can influence its environmental conditions and pH is no exception. Light intensity and photoperiod can cause diurnal pH swings due to photosynthesis, while chemical additions (such as saturated calcium water) have an immediate effect.
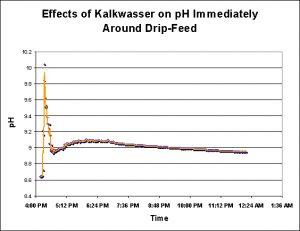
Addition of calcium hydroxide slaked in water (‘kalkwasser’) to the 140 gallon system elevated the pH by about 0.3 standard units. The total amount of kalkwasser addition was ~4.5 gallons, and was drip-fed into the 90-gallon sump over the course of a few hours. pH was measured in the display aquarium, and well away from the kalkwasser’s introduction point (see Figure 10).
To determine the effects to the pH in the close proximity of kalkwasser addition, the pH probe was placed about 1 inch away from the point of drip feed. pH modulations were more extreme here, as would be expected. See Figure 11.
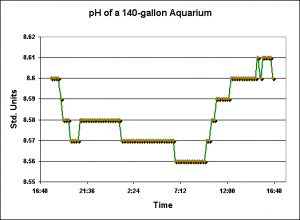
Effects of Refugium Lighting on Night Time pH
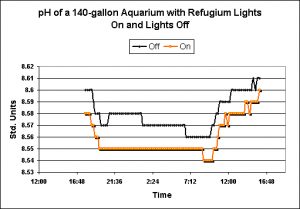
Figure 12. pH values observed in the display tank when lights were either off or on over the 90-gallon refugium.
One of the many attributes of a refugium lighted during the nighttime is purportedly that of pH stability. It is assumed that respiration will offset the effects of photosynthesis and vice versa in a continuous balancing act of nature. See Figure 12 for examples of the effects of a system with refugium lights on, and lights off.
Effects of Recirculation Pump Failure
Murphy’s Law seems especially applicable to reef aquaria where the results of even seemingly minor failures or mistakes can have profound effects.
Salinity
Salinity is the measure of the mass of salts dissolved in a given amount of water. Salinity values are generally 30 to 35 parts per thousand (ppt), although, as with other parameters, are subject to external influences. For instance, the evaporation rate of the Red Sea has led to higher than ‘normal’ salinities, while seawater in other areas is sometimes subject to the influence of surface or ground water.
There are various ways to measure salinity. The method used here is the most precise. Salinity values of the west Hawaii reef flat and aquarium (reported below) were obtained by use of a data logging device (a HQ40D multi-meter manufactured by Hach, Loveland, CO, USA) and a standard conductivity probe.
Salinity: Reef Flat
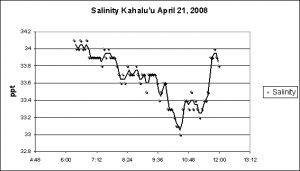
The Kahalu’u reef flat is subjected to a naturally-occurring and substantial inflow of freshwater. No permanent streams exist on the west side of the Big Island; however, rainfall atop the dormant Hualalai volcano filters through fissures in lava rock and migrates downwards to the ocean. This might account for the trend of falling salinity during the morning low tide. See Figure 14.
Salinity: Aquarium
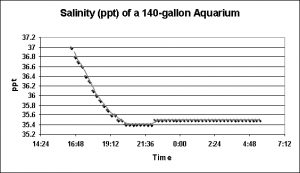
Figure 15 shows the effects of freshwater addition via a drip feed to replace water evaporated from the system. As can be noted, the salinity fell by 1.6 parts per thousand over the course of approximately 4 hours and then stabilized.
Discussion
We will now compare the data collected from various sources, beginning with temperature.
Temperature: More Stable Than Nature
In the cases examined for this article, temperature was determined to be more stable than that observed in nature. No attempt is made to mimic natural seasonal temperatures within this aquarium, although means to do so are available (though not considered inexpensive).
Water has a rather high specific heat, and it is interesting that this small volume of water maintains temperature stability as well as it does. However, when the recirculation pump failed, thus isolating the aquarium with the larger refugium, temperature rose due to heat generated by the lamps and the internal powerheads. This is a good example of a smaller water body’s inability to maintain temperature stability. Interestingly, this is not necessarily a bad thing.
Water temperature has been linked to inducing some corals to spawn (although there is some debate whether it is annual solar radiation with temperature merely being of secondary importance). Deliberate manipulation of physical parameters within aquaria (such as temperature) serves to mimic nature. The modulations seen in the aquarium in these experiments does not approach the annual temperature swings observed on a natural reef. Does this make a difference? This is a question that only the aquarist can answer. Temperature stability is important, but modulations possibly play an important part in closing life cycles of at least some marine invertebrates.
pH: More Stable Than Nature
I was surprised by the results of these investigations. I assumed that pH swings in semi-isolated tidal pools would far exceed that seen in an aquarium. This was not the case. The effects of 5 gallons of settled kalkwasser caused local pH modulations that exceeded anything recorded in three natural reef environments (see Figure 16). Although I did not investigate the effects of a run-away calcium reactor (with a pH lowering excessive carbon dioxide feed), I assume that relatively low pH values are possible in an aquarium this size.
More surprises were noted in the results of the refugium lights-on and lights-off experiments. Upon reflection, the observed results make perfect sense. The process known as ‘reverse daylight photosynthesis’ (or RDP) in theory stabilizes pH (and other parameters such as dissolved oxygen) by ‘balancing’ the effects of photosynthesis and animal/plant respiration. The results noted in this investigation revealed that a refugium illuminated at night does not necessarily negate the effects of respiration of animals in the display aquarium. There are several factors to consider, including refugium size, light intensity in the refugium, respiration rates of the marine algae, fishes and marine invertebrates. Even simple observations of processes involved in ‘balancing’ pH through use of RPD would be time-consuming. At the least, aquarists should be aware that a refugium does not guarantee stability of certain physical and chemical parameters.
Salinity: Less Stable Than Nature
Another surprise. The reef flat at Kahalu’u Beach Park is subject to a lot of fresh water intrusion, and this would certainly have an effect on salinity and water temperature. It has been estimated that portions of the ocean along the west coast of the Big Island of Hawai’i are under the influence of 3 million gallons per day per mile and it is certain that a number of freshwater springs discharge into the semi-protected environment at Kahalu’u. However, the effects of 5 gallons of freshwater dripping into a 140 gallon system caused salinity swings greater than those observed in a natural reef environment.
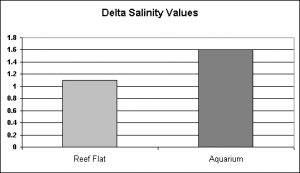
Figure 17. Salinity is less stable in the 140-gallon system than seen in natural environments of a tide pool and reef flat.
This ends this months’ report. Next time we’ll examine how our aquaria compare to nature in regards to dissolved oxygen, ultraviolet radiation, photosynthetically active radiation (PAR), and, to a certain degree, spectral quality.
Questions? Comments? Please leave your comments below, or send them to [email protected].
Acknowledgment
Many thanks to Jan War of NELHA for supplying various data.





0 Comments