Marine flatworms in the phylum Acoelomorpha are nuisance organisms in the marine ornamental trade. I examined the efficacy of three chemical treatments used in the marine ornamental trade to eradicate acoelomorphans in the genera Convolutriloba, Waminoa, and Heterochaerus. Members of the Convolutriloba are particularly problematic in marine aquaria because when exposed to chemical treatments they release toxins into the water that kill corals and fishes. My research had four objectives:
- determine which chemical treatment (Salifert, Coral Rx, or Povidone) was the most effective on the three genera over a 24 hour exposure period,
- determine which coral dip (Povidone or Coral Rx) was the most effective in removing Convolutriloba from live rock substrates,
- determine if Flatworm Exit is toxic to non-target coral reef invertebrates, and
- document the distribution of marine flatworms in the marine ornamental trade across the United States.
Coral Rx (8ml/3.785L) was the most effective chemical treatment, and the mortality for Convolutriloba spp., C. macropyga, and Waminoa sp. was 100% after 24 hours; whereas the mortality for Heterochaerus australis was 80%. Flatworm Exit efficacy increased on convolutrilobids when the concentration was raised to 1.5 times the recommended dosage; the mortality was 90% for Convolutriloba spp. and 76% for C. macropyga. Povidone was the least effective chemical treatment for the both convolutrilobids and H. australis, but at 2ml/3.785L mortality for Waminoa sp was 100%. Coral dip testing showed that Coral Rx (8ml/3.785L) was more effective than Povidone (1ml/3.785L) in removing convolutrilobids. In coral dip tests, Coral Rx and Povidone removed significantly more C. macropyga specimens from live rock than the salt water control (92% and 86%, respectively), but these dips did not remove significantly more
Convolutriloba spp. than the control. Flatworm Exit was not toxic to representatives of the phyla Arthropoda, Mollusca, and Cnidaria; mortality was 0% for all species tested. Acoelomorphs were recorded from 45 cities and 26 states in the United States. In total, 92% (n=44) of aquarists that responded to an online survey had problems with flatworms in their aquaria, and 38% of the aquarists that administered Flatworm Exit to their aquaria lost corals and other invertebrates presumably due to the toxin release from convolutrilobids. Aquarists with acoelomorphs in their aquaria should identify them before applying chemical eradicators, because susceptibility to the chemical treatments varied between genera. Quarantining corals and live rock with coral dips is the best way to deal with marine flatworms because it prevents their introduction, and circumvents the problems associated with convolutrilobid toxin release. Harvesting coral reef taxa to replace livestock lost through
Convolutriloba toxin release is environmentally costly, and should be prevented by using coral dips.
Introduction
Marine flatworms in the phylum Acoelomorpha are pervasive pests in public marine aquaria, the marine ornamental aquaculture industry, and private hobbyist coral reef tanks. Currently, there are two genera of particular importance that thrive in the marine ornamental trade: Convolutriloba and Waminoa (Borneman, 2001; Delbeek and Sprung, 1994, 1997; Nilsen and Fossa, 2002; Shimek, 2004). Both of these genera proliferate rapidly in aquaria and pose specific problems for aquarists. For example, species of Convolutriloba are difficult to remove from coral reef tanks because when aquarists attempt to eradicate them utilizing chemical treatments, these flatworms release a toxin into the water column that reportedly kills both corals and fishes (Delbeek and Sprung, 1997). Species of Waminoa are problematic in aquaria because these facultative commensals collectively settle on both hard and soft coral tissues (Nilsen and Fossa, 2002). Although waminoids
are not predators of corals, their settlement occurs in such large numbers that they compete with their coral hosts for both space and light (Delbeek and Sprung, 1994; 1997). When a population of waminoids is left untreated, large aggregations known as “blooms” occur that are so dense, they block sufficient light from reaching their coral host (Delbeek and Sprung, 1997) (Fig. 1).

Figure 1. Flatworm predators used as biological controls in the marine ornamental trade. A) The six line wrasse Pseudocheilinus hexataenia reportedly predates on acoelomorphs in marine aquaria (from Burgess et al., 1997). B) The nudibranch Chelidonura varians has been observed ingesting covolutrilobids and reduces acoel populations (from Delbeek and Sprung, 1997).
Currently, professional and amateur aquarists utilize either chemical treatments or introduce flatworm predators into aquaria to reduce acoelomorph populations (Delbeek and Sprung, 1997). Two predominant reasons for administering such measures are:
- aquarists believe that marine flatworms can potentially damage coral livestock, and
- marine flatworms are considered aesthetically unappealing (Delbeek and Sprung, 1997).
Currently, there are two classes of chemical treatments used to treat flatworms: tank additives and coral dips. The first class is administered directly to a coral reef tank to treat a bloom, and is referred to as a tank additive. This chemical treatment is added to an aquarium after a flatworm species has been introduced, and the population numbers within that system spike.
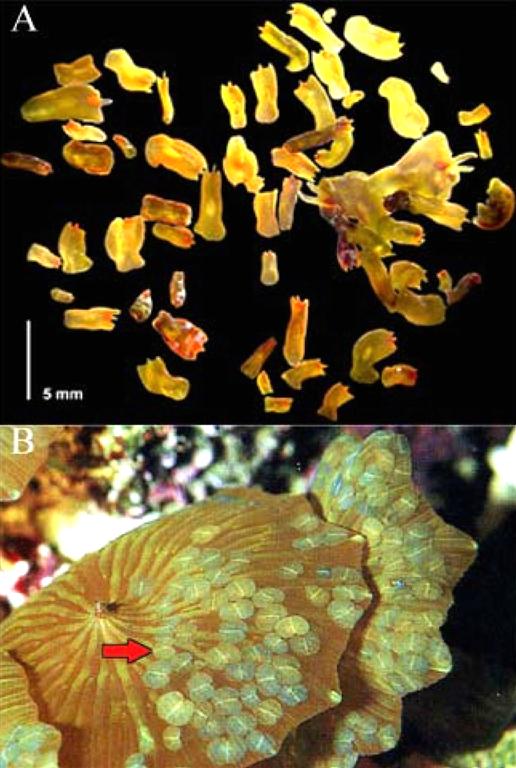
Figure 2. Acoelomorph “blooms” in marine aquaria. A) Convolutriloba flatworms grouped together on glass a panel (from Shannon, 2007). B) Waminoa sp. settled on a Discosoma sp. coral; red arrow indicates Waminoa specimens (from Nilsen and Fossa, 2002).
The second class of chemical treatment is utilized in a preventative protocol used to eradicate flatworms before they are introduced into an aquarium. These chemical treatments are referred to as a coral dips. Coral dips, such as Coral Rx and Povidone® are utilized specifically to remove flatworms that have settled on coral colonies. Newly purchased corals from a retailer are exposed to coral dips to eliminate flatworms that have settled in the cracks of the rock framework of the coral. Aquarists mix the coral dip in containers of salt water and place the coral colony in the solution for 20 minutes; afterward the colony is shaken to remove any remaining worms. Throughout this exposure period, susceptible acoelomorphs fall off of the coral colony and are left behind in the bucket. This process is referred to as “quarantining” in the hobby, and can be repeated several times over two to three days before the coral is finally introduced into a display tank (Delbeek and Sprung, 2005).
The quarantine process is useful because it attempts to stem the spread of flatworms before they can infiltrate a coral reef tank.

Figure 3. Chemical treatments used to eradicate flatworms in the marine ornamental trade. A) Blue Life Flatworm Control is manufactured by Blue Life USA in Los Angeles, California. B) Flatworm Exit made is manufactured by Salifert in Holland. C) Coral Rx is manufactured by The Coral Gardens a private aquaculture company in Hoover, Alabama (from Root, 2008). D) Povidone is an iodine based antiseptic manufactured by Triad Disposables in Hartland, Wisconsin. A and B are tank additives added directly to reef aquaria to treat flatworm blooms, and C and D are coral dips used for ridding corals of flatworms outside coral reef systems.
A distinction exists between the two classes of chemical treatments. Tank additives are applied directly to the water in coral reef aquaria. In contrast, coral dip products are only used briefly in sequestered buckets as a prophylactic measure. Furthermore, coral dips are concentrated products that are used specifically for corals and flatworms alone, and are never administered directly to a tank because their effects on non-target reef tank inhabitants (i.e., fishes and marine invertebrates) are unknown.
Flatworm predators used to reduce blooms in aquaria include the introduction of wrasses in the genus Halichoeres and nudibranchs in the genus Chelidonura. These predators reportedly feed on flatworms located on aquarium live rock, glass panels and corals (Delbeek and Sprung, 1997; Shimek, 2004) (Fig. 2). However, debate exists among aquarists over the effectiveness of these predators in curtailing acoelomorph numbers. For example, professional aquarists at The Pet Barn (Franklin Square, New York) and the Atlantis Marine World Aquarium (Riverhead, New York) recommended introducing the six-line wrasse (Pseudocheilinus hexataenia) and the nudibranch (Chelidonura varians) into aquaria to reduce acoelomorph numbers (Chabla, personal communication; Papparo, personal communication). However, one Convolutriloba researcher observed that this species of wrasse did not consume convolutrilobid species, but merely chewed on individuals before
spitting them back out (Shannon, personal communication). As a result, any tissues that may have been torn and separated from a predated individual will likely regenerate into new flatworms (Pechenik, 2005).
The present investigation examines three chemical treatments used to eradicate flatworm infestations in the marine ornamental trade: Flatworm Exit, Coral Rx, and Povidone (Fig. 3). Although there is anecdotal evidence that these treatments are successful in either eradicating flatworms or preventing them from entering an aquarium, there are no published studies comparing the effectiveness of these chemical treatments across acoelomorph genera. Additionally, there is no quantitative data available to determine the effects of the chemical treatments on non-target invertebrates in coral reef aquaria.
Currently, Salifert Corporation, a subsidiary of Sekha Holding BV of the Netherlands, advertises that the tank additive product “Flatworm Exit” kills all flatworms and that this product is safe for all marine invertebrates and fish in coral reef aquaria. In contrast, Delbeek and Sprung (1997) stated that aquarists’ observed livestock death after administering this product into their tanks. One potential reason for both fish and coral death in closed systems was attributed to the toxins released from convolutrilobid species once they have been exposed to the chemical treatments (Delbeek and Sprung, 1997). For example, when specimens of C. retrogemma die they expel thick yellow bodily secretions into the surrounding water; the secretions have a distinct bromine-like odor (Shannon and Achatz, 2007). These toxins reportedly killed corals in the genus Acropora and reef fishes in the genus Premnas after aquarists administered the recommended dosage of Flatworm
Exit (Chabla, personal communication). Currently, no biochemical analysis of this toxin has been completed (Shannon, personal communication).
Previous investigations have documented that aquatic flatworms can secrete chemical effluents and contain toxic organic compounds in the wild. For example, the freshwater flatworm Dugesia dorotocephala releases chemical cues to alarm conspecifics in response to a predator effluent (Wisenden and Millard, 2001). Similarly, Ritson-Williams et al. (2006) found that two previously unidentified marine polyclad species in the genus Planocerid contained tetrodotoxin (a neurotoxin). One species of Planocerid stored high concentrations of tetrodotoxin (TTX) in the pharynx for a hypothesized feeding function, while another species had high concentrations of TTX throughout the body tissues as a hypothesized defensive function against predators (Ritson-Williams et al., 2006). Toxins associated with feeding and defense behaviors are advantageous for turbellarians in the wild. However, convolutrilobid toxin release in marine aquaria is associated with
the loss of coral reef tank livestock.
The purpose of this investigation was to evaluate the effectiveness of the chemical treatment products used in the marine ornamental trade to eradicate two acoelomorph species in the genus Covolutriloba. Additionally, Waminoa sp. and Heterochaerus australis Haswell 1905, were also exposed to the same chemical treatments to compare their efficacy across representative acoelomorph taxa found in marine aquaria. I divided the research into four objectives:
- determine which chemical treatments (Salifert, Povidone, and Coral Rx) were the most effective on the three flatworm genera over a 24 hour exposure period,
- determine which coral dip was the most effective in removing Convolutriloba from a live rock substrate,
- determine whether Flatworm Exit had short-term deleterious effects (i.e., induce mortality within 72 hours) on non-target reef tank invertebrates, and
- document the distribution of marine flatworms (Platyhelminthes and Acoelomorpha) in the marine ornamental trade across the United States.
Insights gained from this research can be applied toward responsible reef keeping, and are intended to reduce the mortality of coral reef tank livestock that is a result of convolutrilobid toxin release. A review of the biology of acoelomorphs and their impacts on marine ornamental aquaria is provided below.
The Marine Ornamental Trade: Background
The marine ornamental trade is a global industry that harvests coral reef wildlife from over 40 countries around the world (Wabnitz et al., 2003). In a study funded by the United Nations Environmental Programme World Conservation Monitoring Center (UNEP-WCMC), Wabnitz et al. (2003) reported that the hobbyist trade generates between US$ 200-330 million dollars annually. An estimated 1.5 to 2 million hobbyists maintain marine aquaria globally, with 600,000 tanks in the United States (Green, 2003). The main export countries of marine ornamentals species include: the Philippines, Indonesia, the Solomon Islands, Sri Lanka, Australia, Fiji, Maldives, and Palau (Wabnitz et al., 2003). Between 1997 and 2002, a majority of the coral reef biodiversity harvested for the marine ornamental trade was shipped to the United States, the United Kingdom, the Netherlands, Germany and France (Wabnitz et al., 2003). The trade itself is comprised of 99% wild livestock
and 1% farmed livestock (Oliver, 2003).
In 2000, the UNEP-WCMC created the Global Marine Aquarium Database (GMAD), and compiled invoices and trade records from 58 companies (approximately one fifth of all wholesalers in business) and four government management authorities. In August of 2003, their dataset documented that 7.7 million animals were imported and 9.4 million animals were exported (comprising 2,393 species) from 1988 to 2003. The combined hard and soft coral harvest estimates were between 11 to 12 million pieces, or 390,000 pieces per annum (Wabnitz et al., 2003). Coral trade information is pertinent to this investigation because corals are one of the vectors for transporting acoelomorphs into aquaria throughout the trade (Shannon and Achatz, 2007).
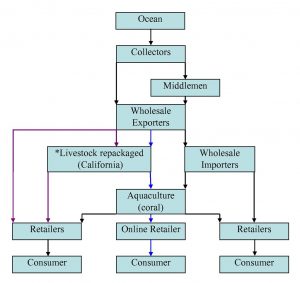
Figure 4. A simplified schematic diagram of the distribution network of livestock in the marine ornamental trade (modified from Green, 2003). Black arrows indicate commonly practiced industry routes (Green, 2003), purple arrows indicate trans-shipping route (Oliver, 2003; Wabnitz et al., 2003), and the blue arrows indicate the coral aquaculture shipping route (Root, personal communication). The stars indicate that trans-shippers open and repackage transport boxes with fresh oxygenated salt water upon arrival in California before they are sent to retailers.
A second vector for acoelomorph introduction is “live rock,” which is also known as “coral rock” or “reef rock.” Live rock consists primarily of the calcium carbonate deposits left behind from scleractinian hard coral skeletons that build on top of one another over time. These same deposits serve as the framework for extant coral reefs (Nilseon and Fossa, 2002). Live rock is highly porous and houses many marine invertebrate taxa including: poriferans, cnidarians, molluscs, bryozoans, annelids, arthropods, echinoderms and a variety of calcareous algae species (Nilseon and Fossa, 2002; Wabnitz et al., 2003; Delbeek and Sprung, 2005). Live rock is intensely exported from Fiji, Tonga, Samoa, the Solomon Islands and the Marshall Islands (Nilseon and Fossa, 2002). In 2001, Fiji harvested and supplied 800 tons of live rock from coral reefs; 95% of which was imported to the United States (Wabnitz et al., 2003).
Live rock is essential to coral reef tanks because it provides an important source of bacteria (e.g., Nitrosomas europaea and Nitrospira spp.) (Sprung and Delbeek, 2005). These bacteria are particularly important because they complete the nitrogen cycle within the tank, and serve as a form of biological filtration (Sprung and Delbeek, 2005). In order to keep all of the aforementioned fauna alive throughout the shipping process, the rocks are kept moist (Wabnitz et al., 2003). Live rock can be purchased in one of two ways, either “cured” or “uncured.” If the rock is cured, it is then subjected to a hypersaline spray for several hours or soaked for several days (Wabnitz et al., 2003). This serves to eliminate some of the larger pest organisms including, unwanted crustaceans and cnidarians, and leaves the coralline algae intact (Delbeek and Sprung, 1994; 2005). Uncured live rock is not treated with any prophylactic chemicals nor subjected to a
saline spray, in order to keep alive the maximum amount of beneficial bacteria. As a result, the most feasible point of entry for acoelomorphs into marine aquaria is through the introduction of un-quarantined corals and uncured live rock.
The Marine Ornamental Trade: Transporting wildlife
Coral reef wildlife goes through several points of human contact before arriving at a retailer (Fig. 4). The trade begins with “collectors” that harvest coral reef inhabitants directly (Green, 2003). Collectors can be indigenous peoples that are self-employed and live near the reefs, or they can be employees of an exporter company hired to dive on the coral reefs (Oliver, 2003). In Southeast Asia, collectors can be single divers, or work cooperatively on boats utilizing long tubes of compressed air connected to the vessel allowing them to stay submerged while harvesting livestock. Typically, once the divers reach the coral colonies, they utilize chisels, screwdrivers, hammers or crowbars to break coral away from the live rock substrate (Wabnitz et al., 2003).
Once specimens are collected, they are brought to exporters, who bag and prepare the livestock for overseas flights that can last up to a maximum of 72 hours (Wabnitz et al., 2003; Cole et al., 1999). Shipments from foreign countries into the United States can take several routes. For example, exporters can send shipments to importers on the west coast of the United States, who then repack specimens for transport to east coast to wholesalers (Root, personal communication). Wholesalers might also buy direct from exporters skipping the repackaging step (Wabnitz et al., 2003).
A third and less common method of importation is known as “trans-shipping,” and cuts out wholesalers completely by combining the orders of several retailers (Oliver, 2003). In this case, the livestock is under the trans-shippers’ care until the retailers pick up their orders. When the retailers receive their livestock, it is unpacked, acclimatized and finally sold to the hobbyist (Oliver, 2003).
Target acoelomorphs: Ecology
Ecological information about Convolutriloba in the wild is limited. The Acoela are exclusively marine, live under stones or among algae, and are commonly found on the bottom mud from littoral zones to deep waters (Hyman, 1951). The genus Convolutriloba contains four species: C. retrogemma Hendleberg and Åkesson 1988, C. hastifera Winsor 1990, C. longifissura Bartolomaeus and Balzer 1997, and C. macropyga Shannon and Achatz 2007. Three of these species were discovered in aquaria (Shannon and Achatz, 2007). Specifically, C. retrogemma was discovered in an aquarium in Gothenberg Sweden, C. longifissura was found in a pet shop in Germany, and C. macropyga was discovered at the Cappuccino Aquarium in Marietta Georgia (Akesson and Hendelberg, 1988; Bartolomaues and Balzer, 1997; Shannon and Achatz, 2007). Only C. hastifera has ever been documented in the wild. Winsor (1990) reported that C.
hastifera specimens were found on the bases of Goniastrea sp. and Platygyra sp. corals from Geoffrey Bay, in Queensland Australia.
Although few details exist about the natural habitat of Convolutriloba, acoel blooms occur in the wild on soft corals (i.e. coral reef lagoons in the Solomon Islands) (Delbeek and Sprung, 1997). In situ information about the natural history, population dynamics, species distribution, and predator prey interactions of the Convolutriloba are still undescribed. As a result, the majority of information about this genus comes from scientific and hobbyist observations in aquaria.
In marine aquaria, Convolutriloba thrive when high nitrate and phosphate levels are coupled with a bright halide lighting system (Delbeek and Sprung, 1994). Halide lights provide a source of energy for Convolutriloba algal endosymbionts. The endosymbionts belong to the genus Tetraselmis, are obligate for host survival, and create photosynthetic products for the host (Hirose and Hirose, 2007; Shannon and Achatz, 2007). Additionally, the introduction of light into an aquarium elicits behavioral responses from this genus. For example, when the aquarium lights are on, convolutrilobids settle in a resting position known as “basking; ” which involves an expansion of lateral parts of the body with the anterior tip indented (Hendelberg and Akesson, 1988). This behavior has been documented in C. retrogemma and C. macropyga (Hendelberg and Akesson, 1988; Shannon and Achatz, 2007).
Shannon (2007) found that C. retrogemma exhibits two different basking positions according to their orientation toward a light source. The first position occurs when the entire body of an acoel is situated on top of a substrate, and is perpendicular to the light source. The second position occurs when an acoel is attached to the side panel of a tank, or parallel to the light source. In each case, the body becomes dorso-ventrally flattened to increase its overall surface area exposed to light. However, in the second position, the anterior end of the body bends away from the side panel to orient half of its body toward the light (Shannon, 2007). In response to a sudden introduction of light, convolutrilobids exhibit negative phototaxis or display a negatively phototactic response (NPR) and will move away from a light source. When subjected to a non-directional source of light, the flatworm exhibits positive photokinesis and will move forward and turn randomly until it finds
a location with lower light intensity (Shannon, 2007).
Little information exists about what the target acoelomorphs consume in the wild. Hyman (1951) noted that members of the Acoela consume diatoms, algae, and swallowed whole protozoan and copepods. In captivity, C. retrogemma feeds on small invertebrates including copepods, Artemia naupli, small polychaetes and rotifers (Akesson and Hendelberg, 1989; Shannon and Achatz, 2007). C. macropyga populations became sexually active when fed micro-invertebrates in conjunction with a light source in a closed system (Shannon and Achatz, 2007). Starvation and light depravation experiments provided evidence that acoelomorphs need to holozoically feed to gain more nutrition than is supplied by their endosymbionts (Akesson and Hendelberg, 1989; Shannon and Achatz, 2007). In fact, without properly administered nutrition mono-specific cultures of asexual populations of convolutrilobids have been noted to crash (Shannon, 2007; Shannon, personal communication).
In contrast to Convolutriloba, flatworms of the genus Waminoa have been well documented in the wild. This genus is potentially comprised of four species: Waminoa sp.1, Waminoa sp.2, W. litus, and W. brickneri; however, only the latter two species have been described (Barneah et al., 2007; Winsor, 1990). Barneah et al. (2007), Ogunlana et al. (2005), and Winsor (1990) documented that W. litus and W. brickneri settle on scleractinians and soft corals in the wild. In coral reefs, waminoids are facultative commensals of corals. For example, W. litus was discovered on a Sarcophyton soft coral in Magnetic Island, Australia, and Waminoa sp.1 and Waminoa sp. 2 were found on unidentified corals in a marine aquarium at the Australian Institute of Marine Science (AIMS) (Barneah et al., 2007; Winsor, 1990). Ogunlana et al. (2005) discovered
W. brickneri associated with the scleractinian coral Plesiastrea laxa at 8-10 m in depth near oil jetties, and on the soft coral Stereonephyta cundabiluensis on a coral reef in the Red Sea.
Barneah et al. (2007) found that W. brickneri settled on 14 species of corals in reef patches in Eilat at depths between 2-50 m. This species displayed an uneven distribution pattern on seven different coral species and the greatest densities were found in order on: Turbinaria sp., Echinophyllia sp., Plesiastrea laxa, Favia favus, Favites pentagona, Acropora hemprichi, and Stylophora pistillata (Barneah et al., 2007). Once waminoids settle on a coral they are referred to as “epizoics,” and have been observed covering the surfaces and expanded polyps of the coral S. pistillata during the day (Barneah et al., 2007). Hobbyist literature documented waminoid specimens on Discosoma and Sarcophyton soft corals in coral reef aquaria (Delbeek and Sprung, 1997; Shimek, 2004).
Winsor (1990) and Achatz and Hooge (2006) documented the known habitats for H. australis in Australia and Africa respectively. In Australia, this convolutid was collected from shallow intertidal rock pools, and sediments in the subtidal zone from a saltwater creek (Winsor, 1990). In Tanzania, this species was collected from pools of water on sand flats high in the intertidal zone, and specimens were reported to occur in the thousands in the sediments (Achatz and Hooge, 2006). Additionally, this species was documented thirty years previously in Mombassa, Kenya, 135 km north of the Tanzania collection site (Achatz and Hooge, 2006). Unfortunately, no details about this species’ life history or ecological role were provided, but Achatz and Hooge (2006) believe that this acoel may be widely distributed throughout the Indian Ocean.
Morphology of Acoela
The order Acoela van Graff 1882, is made up of morphologically diverse, small (~0.5-10mm long), soft bodied acoelomate flatworms that are dorso-ventrally flattened and bilaterally symmetrical (Hooge et al., 2002; Hyman, 1951). Acoels have a solid body form that lacks a coelomic cavity (Tyler, 2004), a gut cavity, and intestines (Hyman, 1951). Because of their small size and flat body shape, they do not require respiratory organs or specialized circulatory systems. As a result, gas exchange occurs via simple diffusion across the body surface (Pechenik, 2005). Furthermore, the Acoela lack an excretory system including an anus or protonephridia; metabolic wastes likely diffuse out of the body (Hyman, 1951; Pechenik, 2005).
The Acoela body shape is vermiform and composed of an epidermal and mesodermal layer (Hyman, 1951; Pechenik, 2005). Both ventral and dorsal body surfaces are covered in locomotory cilia (Hyman, 1951; Tyler, 2004). This external layer contains adhesive organs, chemoreceptors and tangoreceptors which interpret chemical and physical stimuli respectively, and rhabdoid glands that secrete rhabdites (Hyman, 1951; Pechenik, 2005; Shannon and Achatz, 2007). Beneath this layer is a sub-epidermal musculature that consists of circular, longitudinal, and diagonal muscle fibers (Hyman, 1951; Shannon and Achatz, 2007). The Acoela nervous system is composed of a layer in the base of the epidermis outside of the subepidermal musculature. The brain, or cerebral ganglion, can be slightly or deeply bi-lobed and consists of a nervous layer over the statocyst (Hyman, 1951).

Figure 5. Dorsal view of adult target acoelomorphs. A) All four species in Convolutriloba (modified from Shannon, 2007, fig 1.2). B) Specimen of Waminoa sp. collected from the Atlantis Marine World Aquarium, Riverhead, New York. C) Specimen of H. australis collected from Elmont, New York. Black arrow indicates anterior region of acoelomorphs, white arrow indicates caudal lobes, and red arrow indicates eye spots.
The mesoderm is composed of a loose connective tissue matrix called the mesenchyme or parenchyma. Smooth muscle fibers within this layer are important constituents of the pharynx, copulatory apparatus and adhesive structures (Hyman, 1951). Parenchymal tissue performs several vital functions including food assimilation, nutrient transport, and waste product disposal (Hyman, 1951). This tissue also holds the organs in place, creates vacuoles to digest food items, and was recently found to contain a small amount of zoochlorellae endosymbionts in C. longifissura (Hirose and Hirose, 2007; Tyler, 2007).
Feeding occurs through a ventrally located mouth, with or without a pharynx present, (Hooge et al., 2002) that directly opens into the mesenchyme. Within the mesenchyme, phagocytic cells can perform intracellular digestion individually, or they can fuse together to form a digestive syncytium that is distinct from the peripheral mesenchyme (Hyman, 1951). Hyman (1951) noted that acoels ingest diatoms, algae, protozoans and copepods whole. In aquaria, C. macropyga and C. retrogemma feed on Artemia by forming a “capturing funnel” (Hendelberg and Akesson, 1988; Shannon and Achatz, 2007). This occurs when a convolutrilobid lifts its anterior edge off of the substrate and curls its lateral edges and two ventral flaps downward. As the Artemia move into the funnel, the acoel traps its prey by pressing its body back down against the substrate, and then moves forward to bring the prey to the mouth (Shannon and Achatz, 2007).
Morphology of target species
Several important distinctions exist between the target acoelomorphs in the genera Convolutriloba, Waminoa, and Heterochaerus. These include body size, coloration, body morphology, and modes of locomotion. For example, locomotion in convolutrilobids occurs via ciliary action. In addition to this mode of locomotion, Waminoa sp. and H. australis can swim by creating pedal waves with their body musculature. This creates flapping along the lateral edges of the body that is utilized during swimming (Achatz and Hooge, 2006). Another distinction between the target genera includes the obligate endosymbiont taxa they posses. For instance, Convolutriloba harbor zoochlorellae from the genus Tetraselmis (Akesson and Hendelberg, 1989; Shannon and Achatz, 2007; Hirose and Hirose, 2007). In contrast, Waminoa sp. and H. australis harbor dinoflagellate zooxanthellae within their tissues (Ogunlana et al., 2005;
Achatz and Hooge, 2006; Barneah et al., 2007). Additionally, only Convolutriloba secrete toxins into aquaria that negatively affect coral reef tank livestock and con-specifics. Waminoa sp. and H. australis have not been documented to produce such toxins.
All four Convolutriloba can produce tissue toxins that originate from the cells within the rhabdoid glands (Shannon and Achatz, 2007). These ventrally located glands are located just beneath the epidermal layer and are unique to marine turbellarians. They produce thick mucous secretions that were previously hypothesized to protect against desiccation and predation (Hyman, 1951; Pechenik, 2005). In aquaria, convolutrilobids will auto-lyse in response to physical or environmental stressor events including physical contact or temperature fluctuation (Shannon and Achatz, 2007). Once the rhabdoids glands rupture, a subsequent chain reaction causes all the tissues to lyse, including the zoochlorelle endosymbionts (Shannon and Achatz, 2007). Furthermore, if a group of convolutrilobids are kept in a small volume of water, and one individual releases its toxin, the others specimens will also auto-lyse in response (Shannon and Achatz, 2007).
The C. retrogemma body shape assumes a tube-like form, with a rounded anterior tip, and the lateral lobes are folded down and inward (Hendelberg and Akesson, 1988). Individuals vary in size depending upon the age and sexual stage of the animal. Asexually reproducing animals range from 2.5-3mm (excluding buds), and larger sexually mature flatworms can reach up to 5-6mm in length and 4-5mm in width. The mainly green coloration of C. retrogemma is due to its algal endosymbionts, red rhabdoid glands, red pigmentation, and characteristic three caudal lobes that can be observed without a microscope. Hendelberg and Akesson (1988) described this species as “mainly green,” however; Shannon (2007) showed that C. retrogemma can also have more reddish coloration throughout the body (Fig. 5).
Convolutrilobids have two colorless areas at their anterior end, known as eyefields, which detect light fluctuations. The dorsal bi-lobed brain is formed by a pair of ganglia, and there are no medially situated statocysts (Hendelberg and Akesson, 1988; Sikes and Bely, 2008). The nervous system of Convolutriloba is mainly made up of six longitudinal nerve cords. Two of these cords run ventro-laterally, and the other four are dorsally and medially located; the first two cords extend most of the body length, while the other four cords extend half of the body length (Sikes and Bely, 2008). Red rhabdoid glands can be found throughout the body but are concentrated in the median caudal lobe, and the muscle layer is 2-3 cells in thickness (Shannon and Achatz, 2007). The endosymbionts in Convolutriloba lie close to the muscle layer and are surrounded by the peripheral parenchyma (Hendelberg and Akesson, 1988).
The body of C. macropyga is flattened with a shield shape that is rounded anteriorly and indented at the anterior tip. Mature specimens are conspicuously larger than the adults of others species in the genus and can reach 8mm long and 6mm in width when basking (Shannon and Achatz, 2007). When motile, adults can reach up to 10mm in length. Their body coloration is a light green except for a large red pigmentation spot near the posterior end of the animal. In addition to its three characteristic caudal lobes, adults can have 2, 3, or up to 9 separate median lobes that become buds and asexually divide as new individuals (Shannon and Achatz, 2007).
The body shape of Waminoa is highly flattened except for an indentation in the posterior margin that lies close to the reproductive organs. The body coloration is bronze due to the presence of its endoymbionts, with white speckles and scattered white pigment spots. Waminoa body length ranges from 3-4 mm in diameter and 1 mm in thickness. Its epidermis is heavily ciliated, transparent, and densely packed with rhabdoid glands that are found only on the dorsal body wall and lateral sides of the animal (Ogunlana et al., 2005; Barneah et al., 2007; Winsor, 1990). The mouth is ventrally located at the posterior third of the body. W. litus adult specimens collected in the field from Geoffrey Bay Australia lacked eyes, statocysts, and statoliths (Winsor, 1990).
In contrast to Convolutriloba, W. litus and W. brickneri both harbor two distinct dinoflagellate endosymbionts within their parenchyma (Ogunlana et al., 2005; Barneah et al., 2007). The smaller endosymbiont (4-8m) was a member of the genus Synbiodinium, and the larger endosymbiont (11-16m) was in the genus Amphidinum. Two lines of evidence indicate that waminoids do not to predate on corals. First, their digestive syncytium contained no nematocysts from coral tissues. Second, the dinoflagellate endosymbionts harbored inside Waminoa tissues belonged to a clade of zooxanthellae that was distinct from their coral hosts (Ogunlana et al., 2005; Barneah et al., 2007; Barneah et al., 2007).
The body shape of H. australis is spatulate with two elongate latero-caudel lobes. Its coloration is brownish-yellow and adults can reach 4mm in length and 1.5mm in width (Achatz and Hooge, 2006). The epidermal cilia are approximately 8m long, and cover the entire body surface, except near the male and female gonopores. The ventrally located mouth is approximately at half the body length of the animal and numerous zooxanthellae 10-25m wide are found throughout the parenchyma. Instead of eye fields, H. australis has two dark brown ocelli that lie lateral to the statocyst, located approximately 300m behind the anterior tip of the animal (Achatz and Hooge, 2006).
Reproduction
The Acoela are simultaneous hermaphrodites that can reproduce via sexual or asexual reproduction (Hyman, 1951; Pechenik, 2005). The paired gonads are primitively numerous and are scattered throughout the parenchyma (Hyman, 1951; Winsor, 1990). Both male and female gonopores are distinct from one another, and are located on the ventral surface of the acoel body (Hyman, 1951). The female reproductive system consists of a vagina that leads to the female gonopore, which is nearly always located anterior to the male gonopore (Ogunlana et al., 2005; Shannon and Achatz, 2007). Additionally, seminal receptacles for sperm storage may be present in different species (Hyman, 1951). The male reproductive system typically contains a number of testes connected to single symmetrical sperm ducts located on each side of the body. These ducts connect to the copulatory complex which holds the seminal vesicle and penis (Hyman, 1951). Additionally, the Acoela have characteristic spermatozoa
with two incorporated axonemes (Hooge et al. 2002).
Sexual reproduction can occur in two ways. The first method involves hypodermic insemination. This occurs when an acoel pierces a conspecific with a hard needle-shaped structure or hollow penis stylet (Bruggeman, 1986; Hyman, 1951), and injects sperm into the partner’s parenchyma (Pechenik, 2005). Such behavior is termed “penis fencing,” and occurs in acoel flatworms in the wild (Hyman, 1951; Newman and Cannon, 2003). The second method involves copulation, and occurs via insertion of the penis into the female gonopore. During copulation, acoelomorph partners often mutually inseminate and fertilize each other (Hyman, 1951; Tyler, 2004). Copulation is generally preceded by a brief “courtship” display that involves physical contact of the head or body (Hyman, 1951). In the wild, acoels deposit their eggs on variety of substrates by discharging them via the mouth or through a rupture in the epidermis (Ogunlana et al., 2005). Acoel eggs have their own yolk supply, and the
target acoelomorph juveniles exhibit direct development (Hyman, 1951; Pechenik, 2005; Barneah et al., 2007).
The three main categories of asexual reproduction that acoels undergo are architomy, paratomy, and budding (Akesson et al., 2001). Architomy occurs when the offspring separate from a parent before organ differentiation (Akesson et al., 2001). Paratomy is a type of transverse fission that occurs after organ differentiation. Budding is the process where local tissues reorganize and form a new individual (or bud), which later separates from the parent (Akesson et al., 2001).
Convolutriloba exhibit several different modes of budding or fission that are unique to this genus. For example, Hendelberg and Akesson (1988) observed that the cells of C. retrogemma migrate to forms buds at the posterior end of the parent’s body. These buds can form on both sides of the median lobe either singly, or one on each lateral lobe. The main axis of the progeny is reversed 180° relative to that of the mother, so that the heads are facing opposite directions. In the first stage of budding they appear as dark green protrusions as the zoochlorellae migrate toward the developing bud (Shannon and Achatz, 2007). However, after a few days they attain a length approximately one fourth the size of the mother individual (Hendelberg and Akesson, 1988). At this point, the daughter buds develop eye fields and actively move in the opposite direction from the mother via ciliary action.
When the buds are large enough, they attach to a substrate while the mother moves away, and the tissues between the parent and bud tears (Hendelberg and Akesson, 1988). Torn progeny heal and become individuals that are much smaller than the mother. In some cases, tearing occasionally commences before the differentiation of the eye fields, mouth, and organs. However, prematurely shed buds continue to develop into fully grown individuals (Hendelberg and Akesson, 1988).
C. macropyga displays a more prolific mode of budding. In addition to its three caudal lobes, C. macropyga develops up to 9 medial lobes that can each become sites for bud development. Shannon and Achtaz (2007) provide documentation that this species commonly has more than two developing buds distributed between its caudal, lateral, and medial lobes. Furthermore, C. macropyga can support 4-5 individual buds at different stages of development simultaneously (Shannon and Achatz, 2007).
C. longifissura and C. hastifera display two modes of asexual reproduction that are distinct from the other convolutrilobid species. Instead of buds forming on the caudal lobes, C. hastifera asexually reproduces via transverse fission (Akesson et al., 2001). When an individual prepares to undergo fission, its posterior end flattens and adheres to a substrate (Sikes and Bely, 2008). Constrictions form on each side of the acoel’s body that create a future plane of fission, and a full body tear occurs approximately three- quarters of the way down the body length (Akesson et al., 2001; Sikes and Bely, 2008). This creates a larger anterior fragment and a smaller posterior fragment, and each develops into a separate individual.
C. longifissura fission goes one step farther. This acoelomorph asexually reproduces by two sequential orthogonal fissions (Sikes and Bely, 2008). The first transverse fission occurs at the same site as C. hastifera, creating two unequally sized anterior and posterior fragments. However, the posterior fragment then undergoes a longitudinal fission that begins anteriorly and progresses posteriorly, until the fragment is halved and becomes two new individuals (Sikes and Bely, 2008). As a result, C. longifissura creates two new daughters from the parent compared to C. hastifera’s one (Akesson et al., 2001; Sikes and Bely, 2008).
Systematics
Molecular research conducted by Ruiz-Trillo et al. (1999) and Baguna and Riutort (2004) moved the Acoela out of the phylum Platyhelminthes and placed in the newly erected phylum Acoelomorpha. These researchers analyzed the 18S ribosomal DNA and mitochondrial genomes of acoels to provide evidence that the Acoela, along with the sister group Nemertodermatida, are the most basal of the Bilaterians. Recently, a great deal of controversy existed about the placement of the Acoela. For example, Hooge et al. (2002) examined the nuclear 18s rDNA sequences from 16 different acoels in addition to sperm, muscle, and reproductive organ morphology. Hooge et al. (2002) stated that the same data from Ruiz-trillo (1999) study can be used to argue against the monophyly of the Aceolomorpha. Almost a decade after the designation of Acoelomorpha as a phylum, it is clear that the debate over the placement of the Acoela will continue for years to come.
In addition to molecular data, researchers use morphological features to identify species including epidermal ciliary rootlets, an acoel-type statocyst bearing a single statolith, the digestive parenchyma and spermatozoa (Hooge et al., 2002). However, these characters cannot be used alone. For example, although statocysts are common in the Acoela (Tyler 2004), Shannon and Achatz (2007) found that statocysts were only present in C. macropyga juveniles, and were absent from adults. Therefore, additional features including the pharynges, copulatory organs, gonads, brain, sperm structure, and body wall musculature are examined. Once all of these traits are combined with molecular data researchers begin to determine the phylogenic relationships between the 340 acoel species in 20 families (Barneah a et al., 2007; Hooge et al., 2002).
Materials and Methods
Chemical treatments
This investigation attempted to quantify the efficacy of three chemical flatworm controls: Flatworm Exit, Coral Rx, and Povidone. Flatworm Exit is produced by Salifert Corp., a marine aquarium supply company that distributes products to retail hobbyist stores both online and in pet shops around the United States. Neither the box packaging nor the website provided information about the chemical constituents used to make Flatworm Exit. The recommended dosage provided in the Flatworm Exit packaging is 0.2ml/18.925L (4 drops/5 gallons), but the website currently recommends 0.05ml/3.785L (1 drop/gallon). The retail cost for Flatworm Exit is approximately $33.
A second tank additive, Flatworm Control, was created by Blue Life USA and was previously available for purchase online. Blue Life USA is an aquarium supply company based in Los Angeles, California that sells aquarium maintenance chemicals, filtration, salt mixes, lighting products, and live rock. I intended to investigate the efficacy of Flatworm Control on convolutrilobids, but when online complaints arose about the effectiveness of the product, the manufactures responded to online customer reviews and suggested increasing the recommended dosage. As a result, I e-mailed Blue Life USA for a verification of the correct dosage. However, no response was sent and within 1-2 weeks the Flatworm Control product line was removed from the major website. After a second e-mail to the manufacturer, I received communication from a representative that stated that the product was recalled because the correct concentration had not yet been established. Subsequently, this product is no longer for
sale online at major vendors including Marine Depot, and the customer reviews previously posted about Flatworm Control have been removed.
Coral Rx is a coral dip manufactured and sold by Philip Root, the co-founder of The Coral Gardens, which is a private coral aquaculture facility that sells propagated corals to consumers on-line from Hoover, Alabama. Coral Rx is advertised to rid live corals of both acoelomorph and polyclad flatworms. No information about the chemical constituents is provided from the products packaging or online and when contacted the manufacture preferred to keep the nature of the constituents proprietary. Over a period of eight months, the recommended dosage for Coral Rx changed several times. For example, initially the label on the product bottle directed 0.2-0.4ml/3.785L. However, preliminary investigations with this concentration proved to be too dilute and ineffective on the target acoelomorphs. As a result, I contacted The Coral Gardens manufacturer over the phone and was told that the labels were incorrectly printed. At that point the manufacturer recommended increasing the dosage to
8ml/3.785L. The online recommended dosage for this product was 1-2ml/3.785L (20 May 2008), and a separate bottle gave the recommended dosage of 4-8ml/3.785L. In May of 2008, 1oz containers were sold online for $32 U.S. retail before shipping and handling costs.
Povidone is an iodine-based topical antiseptic manufactured by Triad Disposables in Hartland, Wisconsin. This product is re-labeled as Leader® brand 10% Povidone-Iodine solution, and is sold retail in drug stores and pharmacies throughout the United States. Povidone and Lugol’s Iodine are used by private coral aquaculture businesses including The Coral Gardens and The Sound School (New Haven, Connecticut) as coral dips. Aquaculturists in both businesses utilize the trace amounts of iodine in these products as an anti-helminthic dip for live coral (Santiago, personal communication). However, the recommended dosages varied between the two businesses from 0.05ml/3.785L (one drop/gallon) (Santiago, personal communication), to 0.5-1ml/3.785L (10-20 drops/gallon) (The Coral Gardens), and up to 2ml/3.785L (40 drops/gallon) from online resources (AquaCave Inc., 2008).
Tank conditions
Flatworms were harvested from three tanks between January 2008 and September 2008. The first tank was a 151.4L (40 gallons) reef tank at the Atlantis Marine World Aquarium (AMW) in Riverhead, New York, the second was a private 283.9L (75 gallons) reef tank devoted to flatworm culture in Elmont, New York. The third tank was a 90.8L (24 gallons) Aqua-pod at Hofstra University in Hempstead, New York. The AMW tank was kept at 25°C, at 33 ppt on a 11h:13h light-dark cycle supplied by a 96 watt dual power compact light system. The Elmont tank was kept between 23-26.6 °C, at 31-33ppt on a 12h:12h light-dark cycle, and was supplied by a 250 watt 10,000 K metal halide light system, and dual 121.9cm (48 inches) 40 watt actinic lights. The Aqua-pod was kept at 25°C at 30 ppt on a 12h:12h light-dark cycle supplied by a 64 watt compact fluorescent light system. The AMW tank was filled with backwashed ocean water from the Long Island Sound, and the other two tanks were filled with artificial
salt water, made from Instant Ocean® salt mix and reverse osmosis filtered tap water.
Specimen collection and handling
Specimens were siphoned off of aquaria glass panels, live rock, Caribbean gorgonian corals (Waminoa sp. from AMW tank) and plastic aquaculture substrates, using 6mm diameter flexible plastic air-hose tubing. This ensured that the least amount of direct physical contact was sustained to the animals to prevent tissue tearing (Newman and Cannon, 2003). Once siphoned into plastic specimen containers the flatworms were collected with 1-2ml disposable plastic pipettes and individually placed in test chambers. The testing chambers for the Convolutriloba were 1.5ml Eppendorf tubes, and Waminoa sp. and H. australis specimens were tested either in groups of ten in plastic cups, or individually in 1.5ml Eppendorf tubes. Controls were conducted in the same type of testing chambers. The collection cups, pipettes, and Eppendorf tubes were discarded after each trial to avoid the contamination of lysed residues between experimental subjects (Shannon and Achatz,
2007). Shannon and Achatz (2007) found that plastics held higher residual concentrations of the toxin than glassware. Similarly, Newman and Cannon (2003) recommended separating flatworm specimens collected from the field because their mucus could be toxic to one another.

Figure 6. Test chamber used to expose acoelomorphs to chemical treatments. A) Bath chamber utilizes 22.71L (6 gallons) of water, a 50 Watt submersible heater, and 2 air-stones to circulate water and distribute heat evenly. B) 1.5ml Eppendorf tube loaded with Convolutriloba spp. indicated by black arrowhead. Pink arrow indicates plastic cup test chambers, yellow arrows indicate Eppendorf tubes, and green arrows indicate air-stones.
Acoelomorph identification
Target acoelomorphs were photographed with an Olympus DP 11 digital camera connected to an Olympus microscope. Convolutriloba identification was verified by Dr. Shannon from the University of Georgia, Waminoa sp. identification was verified by Dr. Orit Barneah from Tel Aviv University, Israel, and H. australis identification was verified by Dr. Johannes Achatz and Dr. Seth Tyler at the University of Maine. Three of the four species within the Convolutriloba could not be identified based on their external morphology. In fact, the only way to differentiate between C. retrogemma, C. longifissura, and C. hastifera without molecular and histological techniques is to culture the acoels separately in closed systems, and observe their modes of asexual reproduction (Dr. Shannon, personal communication). However, at AMW I observed several Convolutriloba specimens exhibiting the “butterfly stage” of asexual reproduction
found in C. longifissura (Bartolomeus and Balzer, 1997; Akesson and Hendelberg, 2001). In addition, the white speckled dorsal pigmentation that is characteristic of C. hastifera was also observed from specimens from the same tank (Winsor, 1990). Further, the backward budding that is characteristic of C. retrogemma was also observed at AMW. Thus, three out of the four Convolutriloba species were likely mixed together during experimentation. As a result, C. retrogemma, C. longifissura, and C. hastifera will be collectively referred to as Convolutriloba spp. In contrast, C. macropyga was easily indentified based on its body shape, larger body size, increased number of caudel lobes, and the conspicuous large red pigmentation spot featured prominently on its dorso-posterior surface. Throughout experimentation, this species was identified and tested separately from the other three species.
Experimental protocol
Flatworm specimens were separated to the generic or species level before exposure to the chemical treatments. For example, convolutrilobid species (C. macropya, and Convolutriloba spp.) were tested individually in 1.5 ml Eppendorf tubes. Waminoa sp. and H. australis were originally tested in groups of ten because they did not appear to exude any toxic products. No test animals were used twice in experimentation.
All experimental subjects were kept in a water bath between 24-27°C, and were tested in their own tank water to keep the specific gravity and water parameters consistent. Shannon and Achatz (2007) and Shannon (2007) provided informative husbandry techniques to keep these acoelomorphs alive in cultures over prolonged periods. Following their protocol, a submersible aquarium heater was placed in the bath and set to 27°C. The testing chamber itself was a thick plastic storage bin (dimensions: 68.6 x 43.2 x 26.7cm) that contained 22.7-26.5L water, and included a retrofitted holding tray that accomodated 20 test cups at a time or 150 Eppendorf tubes (Fig. 6). Additionally, the bath was aerated with two air-stones to circulate the water and prevent uneven heating pockets from forming around the heater.

Figure 7. Mortality of target acoelomorphs Convolutriloba macropyga (Cm), Convolutriloba spp. (Cs), Waminoa sp. (Wa) and Heterochaerus australis (He) exposed to chemical treatments over 24 hours. A) Flatworms exposed to 0.2ml/18.9L of Flatworm Exit. B) Flatworms exposed to 0.3ml/18.9L of Flatworm Exit. C) Flatworms exposed to 1ml/3.785L of Povidone. D) Flatworms exposed to 2ml/3.785L of Povidone. E) Flatworms exposed to 8ml/3.785L of Coral Rx. Error bars show standard deviation.
The bath protocol was used to control temperature fluctuations and osmoregulatory stress as factors that could contribute to overall mortality rates during exposure to the chemical treatments. Each specimen was tested in cycled tank water because it was the appropriate temperature, buffered with the correct water chemistry, and because cycled water prevents ammonia levels from spiking. All of the tubes’ lids were cut off and exposed to open air, and no water changes or circulation was provided to the specimens inside the testing chambers over the 24 hour period. This protocol allowed me to observe the chemicals effects on flatworms alone, and bypassed the problems associated with specific gravity change and temperature fluctuation that would have occurred using newly created artificial salt water.
Efficacy of chemical treatments on acoelomorphs
Fifty convolutrilobids in five groups of ten were exposed to the chemical treatments (Flatworm Exit, Povidone, and Coral Rx) for 24 hours. The acoels were individually exposed in 1.5ml of liquid in Eppendorf tubes, and left in the bath chamber for the entire experiment. This was accomplished by pippeting the worms directly into the tubes, removing as much water as possible, and then introducing the mixed chemical treatment into the tube.
Waminoa sp. and H. australis speciemens were tested in cups in five groups of ten in 60ml solutions of the chemical treatments. Observational data for both the cups and Eppendorf tubes was recorded every minute for the first twenty minutes for every trial. Additional recordings were made after the first hour, again after 2-3 hours and after 24 hours. After 24 hours each specimen was inspected to determine mortality. Specimen survival was determined by observing either locomotion or a change in body shape. These responses were elicited by:
- using a flashlight to trigger a negatively photo-tactic response (NPR) (Newman and Cannon, 2003; Shannon, 2007),
- shaking or tapping the tube against a hard surface, or
- pippeting a brief strong directional current on the specimens to induce movement (Shannon and Achatz, 2007).
If an acoel was attached to the side of an Eppendorf tube and unresponsive to the previous methods, the chemical treatment was drained from the tube and droplets of salt water were released over the subject’s body. The trickle of water droplets induced quick bodily contractions and expansions if the animal was still alive. Another method to determine mortality involved physically prodding the acoel (Newman and Cannon, 2003). However, this technique was rarely used since this tore the animals’ tissues and was not as reliable as the aforementioned techniques (Newman and Cannon, 2003).
For each four species, the mortality to six chemical treatments (three chemical treatments at two concentrations) and the controls were compared using a chi-square test of independence (SPSS statistical package, SPSS Inc. Chicago; critical value: X2.05, 6 =12.592). If the chi-square test of independence was significant, then the mortality to each chemical treatment was compared to the control using a post hoc chi-square test of independence (critical value: X2.05, 6 =12.592).
Efficacy of coral dips in removal of Convolutriloba
Before the dip experiment began, preliminary investigations were conducted with 50 C. macropyga specimens to determine if their rates of attachment varied according to substrate. Five groups of ten acoels each were left to settle for twenty minutes on plastic from coral aquaculture trays, live rock, live rock placed in a sand bed, live rock with a directional source of light and glass slides. Live rock proved to hold the greatest numbers of specimens. Therefore this substrate was used throughout subsequent experimental trials. Although the target genera have been recorded basking on the glass panels of marine aquariums (Hendelberg and Akesson, 1988; Shannon, 2007) the experimental trials were not conducted with a glass substrate for several reasons. The foremost being, that the settled acoels fell off the glass substrate (microscope slides) when transferred from aquaria to the water surface of the dip cups. As a result, many of the flatworms fell off the slides before the
coral dip experiments could begin.
Live rock was used for the following three reasons. First, it was found to have the best attachment rate. Second, it was easier to apply the worms to the rock surface and account for a known number of flatworms (n =10). Third, live rock and coral are the substrates that convolutrilobids (C. hastifera) cling to in the wild and in coral reef tanks (Winsor, 1990). The live rock fragments used in experimentation were several centimeters in length and broken off of large pieces of cured Fijian live rock. These fragments were sculpted with bone shears (metal clippers) into flat rectangular shaped rocks, which provided a large surface area to apply the flatworms. Five acoels were placed on each side of the fragment, with a total of ten flatworms per rock.
The coral dip experiment was conducted with C. macropyga and Convolutriloba spp. specimens. Once ten acoels were placed on a live rock fragment, the rocks were gently lowered with metal tongs into 60 ml of the experimental dip solution. As soon as the rock touched the bottom of the cup, a stopwatch was used to record time, and a flashlight was used to determine if the specimens were still alive. Observational recordings were taken after one, five, 15, and 20 minutes. After twenty minutes, the total number of worms that fell off of the live rock was recorded. Controls for both species (n=50) utilized the same procedure with saltwater, but no coral dips were added to the cups. Ten trials of ten acoels for both species (n=200) were conducted with Povidone (1ml/3.785L) and Coral Rx (8ml/3.785L).
A total of 500 worms were tested, that consisted of 100 control and 400 experimental subjects. For each species, the drop off rates for Coral Rx, Povidone, and the controls were compared using a chi-square test of independence (critical value: X2.05, 2 =5.991). A post hoc chi-square test of independence was conducted for each species, to compare individual chemical treatment drop off rates to the control (critical value: X2.05, 2 =5.991).
Exposure of non-target species to Flatworm Exit
The mud snail Nassarius obsoletus and the long-wrist hermit crab Pagurus longicarpus were collected during low tide in July 2008 at the Nike Marsh in Long Beach, New York. Aiptasia pallida anemones were purchased from the Carolina Biological Supply Company in August 2008. The average shield length of the hermit crabs was 2.95 ± 0.45mm (n=50). The average shell and aperture length of mud snails was 15.17 ± 0.98mm and 9.3 ± 0.62 mm (n=50) respectively, and the average pedal disc length of the anemones was 9.85 ± 2.73mm (n=50).
Before experimentation began, all of the subjects were acclimated to cycled Instant Ocean® artificial saltwater at 31ppt, at 19°C for 24 hours. Non-target species were exposed to Flatworm Exit over three days during experimentation. The test subjects were exposed to 100% concentration (0.2ml/18.925L) throughout day one, and 75% concentration throughout day two and three. The reduction of Flatworm Exit concentration throughout this protocol simulates the conditions that occur in aquaria. For example, after applying Flatworm Exit to an aquarium, the manufacturer recommends having enough new saltwater to conduct a 25% water change (Salifert.com, 2009). Water changes will dilute the overall concentration of Flatworm Exit in a closed system. As a result, the experimental concentrations are likely higher than that of actual aquaria concentrations, because newly mixed known concentrations are applied every 24 hours, no filtration is provided, and no organic carbon is utilized as
filtration media.
Five groups of ten specimens (n=50) for each species were exposed to the chemical treatment. The hermit crabs and snails were placed in 100ml of solution in plastic cups on a 12h:12h light-dark cycle. Water changes with the appropriate concentration of Flatworm Exit occurred every 24 hours for Aiptasia anemones to avoid pedal disc tearing, and the hermit crabs and snails were transferred into new cups. Survival was determined by tapping the cups or shinning a flash light on the subjects to induce movement. Additionally, controls for each species (n=10) were conducted to determine if the collection and handling methods were responsible for stress related mortalities.
Survey and distribution map of marine flatworms
In order to understand how acoelomorphs affect the hobbyist trade, an online survey was posted on Survey Monkey.com from May to November 2008. This survey collected information from hobbyists around the United States that have encountered marine flatworms in their reef tanks. The survey documented the cities that hobbyists lived, whether flatworms had bloomed in their aquaria, which hobbyists used Flatworm Exit to eradicate their flatworms, and what livestock they lost from convolutrilobid toxin release (Appendix 1). The survey was not exclusive to acoelomorphans, and incorporated hobbyist data regardless of the group of marine flatworm they encountered.
Results
Efficacy of chemical treatments on acoelomorphs
Analysis of the data across chemical treatments for all four species showed that mortality was dependant upon the treatment. In addition, mortality from four of the six dosages was significantly different than control mortality for all four species. The most effective chemical treatments were Coral Rx (8ml/3.785L), Flatworm Exit (0.3ml/18.93L), Povidone (2ml/3.785L), and Flatworm Exit (0.2ml/18.93L). However, the overall effectiveness of these chemical treatments varied between genera.
When individual species were compared to the six chemical treatments and the control, the chi-square test of independence values for the target acoelomorphs were: Convolutriloba spp. (X2=267.1, df=6, P<0.0005), C. macropyga (X2=240.6, df=6, P<0.0005) Waminoa sp. (X2=307.2, df=6, P<0.0005), and H. australis (X2=191.6, df=6, P<0.0005). Flatworm Exit was administered in two dosages, at (0.2ml/18.93L) and (0.3ml/18.93L). The lower dosage produced 96% mortality for Convolutriloba spp., 22% mortality for C. macropyga, and 0% mortality for both Waminoa sp. and H. australis (Fig.7A). The higher dosage produced 90% mortality for Convolutriloba spp., 76% mortality for C. macropyga, 10% mortality for Waminoa sp. and 0% mortality for H. australis (Fig. 7B). The recommended dosage of Flatworm Exit was significantly different in its
effectiveness when compared to the control for Convolutriloba spp. (X2=92.3, P<0.05) and C. macropyga (X2=12.4, P<0.05), but not significantly different from the control for C. macropyga and H. australis. Similarly, the higher concentration of Flatworm Exit was also significantly different in effectiveness than the control for Convolutriloba spp. (X2=81.8, P<0.05) and C. macropyga (X2=61.3, P<0.05), but not significantly different for C. macropyga and H. australis.
At the recommended dosage Flatworm Exit produced visible effects on the target genera. For example, one specimen of Convolutriloba spp. began secreting a yellow discharge three minutes after exposure to Flatworm Exit. That number increased to 10% (n=5) of the specimens within 20 minutes (Table 1). The thick yellow discharge was a viscous fluid that had a pungent odor and remained within several millimeters of the specimens in the Eppendorf tubes. Approximately 30 minutes after exposure, the convolutrilobids also released organic matrices in the tubes that were visible with a flashlight. These long diaphanous matrices were distinct from the yellow discharge, varied in length and thickness, and extended up to several times the body length of the convolutrilobids that secreted them. The matrices were generally spread out in the liquid inside the Eppendorf tubes, and often inhibited specimens from moving freely inside the tube. Some specimens became tangled in their own
matrices which hindered or completely inhibited their movement.
Approximately two hours after exposure to Flatworm Exit, lysed Convolutriloba spp. specimens began disintegrating into a thick yellow collection of tissue fragments. At that point, any introduction of a slight current from a pipette broke the tissues apart. After 24 hours, 70% (n=35) of the Convolutriloba spp. specimens exhibited yellow discharge and 88% (n=44) exhibited matrices within the Eppendorf tubes (Table 1). In contrast, the Convolutriloba spp. controls (n=50) did not secrete the discharge or matrices; instead they exhibited movement, had normal coloration, and displayed a NPR.
| Control | Flatworm Exit (0.2ml) |
Flatworm Exit (0.3ml) |
Coral Rx (0.2ml) |
Coral Rx (8ml) |
Providone (1ml) |
Providone (2ml) |
|
|---|---|---|---|---|---|---|---|
| H. australis | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Convolutrioba spp. | 0 | 75% (35) | 60% (30) | – | 66% (33) | 4% (2) | 26% (13) |
| C. macropyga | 0 | 6% (3) | 0 | 0 | 14% (7) | 0 | 6% (3) |
| Waminoa sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control | Flatworm Exit (0.2ml) |
Flatworm Exit (0.3ml) |
Coral Rx (0.2ml) |
Coral Rx (8ml) |
Providone (1ml) |
Providone (2ml) |
|
|---|---|---|---|---|---|---|---|
| H. australis | 0 | 0 | 0 | 0 | 0 | 0 | 5% (1) |
| Convolutrioba spp. | 0 | 88% (44) | 30% (15) | – | 46% (23) | 68% (34) | 18% (9) |
| C. macropyga | 0 | 28% (14) | 40% (20) | 8% (4) | 50% (25) | 14% (7) | 92% (46) |
| Waminoa sp. | 0 | 0 | 0 | 0 | 15% (3) | 0 | 5% (1) |
The reaction of Waminoa sp. to the recommended dosage of Flatworm Exit was more immediate, but caused no death of flatworms. Within two minutes of exposure, the waminoids flapped their lateral edges rapidly and moved constantly around the cup, swimming sporadically. Ten minutes after exposure, flapping and irregular movements were displayed along with body contractions. Twenty minutes after exposure, activity slowed down and at 30 minutes their bodies began losing coloration. After 24 hours of exposure, specimens of Waminoa sp. lost the rusty coloration in the center of the body, and their lateral margins became a darker shade of brown. By the end of experimentation specimens of Waminoa sp. displayed a slower NPR that took several seconds for individuals to respond, their bodies were nearly translucent, and no matrix or yellow plume was found in their test chambers. Further, when the contents of the test cup were swirled the specimens could not maintain
attachment. In contrast, the controls of Waminoa sp. (n=50) displayed a normal NPR, kept their coloration, and remained adhered to the cup surface when the test cup contents were swirled. One specimen from the control group died.
The immediate effects of Flatworm Exit on H. australis were less severe. This species moved around the test cups for twenty minutes after the initial exposure. After 38 minutes their bodies began to contract, and 6 hours after exposure the contraction was more prevalent. At 18 hours the H. australis specimens contracted to a quarter of their normal size. H. australis specimens did not secrete a yellow discharge or matrix when exposed to either concentrations of Flatworm Exit (Table 1). The controls for this species (n=50) displayed both positive and negative reactions to the NPR test. After 24 hours the H. australis control specimens were actively moving, and their bodies were normal size.
Povidone was administered in two concentrations, 1ml/3.785L and 2ml/3.785L. The lower dosage of Povidone was not significantly different in its effectiveness when compared to the control for all four species. The lower concentration produced 8% mortality for Convolutriloba spp., 0% mortality for C. macropyga, 0% mortality for Waminoa sp., and 2% mortality for H. australis (Fig. 7C). The higher dosage of Povidone was significantly different in effectiveness when compared to the control for two species, and the post hoc chi-square test of independence values were: Convolutriloba spp. (X2=17.6, P<0.05) and Waminoa sp. (X2=96.1, P<0.05). There was no significant difference in effectiveness for either concentration of Povidone when compared to the control for C. macropyga or H. austrailis. The higher concentration was more effective than the lower concentration for three out of
the four species, and mortality increased to 30% for Convolutriloba spp., 12% for C. macropyga, and 100% for Waminoa sp.; but was 0% for H. australis specimens (Fig. 7D).
The higher concentration of Povidone elicited responses from the target acoelomorphs faster than the lower concentration. For example, at 1ml/3.785L, 14% (n=7) of the C. macropyga specimens secreted matrices after 24 hours of exposure. However, at 2ml/3.785L matrix secretion occurred three minutes after exposure; which increased to 92% (n=46) of the specimens after 24 hours (Table 1). In addition, specimens of C. macropyga displayed no yellow discharge when exposed to the lower concentration, but the higher concentration elicited yellow discharges from three specimens. Movement and NPR were displayed sporadically throughout the high and low concentrations trials, and were thus not useful as reliable qualitative indicators of chemical treatment efficacy.
Convolutriloba spp. subjects also displayed yellow discharges when exposed to Povidone. At 1ml/3.785L, 4% (n=2) of the specimens secreted a yellow discharge, which increased to 26% (n=13) at 2ml/3.785L (Table 1). In contrast to C. macropyga, the number of matrices secreted by the specimens of Convolutriloba spp. decreased from 68% (n=34) to 18% (n=9). After 24 hours, NPR occurred at the lower concentration, but not at the higher concentration.
Coral Rx was administered at two concentrations 0.2ml/3.785L and 8ml/3.785L. The lower dosage was not significantly different in effectiveness when compared to the control for all four species, and produced 0% mortality for all four species. The higher dosage was significantly different in effectiveness when compared to the control and the post hoc chi-square test of independence values were: Convolutriloba spp. (X2=100, P<0.05), C. macropyga (X2=100, P<0.05) Waminoa sp. (X2=100, P<0.05), and H. australis (X2=51.9, P<0.05). The higher concentration produced 100% mortality for Convolutriloba spp., C. macropyga, and Waminoa sp., and produced 80% mortality for H. australis (Fig. 7E).
Convolutriloba spp. specimens gave off a yellow discharge within the first minute of initial exposure to the higher dosage. After five minutes of exposure, matrices were visible in the Eppendorf tubes. After 24 hours of exposure to 8ml/3.785L, 66% (n=33) of the Conolutriloba spp. specimens of exhibited yellow discharge and 46% (n=23) secreted matrices in the tubes. Furthermore, after 24 hours 14% (n=7) of the C. macropyga specimens exhibited yellow discharge and 50% (n=25) exhibited matrices. Specimens of Waminoa sp. displayed erratic body contractions within the first minute of exposure to the higher dosage of Coral Rx. The contractions slowed after seven minutes, as did the NPR. After 4 hours the body tissues from these specimens began to degrade, and specimens displayed no movement or NPR. 24 hours after exposure, their body tissues were fully degraded, and three specimens secreted matrices.
Specimens of H. australis displayed continuous movement for the first nine minutes when exposed to the higher dosage of Coral Rx. This activity slowed after ten minutes and the NPR became increasingly slower. After four hours the specimens were observed moving. At 24 hours, the tissues were degraded and three of the four specimens that survived could not swim or display any forward locomotion. Survival after 24 hours was determined by observing body contractions.
Efficacy of coral dips in removal of Convolutriloba
Convolutrilobid drop off rates were dependent upon the species and the chemical treatment. Across treatments the Chi-square test of independence values for Convolutriloba spp. and C. macropyga were (X2=6.6, P<0.05) and (X2= 70.5, P<0.05), respectively (Fig 8). The effectiveness of Povidone and Coral Rx as coral dips on Convolutriloba spp. was not significantly different when compared to control values.
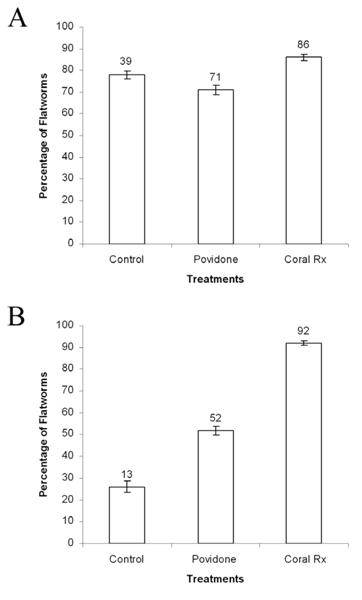
Figure 8. Percentage of Convolutriloba specimens that fell off live rock substrates when exposed to coral dips (control, Povidone, and Coral Rx). A) Specimens of Convolutriloba spp. B) Specimens of C. macropyga. Values above bars are actual numbers, n=50 for control groups and n=100 for experimental groups. Error bars show standard deviation.
In contrast, the effectiveness of these coral dips on C. macropyga was significantly different when compared to control values. The post hoc Chi-square test of independence values for Povidone and Coral Rx were (X2=9.2, P<0.05) and (X2=69.1, P<0.05) respectively. When exposed to Povidone 71% (n=71) of the Convolutriloba spp. and 52% (n=52) of the C. macropyga fell off of the live rock substrate. When exposed to Coral Rx 86% (n=86) of the Convolutriloba spp. and 92% (n=92) of the C. macropyga fell off of the live rock substrate. The controls produced 78% (n=39) drop off for Convolutriloba spp. and 26% (n=13) for C. macropyga (Fig. 8). As a result, Coral Rx and Povidone were significantly more effective in removing C. macropyga from live rock substrates than the control. In contrast, these coral dips were not significantly more effective in removing Convolutriloba spp. from
live rock than the control.
Each coral dip elicited a different response from the Convolutriloba subjects. For example, specimens of C. macropyga exposed to Coral Rx exuded a yellow discharge 15 to 20 minutes after exposure. Convolutriloba spp. exuded yellow discharges 5 minutes after exposure. Povidone did not elicit a yellow discharge from either group. A majority of the flatworms that attempted to move off of the substrate did not travel more than a few centimeters from the live rock.
A clear matrix was exuded from the flatworm subjects and attached them directly to the rock substrate. Most of the specimens that did travel several centimeters from the rock were pulled to the water surface as the rock was removed from the dip cup. At the water surface, the matrices broke and groups of 2 to 10 individuals connected by the viscous matrix sank collectively to the bottom. This phenomenon occurred more frequently with Coral Rx than Povidone.
Exposure of non-target species to Flatworm Exit
P. longicarpus, N. obsoletus, and A. pallida specimens were exposed to the recommended dosage of Flatworm Exit over three days. The mortality was 0% (n=50) for all three species. Specimens responded quickly to a sudden light introduction and tapping of the test cups. The hermit crabs and snails responded to these stimuli by retracting their eyes or withdrawing into their shells, respectively. Anemones reacted to the stimuli by contracting most of their body length. No discernable behavioral differences were observed between the experimental and control groups. In addition, all of the controls survived the acclimation and the experimental protocols.
Survey and distribution map of marine flatworms
In total, 48 individuals responded to the online survey. Responses were comprised from 26 states and 45 cities within the United States (Appendix 2; Fig. 9A). Respondents were concentrated in the Northeast, the Midwest, the South and California on the West Coast. Ninety-two% (n=44) of the respondents had problems with flatworms in their marine aquaria. Of these aquarists, 59% (n=26) used Flatworm Exit to eradicate the flatworms in their tanks (Fig. 9B), and 69% (n=18) needed to dose the tank a second time. After treatment with Flatworm Exit, 38% (n=10) of aquarists that used this chemical treatment reported losing livestock including: corals in the genera Acropora, Stylophora, and Calaustrea, a Sebae anemone Heteractis crispa, and bristle worms from aquaria sand-beds (Appendix 2; Fig. 9C). One aquarist lost the entire tank, and another lost fishes, corals, and invertebrates totaling $1,100 retail. Seventy-six percent (n=26) of the aquarists that
answered why they treated their flatworms, stated that flatworms are “visually unappealing.”
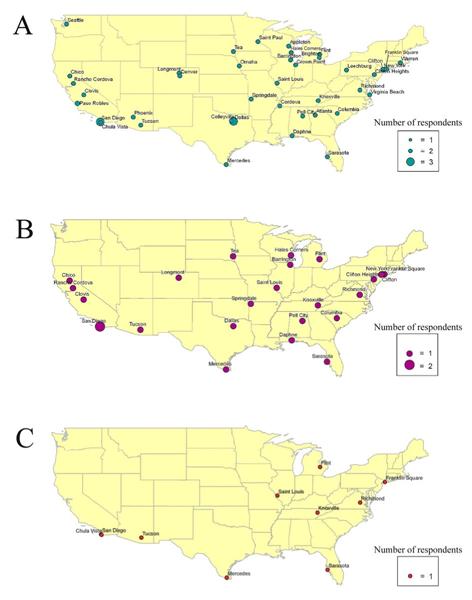
Figure 9. Distribution maps from the flatworm survey. A) Distribution of marine aquaria that were reported to have flatworm infestations (n=44). B) Distribution of aquarists that used Flatworm Exit to eradicate flatworms in their marine aquaria (n=26). C) Distribution of aquarists that lost live stock after using Flatworm Exit (n=10).
In addition, respondents described several other methods they used to remove flatworms from their aquaria. For example, three professional staff aquarists from private and public aquariums used the drug Levamisole to treat their flatworms (Appendix 2). Levamisole has been marketed as an antihelminithic drug since 1965, and is used to rid cattle, sheep, goats, swine and poultry of internal parasites. This drug can be obtained by prescription from a veterinarian and is effective against lungworms and gastrointestinal nematodes (El-Kholy and Kemppainen, 2005; Delbeek and Sprung, 2005). Levamisole is also used to suppress tumor growth in animals and to treat colorectal cancer in humans (Laurie et al., 1989). Delbeek and Sprung (2005) stated that professional aquarists have administered this drug directly to coral reef aquaria to eradicate marine flatworms. However, the three aquarists that used this drug reportedly lost scooter dragonets Synchiropus ocellatus in the
first tank, a blue-masked angelfish Euxiphipops xanthometopon in the second, and 2 sailfin tangs of the genus Zebrasoma in the third (Appendix 2). Survey respondents also reported using flatworm predators that consisted of scooter dragonets and mandarin fishes. Alternatively, two aquarists manually removed flatworms from their aquaria with a siphon.
After 9 months of culturing Convolutriloba species in coral reef aquaria, I observed that living convoltrilobid flatworms imposed no deleterious effects on scleractinians in the genera Caulastrea, Acropora, Pocillopora, or Plerogyra. Further, I did not witness convolutrilobids to settle on or touch scleractinian coral tissues directly. However, the convolutrilobids did settle on dead coral skeletons. In contrast, the soft purple coral Pachyclavularia violacea did become covered with convolutrilobids. Specimens of C. macropyga settled on top of P. violacea in large numbers, and appeared to inhibit full polyp extension.
Discussion
After exposing the target acoelomorphs to the chemical treatments, a wide range of mortality was observed between genera. In most cases, the efficacy of the chemical treatments increased when greater than the recommended dosage was administered to the test subjects (Table 2). Furthermore, the three target genera exhibited different susceptibilities to the chemical treatments. For example, Flatworm Exit was more effective on Convolutriloba spp. and C. macropyga than it was on Waminoa sp., whereas Flatworm Exit was completely ineffective on specimens of H. australis. Coral Rx (8ml/3.785L) eradicated all of the target genera except for four specimens of H. australis. Povidone, (2ml/3.785L), eradicated 0% of the specimens of H. australis and 100% of the Waminoa sp. specimens. As a result, the varied rates of mortality show that the chemical treatments affected the target genera differently.
| Control | Flatworm Exit (0.2ml) |
Flatworm Exit (0.3ml) |
Coral Rx (0.2ml) |
Coral Rx (8ml) |
Providone (1ml) |
Providone (2ml) |
|
|---|---|---|---|---|---|---|---|
| H. australis | 0% (0) | 0% (0) | 0% (0)* | 0% (0) | 80% (16)* | 2% (1) | 0% (0)* |
| Convolutrioba spp. | 0% (0) | 96% (48) | 90% (45) | 0% (0) | 100% (50) | 8% (4) | 30% (15) |
| C. macropyga | 0% (0) | 22% (11) | 76% (38) | 0% (0) | 100% (50) | 0% (0) | 12% (6) |
| Waminoa sp. | 2% (1) | 0% (0) | 10% (2)* | 0% (0) | 100% (20)* | 0% (0) | 100% (20)* |
In addition, mortality rates varied between the species of Convolutriloba. For example, specimens of C. macropyga were more resistant than the Convolutriloba spp. to the high and low concentrations of Flatworm Exit and Povidone. Although Flatworm Exit is advertised to eradicate all flatworms, this chemical treatment did not perform evenly across the target genera. The manufacturer of this product likely did not account for varied levels of susceptibility between genera. This explains the high rates of survivorship for Waminoa sp. and H. australis when exposed to Flatworm Exit.
Applying Flatworm Exit to marine aquaria is only useful if Convolutriloba are present. This chemical treatment performed well in eradicating this genus at 1.5 times the recommended dosage. However, without the Convolutriloba toxin present, the chemical treatment by itself did not eradicate Waminoa sp. or H. australis. When convolutrilobids are present, there are three plausible reasons that explain the observed mortality across different acoelomorph genera in marine aquaria. These reasons include:
- toxin release by Convolutriloba acts synergistically with the chemical treatment to induce mortality,
- toxin released by Convolutriloba alone induces mortality, and
- secreted toxins may degrade the water conditions inside an aquarium, which indirectly induce the mortality of other flatworm genera.
Consequently, Flatworm Exit was not effective by itself in eradicating infestations by multiple genera. Therefore, aquarists should identify the flatworms in their aquaria before applying this chemical treatment, and if Convolutriloba are not present the treatment should not be used.
Target acoelomorph genera can be identified using external morphological features. For example, species in the Convolutriloba have at least three caudal lobes at their posterior end. H. australis has only two symmetrical lateral caudal lobes at its posterior end. The caudal lobes found on Waminoa sp. are indistinct, but its oval body shape, pronounced paired anterior eyespots, and rusty brown coloration make this genus easy to identify. Also, species of Waminoa are found as ecto-commensals of corals of the genera Discosoma and Sarcophyton in coral reef aquaria (Delbeek and Sprung, 1997; Nilsen and Fossa, 2002; Shimek, 2004).
Another reason for not applying Flatworm Exit to marine aquaria is that the chemical composition of this product is unknown. Salifert did not disclose the constituents of this product on the packaging or on the Salifert website. Exposure to Flatworm Exit could be potentially hazardous to the health of both the tank inhabitants and the aquarist. In addition, Salifert has supplied no emergency protocol in case of accidental ingestion or skin exposure. Similarly, it is unknown if the chemical constituents of this product are detrimental to the environment.
Coral Rx (8ml/3.785L) elicited both yellow discharges and matrices from each Convolutriloba species within one to seven minutes. Once a convolutrilobid produces a yellow discharge, it typically auto-lyses completely. As a result, any Convolutriloba exposed to the higher concentration of Coral Rx for 20 minutes will not likely survive, even if placed into an aquarium. In contrast, specimens of Waminoa sp. and H. australis displayed body contractions and movement after 20 minutes of exposure. Further, specimens of H. australis continued to move after four hours of exposure. Therefore, Waminoa sp. individuals still attached to a coral dipped in Coral Rx for 20 minutes may survive in an aquarium. However, further research is needed to determine how long they would survive. Alternatively, it is likely that H. australis individuals will survive in an aquarium after 20 minutes of exposure.
Coral Rx performed well as a coral dip, and it would be advantageous for coral aquaculturists, wholesalers, retailers, aquariums, and hobbyists to use this product when quarantining newly purchased corals. Furthermore, Coral Rx should be utilized when corals are transferred from one aquarium to another to prevent accidental acoelomorph introduction. Coral Rx was developed specifically for corals by a private coral aquaculturist, and the manufacturer claims that the product readily biodegrades and is innocuous to aquarists. However, further research is needed to determine if these statements are accurate.
Povidone was useful as a coral dip in treating C. macropyga, but was ineffective on C. retrogemma. Although Povidone was utilized in a twenty minute protocol, three of the four target species tolerated higher than the recommended dosage for 24 hours. As a result, 70-100% of the target flatworms (i.e., Convolutriloba spp., C. macropyga, and H. australis) that were exposed to Povidone (2ml/3.785L) over 24 hours survived this protocol. Similarly, 92-100% of the target flatworms (i.e., Convolutriloba spp., C. macropyga, Waminoa sp., and H. australis) that were dipped in Povidone (1ml/3.785L) survived. Consequentially, acoelomorphs that do not fall off of a Povidone dipped coral will not be eradicated, and will be introduced into an aquarium. Aquarists that use Povidone should shake the coral colonies vigorously after 20 minutes, because any flatworms introduced into an aquarium can survive. Overall, this product
should not be used as a coral dip because it was less effective than a salt water dip on Convolutriloba spp. specimens. Iodine residue left on corals from this product should be innocuous to aquarium inhabitants because aquarists add this element to aquaria to promote soft coral growth (Delbeek and Sprung, 1994). However, too much iodine is toxic to aquarium livestock (Delbeek and Sprung, 2005).
Future investigations should expose different genera of acoelomorphs to the chemical treatments to determine if mortality and drop off rates vary among taxa. Additionally, mono-specific cultures of the Convolutriloba, as established by Shannon 2007, would provide insight about the efficacy of chemical treatments on individual species. For example, further experimentation could determine if other convolutrilobid species (i.e., C. retrogemma, C. longifissura, and C. hastifera) are resistant to the recommended dosage of Flatworm Exit. Chemical analysis of Flatworm Exit and Coral Rx could elucidate which compounds are responsible for inducing physiological reactions including yellow discharge and matrix secretion. Further, analyzing and identifying the biological toxin produced by this genus could lead to more effective protocols for removing it faster from marine aquaria.
Additional analysis of the acoelomorph matrices could prove useful in determining their function. Matrices secreted by the Convolutriloba were only observed during experimental trials, and did not occur in controls. In the wild, mucus secretion in acoelomorphans and polyclads facilitates movement, is used for prey capture, prevents desiccation, and may provide a chemical defense against predation (Newman and Cannon, 2003; Pechenik, 2005; Wisenden and Millard, 2001). Members of the freshwater flatworm genus Dugesia use chemical cues to locate food (Wisenden and Millard, 2001).
During experimentation the matrices impeded the movement of convolutrilobids. These secretions may be a conspecific alarm cue, a protective adaptation against predation, or a response to the rapid fluctuation of pH or osmotic pressure caused by the chemical treatments (Hickman, 1967; Wisenden and Millard, 2001). Microscopic analysis of the matrix is necessary to determine its origin. For example if the matrices contain rhabdites, then the secretions likely originated from the rhabdoid glands. According to Hickman (1967), gelatinous sheaths of rhabdite secretions form around a marine flatworm’s body when exposed to irritants. However, further research is necessary to better understand the potential functions of the matrix secretions observed in the present study.
The non-target representative invertebrate species exposed to Flatworm Exit are commercially important in the marine ornamental trade. Hermit crabs and snails from Fiji and the Solomon Islands are utilized in coral reef aquaria to clean corals, live rock, glass panels, and sand beds of algal growth and decaying debris. Typically, these invertebrates are sold together as biological “clean up crews” (Delbeek and Sprung, 2005). Hermit crabs and snails, in particular, keep corals healthy by removing algal growth. Therefore, chemical treatments designed to eradicate marine flatworms should not affect the non-target organisms because these invertebrates maintain overall tank health. Representative species from the Arthropoda, Mollusca, and Cnidaria provided strong evidence that Flatworm Exit has no short-term or visible harmful effects on these experimental taxa. This is the first quantitative data showing that this chemical does not cause mortality of these animals.
Future investigations should expose other important marine aquarium taxa (i.e., annelids, echinoderms, and fishes) to Flatworm Exit, to determine if this chemical treatment is safe for all taxa commonly found within coral reef tanks. Additionally, further research should examine the long term sub-lethal affects that Flatworm Exit may have on the reproductive success of vertebrate and invertebrate livestock. Currently, no information exists about how these chemical treatments affect the physiology of the non-target livestock.
Recent hobbyist literature provides information about the trends that occur in the marine ornamental trade (Delbeek and Sprung, 1994; 1997; 2005; Borneman, 2001; Nilson and Fossa, 2002; Shimek, 2004). The survey on nuisance flatworms confirmed some of the hobbyist literature claims. Data from the online survey showed that acoelomorph infestation occured in the marine ornamental trade throughout the United States. Currently, marine flatworms have infiltrated multiple levels of the trade including: wholesalers, retailers, private coral aquaculturists, public aquariums, and hobbyists. As a result, flatworms are spread from one tank to another, allowing multiple species to settle on individual coral colonies. This makes the quarantine process more difficult for aquarists because different acoelomorph genera have varied susceptibilities to coral dips. Therefore, aquarists dealing with flatworms should utilize the following three protocols:
- dip live rock and new coral colonies in Coral Rx before placing them in aquaria,
- siphon out flatworms manually, and
- if a tank additive must be used, take out all of the live stock before applying the chemical treatment.
After applying a tank additive aquarists should conduct a large water change before returning the livestock, to dilute the concentration of convolutrilobid toxin.
In conclusion, I do not recommend administering Flatworm Exit to coral reef aquaria for several reasons. First, this chemical treatment is only effective against Convolutriloba. Second, 38% of the aquarists that used Flatworm Exit reportedly lost livestock after administering this product. Third harvesting coral reef species to replace livestock lost from Convolutriloba toxin release is environmentally costly. Alternatively, responsible reef keepers should prevent acoelomorph infestation by diligently quarantining new corals and live rock before introducing them into aquaria.
Although the two coral dips under investigation varied in their efficacy, aquarists would benefit from using one of the two products to remove any C. macropyga that settle on newly purchased corals. Furthermore, aquarists should at least dip new corals in salt water and shake them vigorously because this will remove 26-78% of the convolutrilobids from live rock. Additionally, introducing natural predators or siphoning flatworms out of an aquarium are advantageous alternatives, because these methods don’t induce toxin release from Convolutriloba. Currently, 99% of the live stock in the marine ornamental trade is harvested from coral reefs in the wild. Therefore, aquarists should take every precaution to protect their livestock and keep it thriving in captivity.
Acknowledgments
I would like to thank my advisor Dr. Jason Williams for his help and support throughout this investigation. I would like to express sincere gratitude to Todd Gardner, Chris Paparo, and Joe Yaiullo at the Atlantis Marine World Aquarium for their continual support and professional expertise. Acoelomorph identification was made possible with help from Drs. Thomas Shannon from the University of Georgia, Orit Barneah from Tel Aviv University, and Seth Tyler and Johannes Achatz from the University of Maine. I would also like to thank Dr. William Ferguson for his dedication and foresight throughout this research. Thanks to Philip Root at The Coral Gardens for supplying marine flatworms and current marine ornamental trade information. I would like to thank Dr. Daniel for his help with the statistical analysis. Finally, thanks to my committee members Drs. Russell Burke and Robert Seagull for their insightful feedback.
References
- Achatz J, Hooge M. (2006). Convolutidae (Acoela) from Tanzania. Zootaxa 1326,1-21.
- Akesson B, Gschwentner R, Hendelberg J, Ladurner P, Müller J, Rieger R. (2001). Fission in Convolutriloba longifissura: asexual reproduction in acoelous turbellarians revisited. Acta Zoologica 82,231-239.
- Akesson B, Hendelberg J. (1989). Nutrition and asexual reproduction in Convolutriloba retrogemma, an acoelous turbellarian in obligate symbiosis with algal cells.13-21.
- Aquacave Inc. (2008) Lugol’s Solution 1 oz. by Kent Marine. http://www.aquacave.com/lugols-solution-1-oz-brby-kent-marine-129.html Accessed 2008 March 5.
- Baguñà J, Riutort M. (2004). Molecular phylogeny of the Platyhelminthes. Canadian Journal of Zoology 82,168-193.
- Barneah O, Brickner I, Hooge M, Weis V, Benayahu Y. (2007). First evidence of maternal transmission of algal endosymbionts at an oocyte stage in a triploblastic host, with observations on reproduction in Waminoa brickneri (Acoelomorpha). Invertebrate Biology 126,113-119.
- Barneah O, Brickner I, Hooge M, Weis V, LaJuenesse TC, Benayahu Y. (2007). Three party symbiosis: acoelomorph worms, corals and unicellular algal symbionts in Eilat (Red Sea). Marine Biology 151,1215-1223.
- Bartolomaeus T, Balzer I. (1997). Convolutriloba longifissura, nov. spec. (Acoela) – first case of longitudinal fission in Plathelminthes. Microfauna Marina 11,7-18.
- Best B, Pomeroy R, Balboa C. (2002). Implications for Coral Reef Management and Policy: Relevant Findings from the 9th International Coral Reef Symposium.
- Borneman E. (2001). Aquarium Corals: Selection, Husbandry, and Natural History. (T.F.H. Publications Inc., neptune City).
- Bruggeman J. (1986). Ultrastructural investigations on the differentiation of genital hard structures in free-living platyhelminths and their phylogenetic significance. Hydrobiologia 132,151-156.
- Burgess W, Axelrod H, Hunziker R. (1997). Dr. Burgess’s mini atlas of marine aquarium fishes mini edition. (T.F.H. Publication Inc., neptune City).
- Cole B, Tamaru C, Bailey R, Brown C, Ako H. (1999). Shipping practices in the marine ornamental fish industry. (Center for tropical and subtropical aquaculture Hawaii, USA). pp 25. 58
- Delbeek C, Sprung J. (1994). The Reef Aquarium A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates (Ricordea Publishing, Coconut Grove).
- Delbeek C, Sprung J. (1997). The Reef Aquarium A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. (Ricordea Publishing, Coconut Grove).
- Delbeek C, Sprung J. (2005). The Reef Aquarium Science, Art, and Technology. (Ricordea Publishing, Coconut Grove).
- El-Kholy H, Kemppainen B. (2005). Levamisole residues in chicken tissues and eggs. Poultry Science Association, Inc. 84,9-13.
- Green E. (2003). International trade in marine aquarium species: using the global marine aquarium database. pp 31-48. In Marine ornamental species: collection, culture and conservation. Cato J, Brown C, eds. Iowa State Press, Ames.
- Hendelberg J, Akesson B. (1988). Convolutriloba retrogemma gen. et sp.n., a turbellarian (Acoela, Platyhelminthes) with reversed polarity or reproductive buds. Fortschritte de Zoologie/Progress in Zoology 36, 321-327.
- Hendelberg J, Akesson B. (1991). Studies of the budding process in Covolutriloba retrogemma (Acoela, Platyhelminthes). Hydrobiologia 227,11-17.
- Hickman C. (1967). Biology of the invertebrates. The C.V. Mosby Company, Saint Louis.
- Hirose E, Hirose M. (2007). Body Colors and Algal Distribution in the Acoel Flatworm Convolutriloba longfissura: Histology and Ultrastructure. Zoological Science 24,1241-1246.
- Hooge M, Haye P, Tyler S, Litvaitis M, Kornfeild I. (2002). Molecular systematics of the Acoela (Acoelomorpha, Platyhelminthes) and its concordance with morphology. Molecular Phylogenetics and Evolution 24,333-342.
- Hyman L. (1951). The Invertebrates: Platyhelminthes and Rhyanchocoela The acoelomate Bilateria. McGraw-Hill Book Company Inc., New York.
- Laurie J, Moertel C, Fleming T, Wieand H, Leigh J, McCormack G, Gerstner J, Krook J, Mallidard J, Twito D, Morton R, Tschetter L, Barlow J (1989). Surgical adjuvant therapy of large-bowel carcinoma: an evalutation of levamisole and the combination of levamisole and fluorouracil. Journal of clinical oncology 7,1447-1456. 59
- McMurrich J. (1896). A text-book of invertebrate morphology. Henry Holt and Company, New York.
- Newman L, Cannon L. (2003). Marine Flatworms: The World Of Polyclads. CSIRO Publishing, Collingwood.
- Nilsen A, Fossa S. (2002). Reef Secrets: starting right,selecting fishes & invertbrates, advanced biotope techniques. (T.F.H. Publications, Inc., Charlotte, VT).
- Ogunlana M, Hooge M, Tekle Y, Benayahu Y, Barneah O, Tyler S. (2005). Waminoa brickneri n.sp. (Acoela: Acoelomorpha) associated with corals in the Red Sea. Zootaxa 1008,1-11.
- Oliver K. (2003). World trade in ornamental species. In Marine Ornamental Species: Collection, Culture and Conservation. Cato J, Brown C, eds. (Iowa State Press, Ames). pp 49-63.
- Pechenik J. (2005). Biology of the invertebrates, fifth edition. (McGraw-Hill, Boston).
- Ritson-Williams R, Yotsu-Yamashita M, Paul V. (2006). Ecological fucntions of tetrodotoxin in a deadly polyclad flatworm. In PNAS. (The National Academy of Sciences USA). pp 3176-3179.
- Root P. (2008). Coral Rx first aid for your corals. (The Coral Gardens) http://www.coralrx.com/coralstore/ Accessed 2009 January 20.
- Ruiz-Trillo I, Paps J, Loukota M, Ribera C, Jondelius U, Baguñà J, Riutort M. (2002). A Phylogenetic Analysis of Myosin Heavy Chain Type II Sequences Corroborates That Acoela and Nemertodermatida Are Basal Bilaterians. In Proceedings of the National Academy of Sciences of the United States of America. (National Academy of Sciences). pp 11246-11251.
- Ruiz-Trillo I, Riutort M, Littlewood T, Herniou E, Baguna J. (1999). Acoel Flatworms: Earliest Extant Bilaterian Metazoans, Not Members of Platyhelminthes. Science 283,1919-1923.
- Shannon T. (2007). Photosmoregulation: evidence of host behavioral photoregulation of an algal endosymbiont by the acoel Convolutriloba retrogemma as a means of non-metabolic osmoregulation. (University of Georgia). pp 178.
- Shannon T, Achatz J. (2007). Convolutriloba macropyga sp. nov., an uncommonly fecund acoel (Acoelomorpha) discovered in tropical aquaria. Zootaxa 1525,1-17.
- Shimek R. (2004). Marine Invertebrates: 500 + Essential to know aquarium species. (T.F.H. Publications,Inc., neptune City). 60
- Sikes J, Bely A. (2008). Radical modification of the A-P axis and the evolution of asexual reproduction in Convolutriloba acoels. Evolution and Development 10,619-631.
- Speigel M, Stephens L. (2008). Schuam’s outline of theory and problems of statistics. McGraw-Hill, New York.
- Tyler S. (2004). Platyhelminthes and Acoelomorpha, phyla of controversy. (University of Maine). http://devbio.umesci.maine.edu/styler/globalworming/platyhelm.htm Accessed 2008 October 28.
- Wabnitz C, Taylor M, Green E, Razak T. (2003). From Ocean to Aquarium: The global trade in marine ornamental species. In UNEP World Conservation Monitoring Centre 2003. pp 1-66.
- Winsor L. (1990). Marine Turbellaria (Acoela) from north Queensland. Memoirs of the Queensland Museum 28,785-800.
- Wisenden B, Millard M. (2001). Aquatic flatworms use chemical cues from injured conspecifics to assess predation risk and to associate risk with novel cues. Animal Behavior 62,761-766.
Appendix 1. Survey template used to collect data for distribution map. (Surveymonkey.com 2008)
| Respondent # | Flatworms | Levamisole | Flatworm Exit | Lost livestock | Animals lost | State | City |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 1 | Brittlestars, misc invertebrates | NE | Omaha |
| 2 | 1 | 0 | 0 | 0 | VA | Virginia Beach | |
| 3 | 1 | 0 | 1 | 0 | AL | Pell City | |
| 4 | 1 | 0 | 0 | 0 | AZ | Phoenix | |
| 5 | 1 | 0 | 0 | 0 | TN | Cordova | |
| 6 | 1 | 1 | 0 | 1 | Blue masked angelfish | CA | La Jolla |
| 7 | 1 | 0 | 1 | 0 | PA | Clifton Heights | |
| 8 | 1 | 0 | 1 | 0 | SD | Tea | |
| 9 | 1 | 0 | 1 | 0 | CO | Longmont | |
| 10 | 0 | 0 | 0 | 0 | HI | Kailua | |
| 11 | 1 | 0 | 0 | 0 | WI | Appleton | |
| 12 | 1 | 0 | 1 | 1 | Acropora cervicornis | FL | Sarasota |
| 13 | 1 | 0 | 0 | 0 | WA | Seattle | |
| 14 | 1 | 0 | 1 | 0 | MN | St. Paul | |
| 15 | 1 | 1 | 0 | 1 | 2 sailfin tangs, brittle star | CO | Denver |
| 16 | 1 | 0 | 0 | 0 | TX | Dallas | |
| 17 | 1 | 0 | 1 | 0 | SC | Columbia | |
| 18 | 1 | 0 | 0 | 0 | GA | Atlanta | |
| 19 | 1 | 0 | 1 | 0 | TX | Dallas | |
| 20 | 1 | 0 | 0 | 0 | MI | Brighton | |
| 21 | 0 | 0 | 0 | 0 | CA | Roseville | |
| 22 | 1 | 0 | 1 | 1 | Misc. invertebrates | MI | Flint |
| 23 | 1 | 0 | 1 | 0 | CA | Rancho Cordova | |
| 24 | 1 | 0 | 1 | 1 | TX | Mercedes | |
| 25 | 0 | 0 | 0 | 0 | OR | Portland | |
| 26 | 1 | 0 | 0 | 0 | PA | Leechburg | |
| 27 | 1 | 0 | 0 | 0 | RI | Warren | |
| 28 | 1 | 0 | 1 | 1 | Bristle worms | CA | San Diego |
| 29 | 1 | 0 | 1 | 1 | Caulastrea | CA | Chula Vista |
| 30 | 1 | 0 | 1 | 0 | CA | San Diego | |
| 31 | 1 | 0 | 1 | 1 | NY | Franklin Square | |
| 32 | 1 | 0 | 1 | 1 | Coral, fish, invertebrates | VA | Richmond |
| 33 | 0 | 0 | 0 | 0 | Canada | Toronto | |
| 34 | 1 | 0 | 0 | 0 | TX | Colleyville | |
| 35 | 1 | 0 | 1 | 0 | CA | Chico | |
| 36 | 1 | 0 | 1 | 1 | Sebae anemone, Calaustrea | AZ | Tucson |
| 37 | 1 | 0 | 1 | 1 | Acropora, Stylophora | MS | St. Louis |
| 38 | 1 | 0 | 1 | 0 | IL | Barrington | |
| 39 | 1 | 0 | 1 | 1 | Entire tank | TN | Knoxville |
| 40 | 1 | 0 | 1 | 0 | IL | Barrington | |
| 41 | 1 | 0 | 1 | 0 | CA | Clovis | |
| 42 | 1 | 0 | 0 | 0 | TX | Dallas | |
| 43 | 1 | 0 | 0 | 0 | IN | Crown Point | |
| 44 | 1 | 0 | 0 | 0 | NY | Brooklyn | |
| 45 | 1 | 0 | 0 | 0 | CA | Paso Robles | |
| 46 | 1 | 0 | 1 | 0 | AL | Daphne | |
| 47 | 1 | 0 | 1 | 0 | WI | Hales Corners | |
| 48 | 1 | 0 | 1 | 0 | NJ | Clifton | |
| TOTALS | 44 | 3 | 26 | 13 |



Why is there no author credit for this master’s thesis? I spent years writing it.