
A portion of the reef crest at Honaunau, Big Island of Hawaii. Pocillopora meandrina and Porites lobata are the dominant coral species here.
In Part One of this series, we investigated conditions on various portions of Hawaiian reefs and compared them to those observed in a small aquarium. These parameters included pH, temperature and salinity and it was found that some variables are more stable in an aquarium. See here for details: http://www.advancedaquarist.com/2009/6/aafeature This time we’ll examine Photosynthetically Active Radiation (PAR), ultraviolet radiation (UVR), dissolved oxygen (DO) and oxidation-reduction potential (ORP).
Description of the Aquarium System
Aquaria data were collected from a system totaling about 140 gallons in total volume. The display aquarium holds approximately 55 gallons and contains marine algae and a number of photosynthetic invertebrates. This tank is illuminated for about 12 hours with six 39-watt T5 fluorescent lamps. An overflow allows water to gravitate to a 100 gallon Rubbermaid™ tub (holding about 85 gallons of water). This sump contains marine algae and is illuminated with a fluorescent lamp at night, when the display aquarium lights are off. An ETS™ model 800 protein skimmer is driven with a MagDrive 900™ pump and operates continuously. An Ehiem™ 1250 water pump provides water circulation between sump and display tanks.
The experiments in which the metal halide lamp performance was observed involved a 250-watt double-ended metal halide lamp (Ushio 14,000K) housed in a commercially-available luminaire (light fixture, manufactured by Sunlight Supply). This luminaire was suspended above a 100-gallon Rubbermaid tub, and the distance from the lamp to the water surface was approximately 7 inches. The PAR and UV sensors were at a depth of ~6 inches.
We’ll resume our discussion of various physical parameters beginning with Photosynthetically Active Radiation, or PAR.
Light (Photosynthetically Active Radiation, or PAR)
Our global ecosystem is driven by the energy received from the sun. And, as we all know, corals and other symbiotic invertebrates require light in order to survive and thrive. Light must be of proper intensity in order for photosynthesis in zooxanthellae, zoochlorae, and marine ‘plants’ to proceed. Too little light, and the compensation point is not reached, and the animal/algae will rely on heterotrophy or stored reserves (starches, fats) to survive. On the other hand, too much light will cause photoinhibition where the rate of photosynthesis is inhibited. In a worst case scenario, the symbiotic invertebrate will expel its zooxanthellae in a last ditch effort to survive.
Photosynthetically Active Radiation (PAR) is the metric used here to describe sunlight intensity. PAR is usually reported in units called micromole photons per square meter per second (molm²sec). Maximum PAR at noon on a clear day here in Hawaii is ~2,200 molm²sec.
PAR was measured with a WatchDog™ data logger and a water-proofed PAR sensor manufactured by Spectrum Technologies, Inc., Plainfield, Illinois. In order to compare the total number of photons falling upon a given area, we can use the information gathered from the data logger and calculate the Daily Light Integer (DLI). Fortunately, the Spectrum Technologies’ software is capable of generating this information in a report and relieves us of tedium involved with manually calculations.
However, for those interested in calculating DLI, this is the formula:
PAR (micromole photons per square meter per second) times 60 seconds per minute times 60 minutes per hour times number of hours in photoperiod divided by 1,000,000 equals Mol photons per photoperiod, or day.

Figure 1. PAR above and below water (~1 meter depth) on a Hawaiian reef flat. PAR: Aquarium, Low Light Level
Estimating DLI for an aquarium is straightforward, although it might involve several calculations when using fluorescent lamps to transition to mid-day metal halide lighting and back to fluorescent lamps for the transition to dusk and darkness.
PAR: Reef Flat
This particular reef flat is at the Kahalu’u Beach Park on Ali’i Drive in Kailua-Kona, Big Island of Hawai’i and measurements presented are for a typical spring day (see Figure 1). Note that the underwater measurements sometimes exceed those seen above the water’s surface. These PAR sensors took a snapshot every minute, and the snapshot’s length is very short, thus allowing the sensor to see the ‘glitter lines’ or lensing effect of the wave action. The sharp drop in light energy at about noon is due to cloud cover.
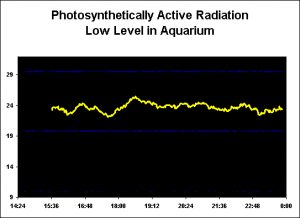
Figure 2. Variations in the low-light field within an aquarium during the course of about 10.5 hours, with no water surface agitation. PAR: Aquarium, High Light Level
Figure 2 shows time-course PAR values in an aquarium lighted with a single T5 lamp. Note that these PAR values are not sufficient to maintain many, if not most, photosynthetic invertebrates and is presented here only to demonstrate variations in the light field at low PAR.
For these observations, a 250-watt single-ended metal halide lamp was housed in a reflector suspended over a 100-gallon Rubbermaid sump. Circulation and water surface agitation was created by a modified powerhead (see here for a Product Review): http://www.advancedaquarist.com/2007/12/review1/
A WatchDog data logger recorded PAR values every minute. See Figure 3.

Figure 3. Data obtained with a data logger during a portion of the 100-hour burn-in of a metal halide lamp. There was no shield (splash guard) on the fixture.
Ultraviolet Radiation (UVR)
Ultraviolet Radiation (UVR) was measured with a WatchDog™ data logger and a water-proofed UVR sensor manufactured by Spectrum Technologies, Inc., Plainfield, Illinois. This sensor reports both UV-A and UV-B (range of 280nm to 400nm) as micromole photons per square meter per second (molm²sec). Maximum UVR as reported by this instrument on a clear day at noon is ~250 molm²sec.
Figure 7 shows the UVR portion of the solar spectrum as seen on a sunny day at sea level. This information was gathered with an Ocean Optics™ USB-2000 spectrometer.
UV Radiation and Aquarium Lamps
While sunlight is composed of broadband ultraviolet radiation, lamps manufactured for aquaria (or any other purpose for that matter) generally do not mimic sunlight’s ultraviolet radiation characteristics.
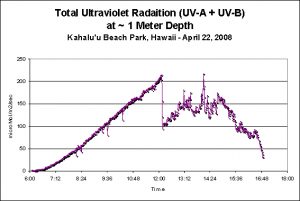
Figure 4. Underwater Ultraviolet Radiation over the course of a typical April day on the west coast of the Big Island of Hawaii.
The element mercury is found in most aquaria lamps (including fluorescent and power compacts, metal halides and mercury vapors) causes spectral signatures that are ‘spiky’ usually with the largest output at 365nm (see Figure 7). Some specialty lamps (such as those for terrariums containing reptiles and ultraviolet disinfection) can produce large amounts of UV-B and UV-C, respectively.
UVR: Reef Flat
A WatchDog data logger diligently logged UVR every 60 seconds during an April day at Kahalu’u Beach Park. The UV sensor was in about 0.3 meter at low tide (about 9 am) and about 1.5 m at high tide (about 5:30 pm).
UVR: Aquarium, Low Light Level
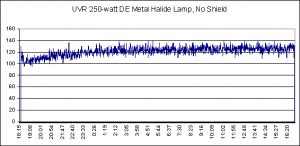
Figure 5. UVR data (UV-A + UV-B) obtained during a portion of the 100-hour burn-in of a metal halide lamp. No shield or splash guard was on the fixture.
Ultraviolet radiation generated by the single T5 lamp was below the instrument’s detection limit (or less than1 micromol per square meter per second UV-A plus UV-B; data not shown).
UVR: Aquarium, High Light
A 250-watt metal halide lamp was suspended over a 100-gallon Rubbermaid tub, with egg crate material resting on the tub’s shallowest shoulder. Vigorous water surface agitation was provided by a propeller pump. A WatchDog data logger monitored and recorded UVR (UV-A + UV-B) every 60 seconds. This data logger was deployed twice – once to record UVR from an unshielded metal halide lamp (where UV is absorbed to a degree by a material placed between the lamp and the water; see Figure 5) and again for a shielded lamp (Figure 6).
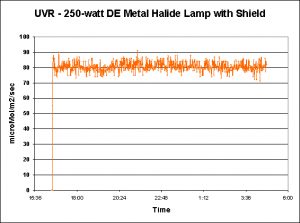
Figure 6. Another data set, with conditions similar to those of Figure 5, except that a splash guard or shield was on the luminaire. The shield removes only ~40% of the UVR.
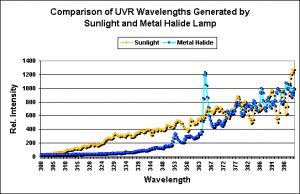
Figure 7. In this case, the spectral characteristics of natural sunlight and artificial light (250-watt Ushio 14,000K metal halide lamp) are surprisingly similar at ~375nm and above.
Dissolved Oxygen (DO)
Oxygen is necessary for life. Minimum Dissolved Oxygen (DO) concentration of aquarium water is generally considered to be about 2 milligrams per liter (mg/l). This is not difficult to obtain as DO concentrations of ~4 mg/l have been observed in aquaria filtered only by sub-sand filters. Those aquaria using protein skimmers usually have concentrations around saturation (6-7 mg/l, depending upon water temperature, barometric pressure, altitude, salinity, etc.). Those systems using algal scrubbers (or contain much algae and/or symbiotic invertebrates) can become supersaturated with DO.
Dissolved Oxygen values of the west Hawaii tide pool and aquarium (reported below) were obtained by use of a data logging device (a HQ40D multi-meter manufactured by Hach, Loveland, CO, USA) and a luminescent dissolved oxygen probe.
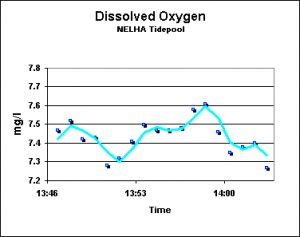
Dissolved Oxygen: Tide Pool (NELHA)
Dissolved oxygen was measured at low tide in a semi-isolated tide pool located near the Natural Energy Lab. Waves were occasionally high enough to push water into and out of the pool, creating a slow water exchange. As Figure 8 shows, the water was at, or slightly over, the saturation point.
Dissolved Oxygen: Aquarium
As Figure 9 shows, dissolved oxygen rose above the saturation point during the period lights were on over the display aquaria.
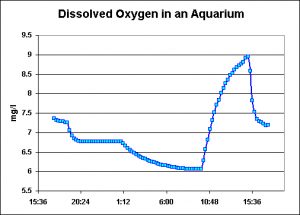
Figure 9. Dissolved Oxygen in an aquarium. Note the rise in pH when the lights come on at about 10 am.
Oxidation-Reduction Potential (ORP)
Oxidation-reduction Potential (ORP) or Reduction-Oxidation Potential (Redox) mediates many reactions in water, and in the early days of reef aquaria was popularly proposed to report the purity of aquarium water. Its use seems to have fallen out of favor in the last decade or so, and it has been my observation that few hobbyists rely upon it as an indicator.
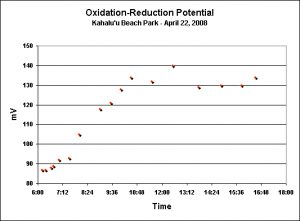
ORP: Reef Flat
I don’t monitor ORP in any aquarium, but thought it would be interesting to present this information anyways. These measurements were made the day before the seasonal Pocillopora meandrina spawnings at Kahalu’u Beach Park. See Figure 10.
In the early days of reefkeeping, there were those advocating the use of ozone to clarify aquarium water, and, though recommendations varied, ORP (or redox) values of 350-400 mV were common.
Discussion
Comparison of Photosynthetically Active Radiation (PAR) Doses in Nature and Aquaria
The Daily Light Integer reports the total number of photons falling upon a surface over a given period of time (most often the entire photoperiod). As mentioned in a previous article, DLI is analogous to the inches of rainfall per day, while instantaneous PAR values give us an idea of the number of raindrops falling per second. Using the formula for Daily Light Integer (DLI – see www.advancedaquarist.com/ for details), these DLIs were calculated for a natural reef flat and an aquarium, with the photoperiod being standardized to 597 minutes (9.95 hours):
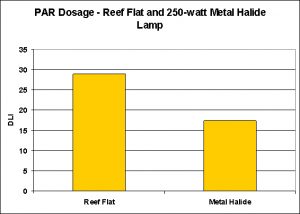
Figure 11. The amount of light generated by a 250-watt metal halide lamp was ~60% of the PAR falling upon a coral on a reef flat during an ‘average’ spring day in Hawaii.
- PAR, Reef Flat, DLI = 29.03/597 minutes
- PAR, Metal Halide (unshielded) DLI = 17.46/597 minutes
See Figure 11.
It is also obvious that the amount of light produced by both a T5 lamp (‘low’ light) and a metal halide lamp (‘high’ light) is not consistent during the photoperiod. Although the cause of this has not been investigated, it is felt that variations in the electrical supply (line voltage) are responsible. These observations were made when no water surface agitation was taking place, so we can rule out fluctuations due to the waves’ ‘lensing’ effects (‘glitter lines’).
However, when water movement causes surface agitation, PAR values can easily double within fractions of a second. Note that these observations were made with a data logger recording values with a ‘shutter speed’ (integration time) of just fractions of a second. Many PAR meters average measurements, or report PAR values seen over a longer integration time. Nonetheless, glitter lines do play a part in the rapidly changing PAR values reported by many hobbyists.
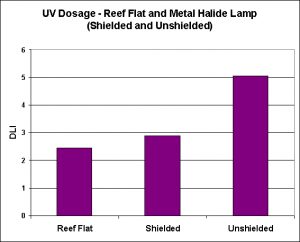
Figure 12. The dosage of Ultraviolet Radiation (UV-A + UV-B) of a reef flat and a shielded and unshielded metal halide lamp.
Comparison of UVR Doses in Nature and Aquaria
When using artificial light exclusively, the amount of ultraviolet radiation actually falling upon a captive organism within an aquarium is dependent upon many factors. The type of light source (metal halide, fluorescent lamp, LED, etc.) and the amount of UVR generated is the primary factor. The geometry of the reflective surface and the material used as the reflective surface can focus (or not) the UVR. The material used to shield the lamp(s) from dust, salt spray, splashes, etc. can attenuate or eliminate the amount of UVR actually reaching the aquarium water surface. Once the UVR has entered the water column, its intensity can be weakened by selective absorption by organic compounds (such as those that lend a yellow tinge to the water). With these caveats in mind, these are the UVR DLIs calculated for a reef flat and an aquarium illuminated with a metal halide lamp (shielded and unshielded):
- UVR, Reef Flat: 2.45 mol/597 minutes
- UVR, Metal Halide (Unshielded): 5.06/597 minutes
- UVR, Metal Halide (Shielded): 2.89/597 minutes
See Figure 12.
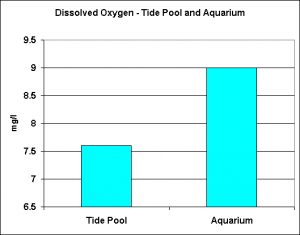
Figure 13. Maximal Dissolved Oxygen levels seen in a tide pool and an aquarium.
Dissolved Oxygen
Figure 13 represents observed dissolved oxygen values in a natural tide pool and an aquarium containing lots of photosynthetic growths.
Conclusions
Under the conditions in these experiments, a single 250-watt DE metal halide lamp suspended ~7 inches above the water surface of an aquarium (and the sensor being at a depth of ~6″) delivered 60% of the total PAR experienced by reef flat corals at Kahalu’u Beach Park in Hawaii on a typical spring day. Under different circumstances (higher wattage, or one producing more PAR; lessened distance from the lamp to the water surface, etc.), it would be possible to deliver the same number of photons (light energy) to a coral within an aquarium.
Light intensity in an aquarium lighted by artificial light sources is subject to much variation due to line voltage, water surface motion and so on.
To my surprise, the amount of ultraviolet radiation delivered by the unshielded and shielded metal halide lamp exceeded that seen on a Hawaiian reef flat. Since the shield on this particular light fixture is made of glass (and not coated with UV-absorbing material), the amount of UV transmitted is relatively high. A shielded made of acrylic or glass with a UV-absorbing coating would most likely transmit much less UV. In case the total amount of UV delivered to the underwater sensor was higher than that observed in nature on one given day. When we examine the spectral characteristics of natural and artificially-generated UV radiation, we note differences. In particular, the spectral signature indicates that UV-B generated by the metal halide is not as great as that seen by corals on the reef flat, but UV-A exceeds natural amounts at 365nm, and is more or less consistent with water-filter UV at wavelengths at ~370nm to 400nm.
Interestingly, the highest amount of dissolved oxygen seen in a closed-system aquarium exceeded that observed in a semi-isolated tide pool during the afternoon. The aquarium water became supersaturated with oxygen even when the PAR generated by T5 lamps was relatively low (estimated to be ~150 molm²sec). Bear in mind that dissolved oxygen in an aquarium is dependent upon many factors, including light intensity, amount of algae and/or photosynthetic invertebrates, nutrient availability, water motion, oxygen demand of aquarium inhabitants, etc.). However, it is possible for oxygen levels to reach, and exceed, the saturation point under certain conditions. It would be interesting to monitor pH in a nano-reef – pH modulations must be terrific!
The oxidation-reduction potential values varied during the morning hours, with a general trend of rising values seen during the observed time-frame. At no time did the ORP values reach those recommended in the early 1990’s for reef aquaria.
That does it for this time. I’ve got too many projects just completed or in the works to decide next month’s subject, so visit again soon for a surprise.
Comments? Questions? I am best reached at [email protected].



0 Comments