The concluding glimpse into the unseen world of fish gut and gill microbiomes with reference to the impacts of diet, pre-, and pro-biotics. Around 20 kg of farmed finfish is consumed by each person per annum in countries that exploit sophisticated aquaculture (Amillano-Cisneros et al. 2023) and thus a wealth of open-source literature is at our disposal.
The intestinal communities of saltwater teleosts, anadromous salmonids, and catadromous and saltwater eels are influenced by nourishment, life stage, season, and salinity. Distinct omni-, plankti-, herbi-, pisci-, or inverti-vore-adapted alimentary canals share common elements which are featured in figure 1. (Edgerton et al. 2018). Buccal and pharyngeal cavities proceed into the fore-, mid-, and hind-gut. Immediately behind the opercula occurs the foregut comprising the oesophagus, stomach, and pyloric caeca where 20 percent of fish such as Gobiidae and Blennidae lack conventional stomachs (Wilson & Castro 2010). However those fish often have compensatory rudiments such as pharyngeal teeth or pockets, oesophageal secretory glands, or muscular gizzards (Kapoor & Khawna 1993, cited in Edgerton et al. 2018; Stevens & Hume 2004, cited in Edgerton et al. 2018) where a lack of gastric acidification assists microbial recruitment (Ringø et al. 2016). Stomachs are U-, Y-shaped, or straight which are rare yet occur in mullet (Mugil species), anchovy (Engraulis species), and menhaden (Brevoortia species). Omni- and carni-vores such as salmonids and seabass (Dicentrarchus species) retain the former while Y-shaped stomachs occur in piscivorous fish that swallow their prey whole such as lionfish (Scorpaenidae), groupers (Serranidae), or eels (Anguillidae, Congridae, and Muraenidae; Mihalitsis & Bellwood 2017; Edgerton et al. 2018).
Most absorption occurs in the midgut where surface area is elevated by the pyloric caeca while lumen expansion frequently demarcates the isthmus of the hindgut. The alimentary canal is roughly equal to body length in dedicated carnivores, while it is one to three and three times longer in omnivorous and herbivorous fish (Bone et al. 1995, cited in Edgerton et al. 2018; Karachle & Stergiou 2010). Nevertheless the gut is ~20 times body length in species of Tilapia (Ray & Ringø 2014, cited in Ringø et al. 2016).
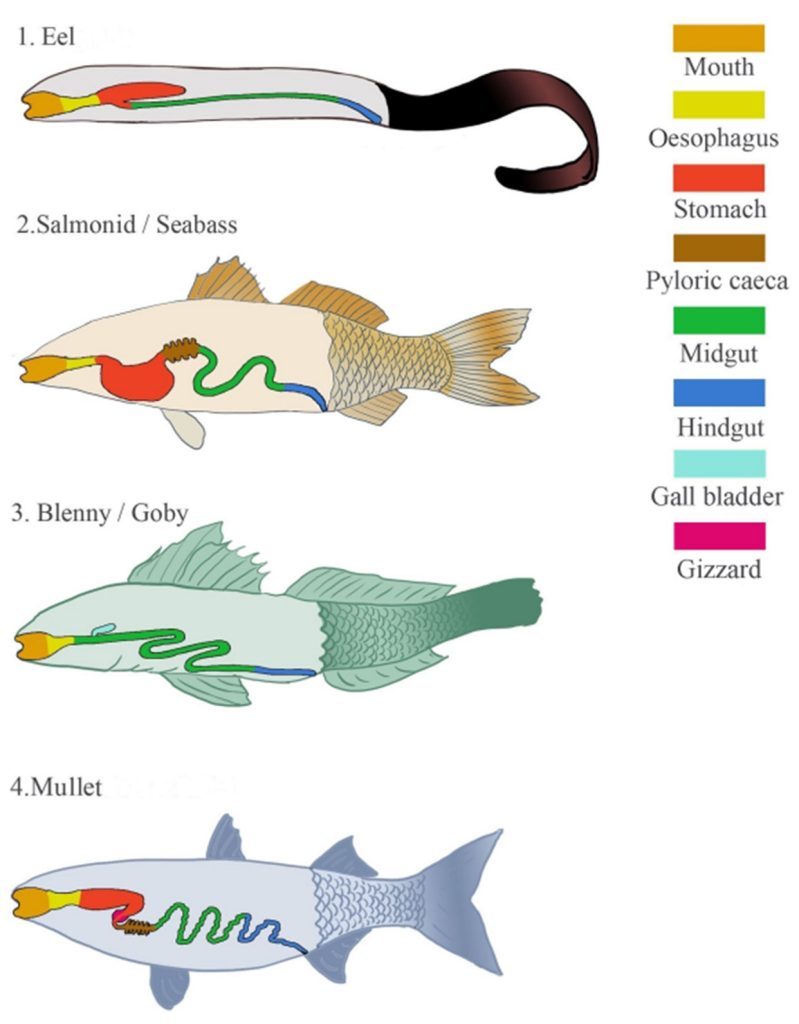
Fig 1. Diagrams of the distinct gut anatomies of marine, anadromous, and catadromous teleosts. Image courtesy of Edgerton et al. 2018 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
Fish microbiomes are diverse and much like corals comprise bacteria, fungi, yeasts, chromists, protozoa, bacteriophages, viruses, and Archaea, where Bacteria are the dominant kingdom in teleostean gastrointestinal tracts (GITs) whereas around 90 percent of their gut prokaryotes are members of the phyla Bacteroidetes, Firmicutes, and Proteobacteria (Rombout et al. 2011, cited in Edgerton et al. 2018; Ghanbari et al. 2015, cited in Edgerton et al. 2018). Some are transient allochthonous digestion-associated taxa, whereas established core autochthonous communities inhabit mucus-secreting microvillus-adorned enterocytes which sustain an efficacious functional barrier (Fig 2.; Fig 3.; Ringø et al. 2016; Edgerton et al. 2018). Lumen aerobic to anaerobic abundances range from 104 to 109 colony forming units (CFU) per gram (g-1; Skrodenyte-ArbaCIauskiene 2007) which is dwarfed by those of warm-blooded vertebrates (Nayak 2010) yet the highest density of fish microbes inhabit their gut. Prokaryotic abundances are around 105 in the stomach, 104 in the pyloric caeca, and 106 in the intestines where microbial hydrolytic enzymes are proportional to cell biomass (Das et al. 2014) whereas studies of contrasting fish have found this trend reversed or absent (Navarrete et al. 2009; Zhou et al. 2009). Nevertheless distinct communities zone within each section of the alimentary canal (Edgerton et al. 2018). A recent culture-dependent study suggested Actinobacteria, Firmicutes, and Proteobacteria dominated the stomach consortia of gilthead seabream (Sparus aurata; Estruch et al. 2015). The genera Aeromonas, Enterobacter, Photobacterium, Pseudomonas, and Vibrio are prolific enzyme synthesisers ranging from proteases to chitinases, however such analysis must differentiate between allochthonous and autochthonous affiliates and “friend” or “foe” (Edgerton et al. 2018).
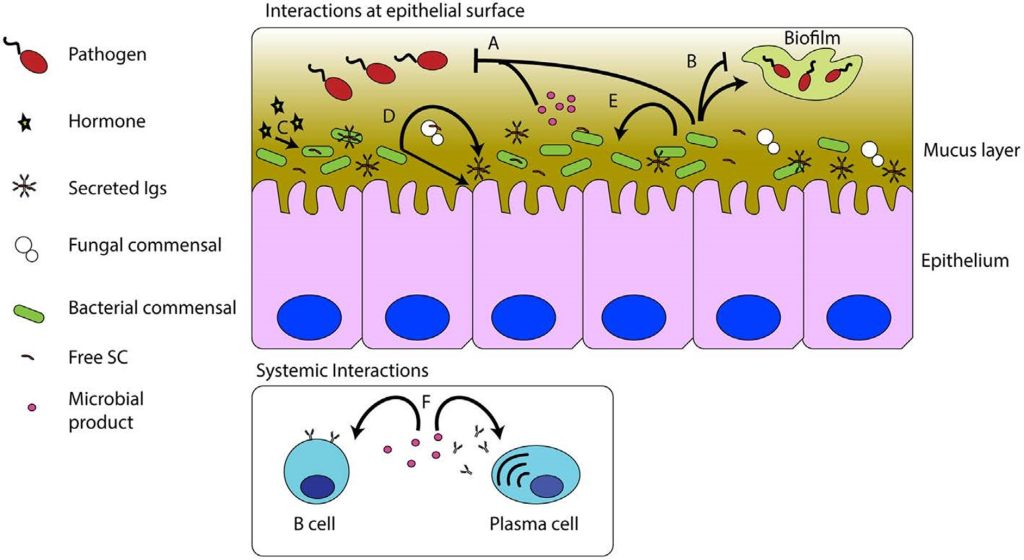
Fig 2. Fish gut mucosal microbiome, pathogen, and immunity dynamics illustrating the gut epithelium (enterocytes) with miniature surface area-optimising finger-like projections called villi, where mutualists prohibit pathogenic invasion [A] and moderate biofilms [B] while fish hormones such as cortisol impact commensals [C] which induce the secretory antibody sIgT and recycle sloughed epithelium [D]. Microbiome affiliates interact [E] while their metabolism degrades mucus and other biochemicals which generates secondary metabolites that regulate antibody production through B-cell modulation [F]. Image courtesy of Kelly & Salinas 2017 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
Ostensibly sterile yolk sac-bound larvae initially absorb chorion-associated bacteria which colonise their nascent GIT. Further acquisitions originate from saltwater teleostean drinking and their food (Hansen & Olafsen 1999, cited in Edgerton et al. 2018) while their affiliates develop as they grow like human gut microflora (Yatsunenko et al. 2012; Edgerton et al. 2018). The gut communities of salt- and fresh-water fry are populated in order of prevalence by Vibrio, Pseudomonas, Cytophaga, and Flavobacterium species accompanied by Enterobacteriaceae (Edgerton et al. 2018).
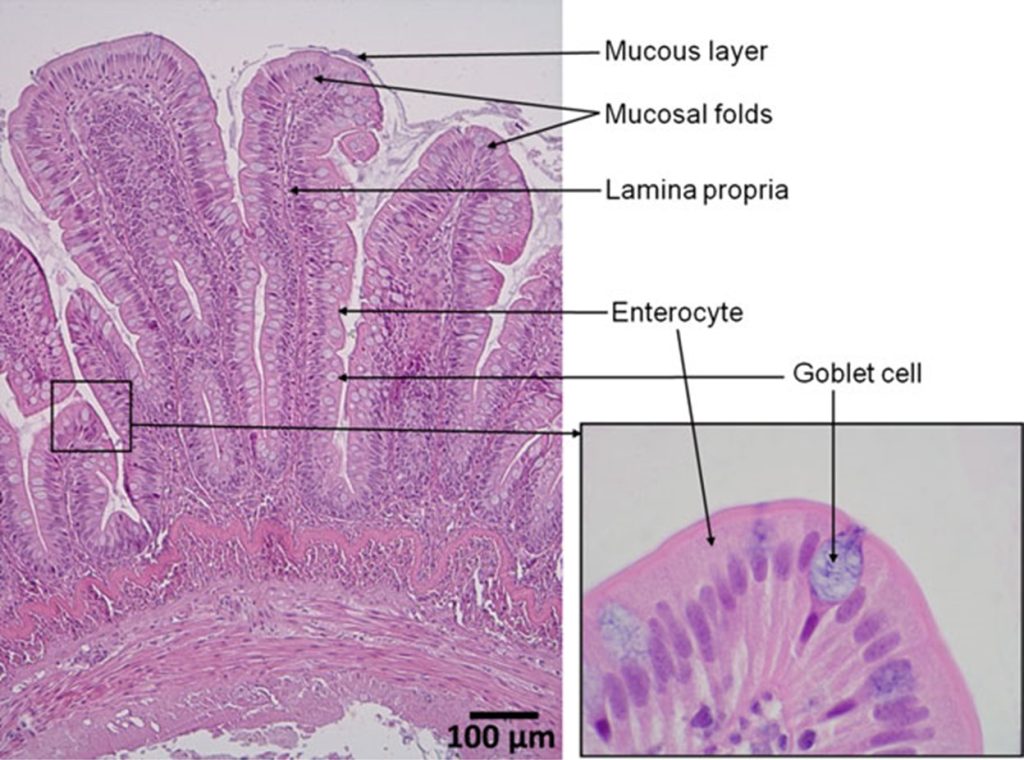
Fig 3. Micrographs of the midgut wall of Atlantic salmon (Salmo salar) stained with haematoxylin and eosin featuring mucus-and lysozyme-secreting goblets cells which proliferate throughout effective probiotic administration (Carnevali et al. 2017). Images M. Penn © courtesy of Ringø et al. 2016 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
Most alimentary populations are established by day 50 (Larsen 2014) where trophic rank, host phylogeny, and habitat including the wild, aquarium, or mariculture remain the utmost determinants (Sullam et al. 2012, cited in Edgerton et al. 2018; Siriyappagouder et al. 2018). Dietary inoculants cannot fail to influence transient or core communities, while the gut microbiota of herbivorous fish retain anaerobic digestive competencies conferred by members of the phylum Firmicutes and class Clostridia (Mouchet et al. 2012, cited in Edgerton et al. 2018; Zarkasi et al. 2016). Potentially harmful Vibrio, Photobacterium, and Clostridium species were widespread along with noteworthy Desulfovibrio (Edgerton et al. 2018) where cellulose algal cell walls, plant starches and sugars are fermented to form volatile fatty acids (Moyle 2000). Nonetheless anaerobic digestion yields nominal nourishment in cold water species (Ringø et al. 2016). The gut biota of omnivores comprise the genera Clostridium, Corynebacterium, Mycoplasma, Photobacterium, Propionibacterium, Pseudomonas, and Staphylococcus, whereas the greatest diversity occurs in carnivores where Corynebacterium, Propionibacterium, and Staphylococcus were absent where the genera Acinetobacter, Aeromonas, Bacillus, Cetobacterium, Escherichia, and lactic acid bacteria (LAB) like Lactobacillus and Lactococcus appeared exclusive, while pisci-/inverti-vores underperform and cannot adapt to several types of plant-derived nourishment (Fournier et al. 2004). Moreover switching from fishmeal to vegetable protein adversely affects non-herbivorous fish which instigates numerous life-threatening disorders associated with shifts in gut microbiota. Downturns in LAB may be accompanied by successions of atypical Escherichia and Propionibacterium species (Green et al. 2013; Edgerton et al. 2018). The GITs of zooplanktivores were dominated by Achromobacter, Alteromonas, Endozoicomonas, Pseudomonas, Shewanella, and Vibrio species including V. harveyi (Edgerton et al. 2018) although bacterial virulence is often strain-specific. Remarkably clades of Endozoicomonas are cross-taxa core affiliates of healthy hermatypic corals (Pollock et al. 2018; McCauley et al. 2022) so mysid or brine shrimp-eating fish may provide welcome inoculums yet V. harveyi are the aetiological agents of white band disease of corals (Richardson et al. 1998).
Seasonal variances occur in summer and autumn when temperatures soar despite the availability of prey items changing throughout the year. One study discovered that elevated temperature diminished LAB and caused upsurges of conceivably detrimental Acinetobacter and Vibrio species (Neuman et al. 2016). A wealth of evidence suggests that fish conventionally retain enduring core microbiota where captivity has recognised impacts, while comparative baseline data for wild teleosts is lacking (Edgerton et al. 2018).
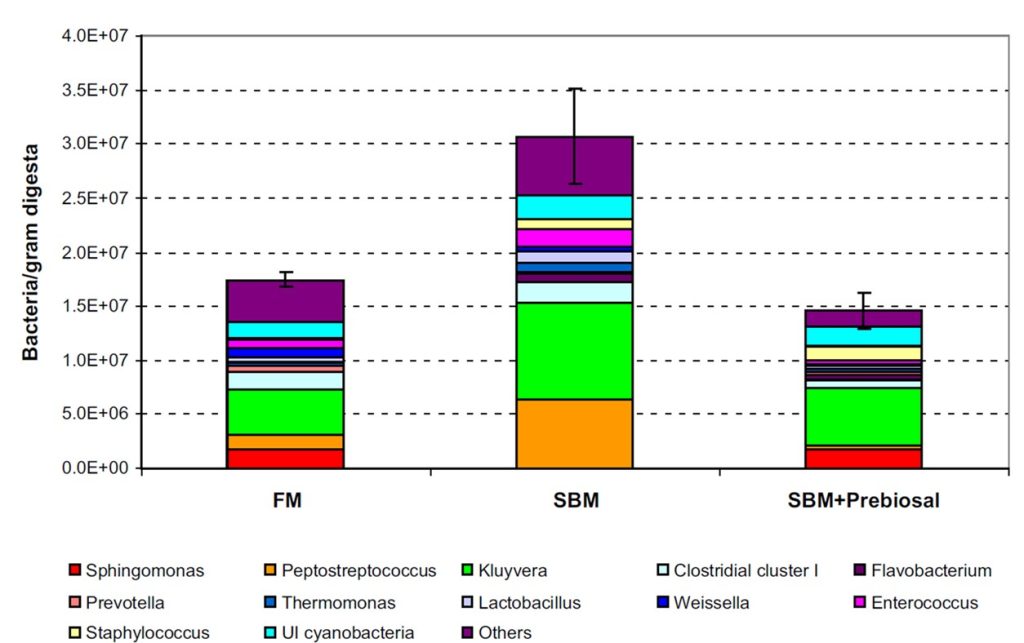
Fig 4. Profiles of the gut biota of Atlantic salmon raised for six weeks on diets comprising fishmeal, soybean meal, and soybean meal supplemented with a proprietary prebiotic derived from marine and terrestrial complex carbohydrates called EWOS Prebiosal®. Image and analyses courtesy of Ringø et al. 2016 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
Dietary Manipulation
Sugar metabolism in finfish is akin to that of a diabetic mammal insofar as digestible or simple sugars like glucose cause hyperglycaemia. Fish insulin can manage serum concentrations, but it is not used for that purpose most likely because of the elevated glycogen reserves of teleostean brains (Roberts 1989) whereas mammalian intellects operate on glucose or ketones. The nervous system of starved fish likely depends upon gluconeogenesis rather than glycogenolysis (Roberts 1989) whereas marine carbohydrates are primarily produced by photosynthetic autotrophs (algae; Moyle 2000). Nevertheless insulin-like growth factors govern finfish anabolism where the water- or prey-borne probiotic Lactococcus rhamnosus escalated development and survivorship of tank bred common clownfish (Amphiprion ocellaris; Carnevali et al. 2017).
Studies are underway to ascertain the impacts of dietary proteins, lipids, and pre-, and pro-biotics (Ringø et al. 2016; Carnevali et al. 2017; Edgerton et al. 2018; Amillano-Cisneros et al. 2023) where most teleostean energy arises from the former two. Protein ingestion is related to growth irrespective of feeding specialisations (Roberts 1989) which must be of the correct kind and proportional insofar as essential amino acids must be consumed and elevated protein can be harmful (Wilson, personal communication), whereas deficiencies can stimulate escalations of microbial diversity (Fig 4.; Ringø et al. 2016; Zarkasi et al. 2016). Aquaculture feeds previously comprised up to 70 percent protein (Roberts 1989) yet from 30 to 37 percent is considered ample today because 12 to 16 percent of protein-derived energy is expended for its digestion and assimilation. Merely ~21 percent of dietary nitrogen is converted to ammonia which indicates around 80 percent of protein is used for energy and growth (Moyle 2000).
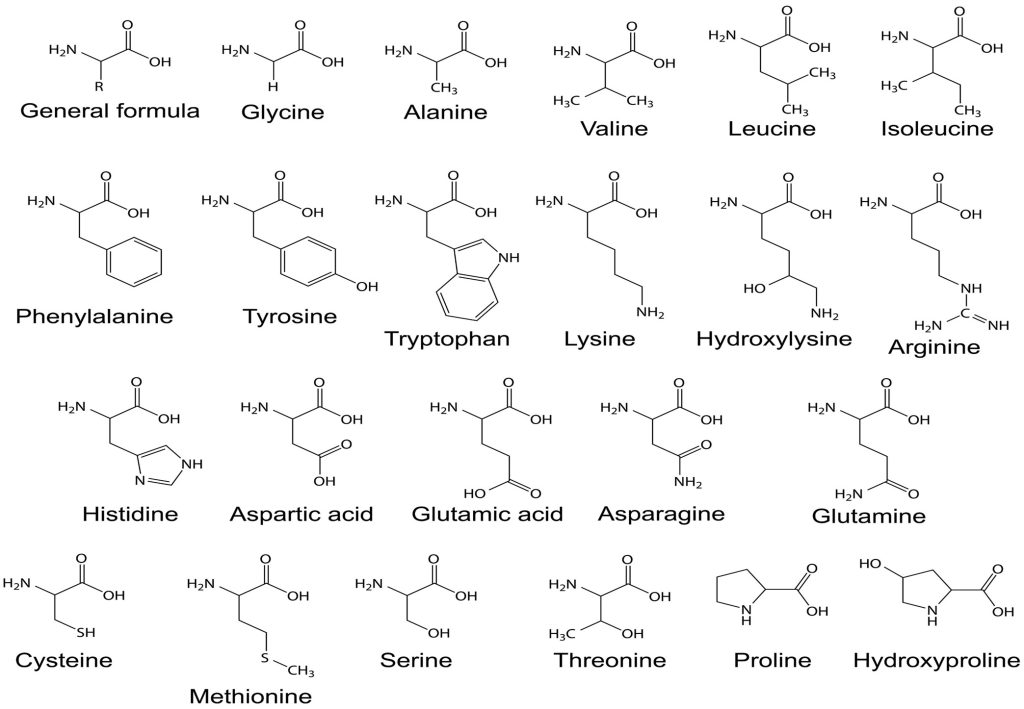
Fig 5. The structural formulas of 22 amino acids where their various R-groups (sidechains) determine protein folds. Some are essential because they cannot be synthesised by the body and must be ingested. A molecule of water is liberated when the hydroxyl (-OH) of an amino acid is bonded to the following amino group (-NH2), which is known as a condensation reaction, whereas proteases and peptidases cleave by adding water (hydrolysis).
Hydrolytic proteases digest proteins into peptides while peptidases cleave them into their constituent amino acids, where abridged proteins (oligopeptides) impact gut biota as well as epithelial function (Swiatecka et al. 2012). Such oligomers are exploited by several bacteria which nutrify a subpopulation whereas many retain antimicrobial competencies while some prove deleterious to growth (Sila et al. 2014, cited in Edgerton et al. 2018; Delcroix et al. 2015). Short peptides and amino acids play important roles in defence because the latter may be crucial for immune signalling (Kiron 2012, cited in Edgerton et al. 2018). Arginine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, and valine are essential amnio acids that must be ingested because they cannot be synthesised by fish (Fig 5.; Roberts 1989). Nevertheless, insufficient evidence to predict outcomes makes protein supplementation or substitution likely injurious (Delcroix et al. 2015).
The ratio of aerobic to anaerobic gut prokaryotes remained stable when goldfish/carp hybrids were fed 30 percent soybean meal instead of fishmeal, while reductions in the ratio of Proteobacteria to Firmicutes occurred in rainbow trout. 20 percent soybean meal fed to Atlantic salmon increased Lactococcus lactis subspecies lactis in the midgut and reduced hindgut Weissella confusa, whereas microbial richness increased significantly in salmon fed a control diet with merely 10 percent soybean meal, while bacterial profusion was somewhat normalised by the commercial prebiotic Prebiosal® (Fig 4.; Ringø et al. 2016).
Fats (lipids) from which finfish energy is easier to derive (Roberts 1989) are stored as triglycerides (Moyle 2000). They elevate gut microfloral diversity (Lesel et al. 1989, cited in Edgerton et al. 2018) while the marine polyunsaturated fatty acids (PUFAs) such as arachidonic, eicosapentaenoic, and docosahexaenoic (Edgerton et al. 2018) with multiple carbon-to-carbon double covalent bonds (C=C) constitute ~10 to 20 percent of fish anatomy including cell membranes which offer greater fluidity at low temperatures (Roberts 1989). However they are susceptible to autooxidation which demands proprietary feeds exploit antioxidants like vitamin E (Noga 2010) whereas the fat content of fish with diminished reserves is replaced by water (Roberts 1989; Moyle 2000). 10 to 20 percent dietary lipid optimises protein assimilation because it is “spared” for growth rather than energy; however surplus triggers fatty liver disease and mortality in Salmonidae. Substitutions with plant oils such as linseed, soybean, and sunflower conferred resistance to several pathogenic bacteria (Ringø et al. 2002; Ringø et al. 2016) while PUFA-synthesising prokaryotes are ubiquitous in saltwater which include Shewanella and Vibrio species isolated from fish and invertebrates (Monroig et al. 2013). 1 percent vegetable PUFAs enhance feed conversion while fish vitamin and mineral requirements remain analogous to those of humans (Roberts 1989). Various plant oils provoke hindgut microbiome reshuffling while their impact remains unclear. 7 percent linoleic acid shows merit as a prebiotic because it stimulates the growth of the LAB Carnobacterium piscicola which moderates the pathogen Aeromonas salmonicida subspecies salmonicida (Ringø et al. 2016).
Dietary modifications are more likely to cause harm thanks to natural selection, and objective studies evaluate a range of homeostatic physiological parameters which cannot be visually appraised. Walnut, peanut, and olive oils contain omega-3 and -6, and mere traces applied to feeds may exert significant impacts but do not dose water directly and remain vigilant for protein skimmer foam flattening which suggests perilous overuse. Long-term administration is necessary to influence alimentary health.
Probiotics are beneficial microorganisms that confer wellbeing when fed above therapeutic thresholds (106 to 107 CFU g-1), which were initially used in mariculture as surrogates for antibiotics (Carnevali et al. 2017; Amillano-Cisneros et al. 2023). They are thus the present-day remedy for several diseases where gram positive and negative bacteria, yeasts, and bacteriophages have proven efficacious (Akhter et al. 2015, cited in Edgerton et al. 2018). LAB including Carnobacterium, Lactobacillus, and Lactococcus species and the genera Aeromonas, Bacillus, Clostridium, Enterobacter, Enterococcus, Leuconostoc, Pseudomonas, Saccharomyces, Shewanella, and Vibrio were the most frequently used amongst the 60 or so studies investigating finfish manipulations (Merrifield & Carnevali 2014, cited in Carnevali et al. 2017; Edgerton et al. 2018). Bifidobacterium and Lactobacillus species manufacture energetic fatty acid oligomers like acetic, butyric, and propionic that beneficially supress GI pH (Amillano-Cisneros et al. 2023). Many probiotic investigations have observed enhanced anabolism and immunity with expansions of microbial richness and diversity (Lobo et al. 2014; Cordero et al. 2015; Carnevali et al. 2017) where environmental Bacillus antagonise the pathogen Aeromonas hydrophila, increase survival, and diminish ammonia, nitrate, and phosphate (Lalloo et al. 2007; Carnevali et al. 2017). Advantageous microbes offer digestion-augmenting amylases, lipases, and proteases as well as minerals, co-factors, vitamins, and amino and fatty acids, while they condition gut epithelium, raise mucus-secreting goblet cells, modulate cytokines, and prompt leucocyte differentiation and heterogeneity in gut-associated lymphoid tissue (GALT; Fig 2.; Fig 3.; Carnevali et al. 2017; Amillano-Cisneros et al. 2023). However, leaching prior to ingestion and a loss of viability during shipment and storage remain central challenges (Edgerton et al. 2018) while Carnevali and colleagues reviewed the use of probiotics in ornamental aquaculture where they increased procreative competence (fecundity; Carnevali et al. 2017).
Several human probiotics comprise LAB including strains of Lactococcus that are available as capsule-enveloped granules; however our alimentary canals differ from those of fish, many aquaculture studies are performed in cold water, and bacteria are temperature-dependent. Notwithstanding tropical temperatures are more akin to our own, albeit studies examine strains that are isolated from fish (Carnevali et al. 2017).
Dietary prebiotics stimulate the growth of beneficial microbes because they cannot be absorbed or digested by fish enzymes; several of which are fruit and grain derivatives (Amillano-Cisneros et al. 2023). They increase performance in all areas including immunity and present none of the challenges associated with live microorganisms. Various studies have appraised arabinoxylo-oligosaccharides, β-glucans, fructo-oligosaccharides, galacto-oligosaccharides, inulin, isomalto-oligosaccharides, lacto-sucrose, lactulose, lactylol, mannan-oligosaccharides, oligofructose, stachyose, transgalacto-oligosaccharides, and xylo-oligosaccharides (Torrecillas et al. 2014; Ringø et al. 2016; Amillano-Cisneros et al. 2023). Several exhibit synergy when fed with probiotics as synbiotic blends (Cerezuela et al. 2011) while an early study suggested probiotics are active in the digestive midgut whereas prebiotics influence hindgut biota (Fig 6.; Gibson & Roberfroid 1995, cited in Edgerton et al. 2018).
Pre- and pro-biotics are approved for finfish culture in Argentina, Brazil, the European Union, Japan, and the United States of America, which are not equivalents insofar as probiotic densities must be applied within the therapeutic range and handling and implementation protocols must sustain their viability, whereas prebiotics must be significant feed ingredients ranging from 0.2 to 2.5 percent (Amillano-Cisneros et al. 2023).
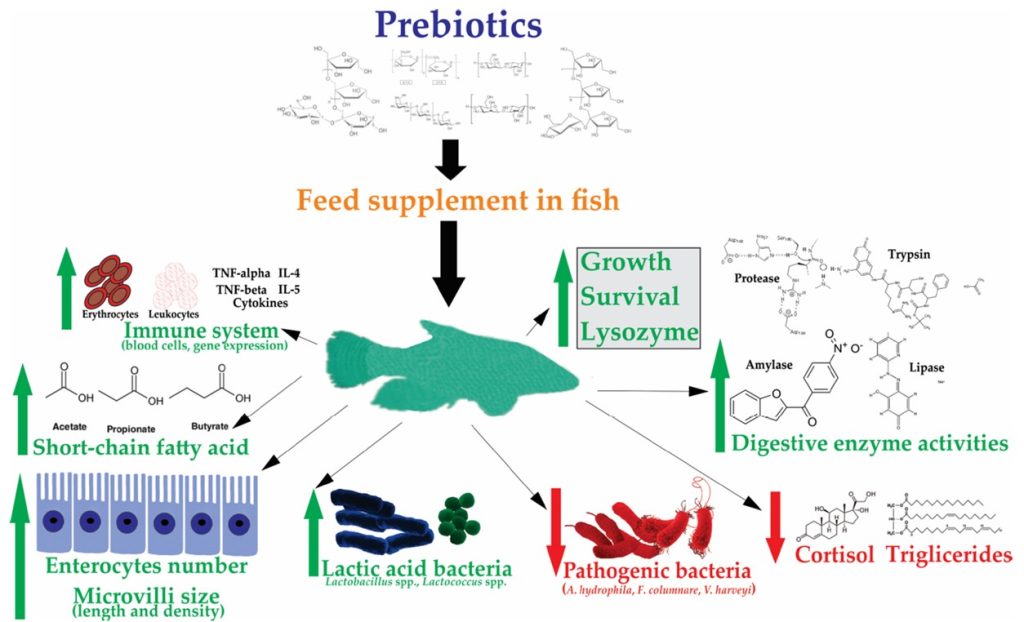
Fig 6. Prebiotic dynamics courtesy of Amillano-Cisneros et al. 2023 and the Creative Commons Attribution License (CC BY) https://creativecommons.org/licenses/by/4.0/
Prebiotics have instigated sporadic reductions in overall performance, microvilli erosion/degradation, and enteritis, while inulin is the most investigated yet it may harm enterocytes while its incomplete hydrolysis yields more advantageous oligofructose (Fig 3.; Ringø et al. 2016; Amillano-Cisneros et al. 2023). Galacto-oligosaccharides are the next most widely studied, whereas fructo-oligosaccharides appear to benefit Tilapia species and white leg shrimp (Ringø et al. 2016; Amillano-Cisneros et al. 2023). Research affirms mannan-oligosaccharides optimise digestive efficacy by bolstering villi length and integrity, and they interfere with the lectin-mediated binding of predominantly gram negative pathogens to the glycoconjugate receptors of the gut mucosae, while they support the biosynthesis of bacteriocins and pH- and pathogen-diminishing acetate and lactate (Ringø et al. 2016). β-glucans and mannan-oligosaccharides are the immunostimulant pathogen-suppressive constituents of brewer’s yeast that enhance growth, survival, and microvilli density. Arabinoxylan-oligosaccharides, gluco-oligosaccharides, and xylo-oligosaccharides extend villi, elevate immunity and endurance, accelerate maturity, and upregulate host lipase and amylase activities. Nevertheless a universally advantageous fish prebiotic is unlikely to exist due to the digestive anatomies and strategies employed by 25,000 distinct teleostean species (Amillano-Cisneros et al. 2023).
Chitosan from fungal cell walls acts as a potent prebiotic and biocidal antioxidant, while chitin from annelid worms, fish scales, and the exoskeletons of insects, arthropods, and crustaceans warrant further investigation. Derivatives of the algal genera Ascophyllum, Fucus, Laminaria, Palmaria, Sargassum, and Undaria like alginate, laminarian, fucoidan, and xylan confer all conventional prebiotic gains which may act synergistically (Fig 6.; Amillano-Cisneros et al. 2023).
Prebiotics appear the most accessible to aquarists and there are few risks if conventional feeds are supplemented with ~1 percent derivatives of chitin or the refractory insoluble particulates of deceased food-grade plants, yeasts or fungi, where gelatine food binders (Bartelme 2004) may prove useful which are proteins that may exert deleterious effects. Nevertheless, long-term administration is necessary and inadequate doses have on occasion proven injurious (Amillano-Cisneros et al. 2023) and they must be used exclusively in fish-only systems exploiting merely dedicated aerobic filtration lacking deep substrate, sump bricks, or microporous rock. Brewer’s yeast is a human immunostimulant that may be fed indefinitely to omni- and herbi-vores without waning defence. It contains dietary precursors to the essential co-factors and cell energy-supplying organic molecules NADH/NADPH (Oliva-Teles & Goncalves 2001; Li & Gatlin 2003; Elhassan et al. 2017; Orlandi et al. 2020; Moon 2021). Profoundly immunogenic brewer’s yeast quashes pathogens and amplifies beneficial microbes (Ringø et al. 2016).
Prebiotics may cause harm in reefs because they are potent sources of organic carbon, where soluble organics feed microaerobic zones which makes them anoxic while they stimulate blooms of oxygen respiring heterotrophs. Nevertheless, we can opt for those that are insoluble and particulate. Carbon dosing kills SPS hermatypes (Kline et al. 2006) and augmenting anaerobia and diminishing dissolved oxygen (DO) conceivably leads to the surfacing of putrefying anoxic regions that liberate waterborne deadly hydrogen sulphide gas (H2S). Small quantities have significant impacts where the most robust of life support systems have collapsed.
Slime mats of cyanobacteria alert aquarists to diminished DO insofar as oxygen is inhibitory to the enzyme nitrogenase that cleaves the nitrogen-nitrogen triple bond (N≡N) and assimilates ammonium (NH4+; diazotrophy). So cyanobacteria find it hard to subsist in reefs optimised for degas/regas with plentiful DO (~8 mg l-1; Fig 7.; Wilson, personal communication; Aslett 2024).

Fig 7. Maroon slime algae (cyanobacteria). Image courtesy of Arvind ©, a Reef2Reef.com forum contributor.
Ensure prebiotics destined for reefs are water insoluble by stirring a quarter of a teaspoon in 250 millilitres of reverse osmosis water, draining through a coffee filter, and drying. Proteins are denatured by heat and desiccation restores stability whereas prebiotics are generally heat-stable complex carbohydrates (Amillano-Cisneros et al. 2023).
From their conspicuous exterior surfaces to their alimentary canals, animal epidermal integuments protect their viscera from biotic incursions, where mutualist and commensal microorganisms help maintain an operational barrier, while they provide useful secondary metabolites including vitamins and bactericides which assist in pathogen suppression. Moreover teleostean surfaces are awash with immune system-derived antibodies, complement, lysozyme, reactive oxygen species (ROS), and phagocytic and killing “white” leucocytes that may freely permeate all mucus-secreting surfaces including those of the gill. Furthermore gill-associated lymphoid tissue (GIALT) enhances brachial immunological status (Ringø et al. 2016; Yu et al. 2020).
A Modern Perspective
The community compositions of each microbiome are thought to be intimately related to the biochemical profile of the mucus, where only microbes that gain nourishment can survive. They are rapid-growing heterotrophs which assimilate mucosal organic carbon yet can shunt electrons between inorganic molecules to generate energy. Furthermore each consortium is functionally redundant insofar as its metabolome is sustained by numerous affiliates where their roles endure despite the loss of several taxa.
Their brief doubling and raised mutation ensure their communities retain rapid adaptation competencies which tailors them to the prevailing conditions. Such metagenomic plasticity confers an inertia which safeguards against environmental change.
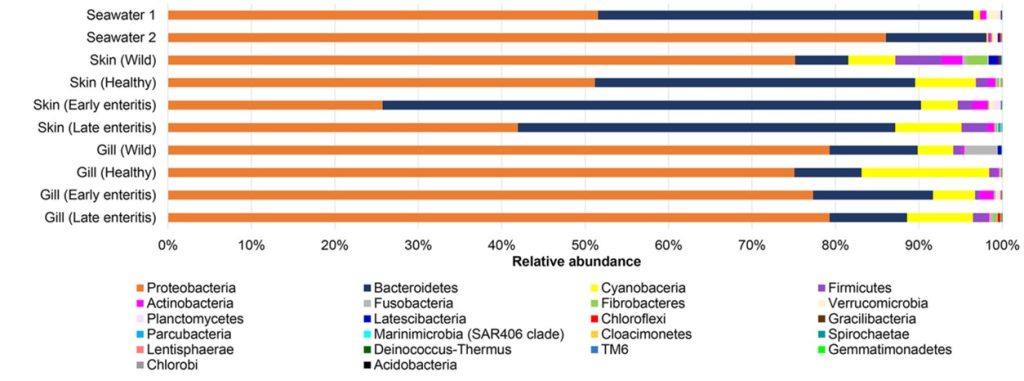
Fig 8. Comparative phylogenetic analyses of the microbial profiles of seawater, skin, and gills of healthy and diseased yellowtail kingfish (Seriola lalandi). Image courtesy of Legrand et al. 2018 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
Abiotic or biotic perturbations that exceed the “buffering” capacity of the consortium demand microbial purging known as dysbiosis. The host therefore modulates its mucosal microbiomes by flooding them with antibacterials, antibodies, and ROS while preserving a microbe-specific biochemical profile. Fish initiate sloughing of a cross-section of their microbiota with a view to recruiting environmental microbes which confer protection and metabolic function in the face of anomalous conditions.
The surrounding seawater and the microbial consortia of the skin and gill of healthy wild and cultured yellowtail kingfish (Seriola lalandi) and those suffering from early- to late-stage enteritis were studied (Legrand et al. 2018). 377 of the 930 operational taxonomic units (OTUs) were only found on the skin and gills while 77 were unique to seawater. 20, 61, and 395 were common to skin and water, gills and water, and all three respectively. The prokaryotic community compositions of wild, cultured, early enteritis, healthy, and late enteritis groups were distinct yet the microbiomes of the latter two were comparable (Legrand et al. 2018).
22 phyla, 50 classes, 109 orders, 189 families, and 380 genera were discovered where Bacteroidetes and Proteobacteria constituted over 80 percent (Fig 8.). 14 prevailing OTUs appeared in over 50 percent of reads which comprised mostly unculturable or unclassified phylotypes (Table 1.).
The microbiomes of healthy gills were monopolised by the phyla β-proteobacteria SC-I-84 and Cyanobacteria of the order Chloroplast, while the former mostly comprised the genera Alcaligenes, Candidatus Glomeribacter, and Ferrovum of the order Neisseriales. However six Nitrosomonas species were more abundant in gills which are associated with ammonia oxidation (Fig 9.). The ratio of the phyla Proteobacteria to Bacteroidetes in healthy gills was 10 but it was two in the skin of undiseased farmed fish, albeit wild fish had ratios of 14 and four (Legrand et al. 2018). The mean microbial richness was higher in healthy gills (485 OTUs) compared to skin (453), yet fewer phylotypes dominated the gills compared to skin communities. Elevated diversity was stable in healthy skin and gills where fewer lineages prevailed which was reflected in the higher incidence of β-proteobacteria (Legrand et al. 2018).
The gill microbiomes were perturbed by the onset of enteritis which initially caused more significant community shifts that stabilised and reverted in late pathology (Legrand et al. 2018) which may epitomise the efficacy of initial dysbiosis followed by selective recruitment. 666 OTUs were shared across all states from healthy to chronic enteritis from the 817 detected in farmed fish gills, where the profusions of 50 percent (409) remained stable. 10, 33, and 32 OTUs of the variable phylotypes (408) appeared unique to healthy, early to mid, and late enteritis fish respectively. The gill microbiomes of healthy and late diseased fish were more alike than other groups excluding Alteromonas, Arcobacter, Desulfobacter and an unclassified species of the order Rhodobacterales (Fig 9.; Legrand et al. 2018).
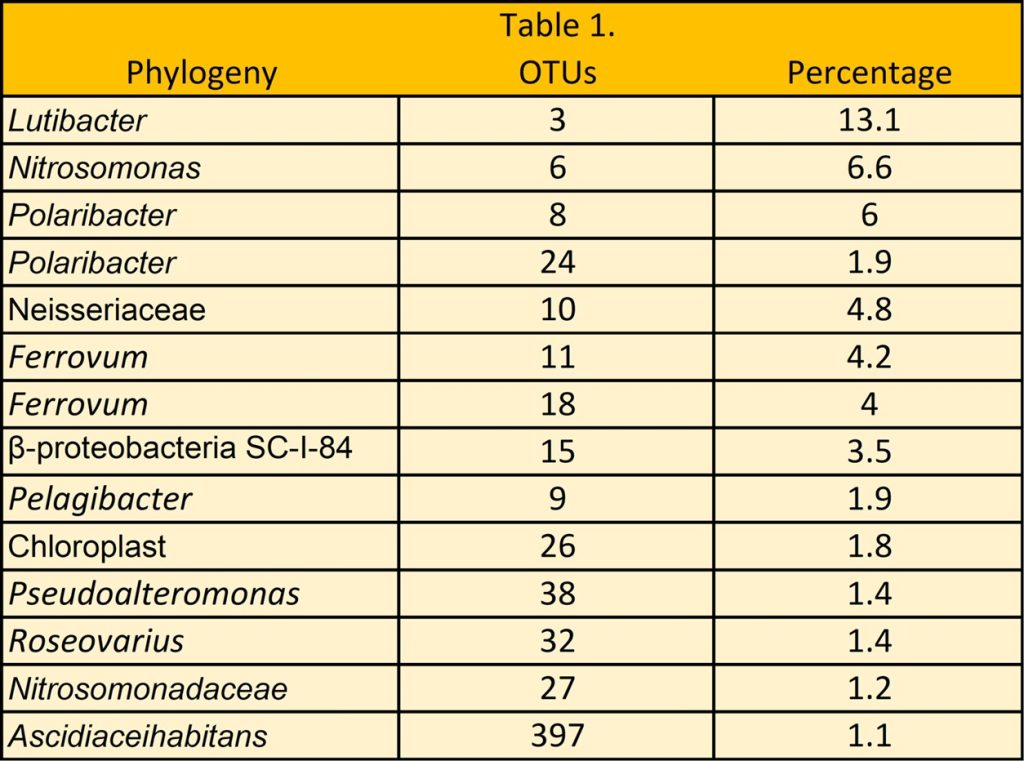
Table 1. Adapted from Legrand et al. 2018.
The gill consortium of healthy kingfish comprised the α-proteobacteria Ascidiaceihabitans, Roseovarius, and unclassified species of the order Rhodobacterales; the β-proteobacteria Candidatus Glomeribacter and Polynucleobacter species; the γ-proteobacteria Alteromonas, Cobetia, Glaciecola, Halomonas, Idiomarina, Marinobacter, Photobacterium, Pseudoalteromonas, and Psychrobacter species; unclassified Bacteroidetes of the order Flavobacteriales; species of Cyanobacteria of the order Chloroplast; and the Firmicutes genus Salimicrobium. Although α-proteobacteria of the genera Loktanella, Marivita, Pelagibacter, Planktomarina; the β-proteobacteria Simplicispira species; unclassified γ-proteobacteria of the orders Cellvibrionales and Oceanospirillales as well as Litoricola and Pseudospirillum species; Bacteroidetes of the marine group NS5 and unclassified species of the order Sphingobacteriales; Cyanobacteria of the order Chloroplast; the Actinobacteria genera Candidatus Aquiluna and Microcella, and unclassified Planctomycetes dominated the gill communities of fish with onset enteritis (Fig 9.; Legrand et al. 2018).
Mean species richness was highest in the gills of chronically diseased fish (514 OTUs) which was comparatively diminished in healthy (485) and the early enteritis group (435). The taxonomic diversity and the Proteobacteria to Bacteroidetes ratios were elevated in the gills of healthy and late-stage enteritis fish compared to those with initial pathology. The gill communities of the kingfish in the former categories exhibited unevenness and a rise in a select number of species compared with the consistency and marginally less diverse populations of the latter (Legrand et al. 2018).
We may assume a holistic perspective to marine husbandry with a comprehensive perception of the intricate microscopic relationships that underpin our captive reefs (Aslett 2024). It has taken 40 years to embrace the “One World – One Health” paradigm which proposes our mere survival and wellbeing are related to our environment, where corals have been described as coalmine canaries alerting us to climate change (Michael et al. 2021). This cutting-edge discipline is advancing at breakneck speed, where seven years should be ample to develop ergonomic and efficacious domestic pre- and pro-biotic manipulations for reef fish and invertebrates.
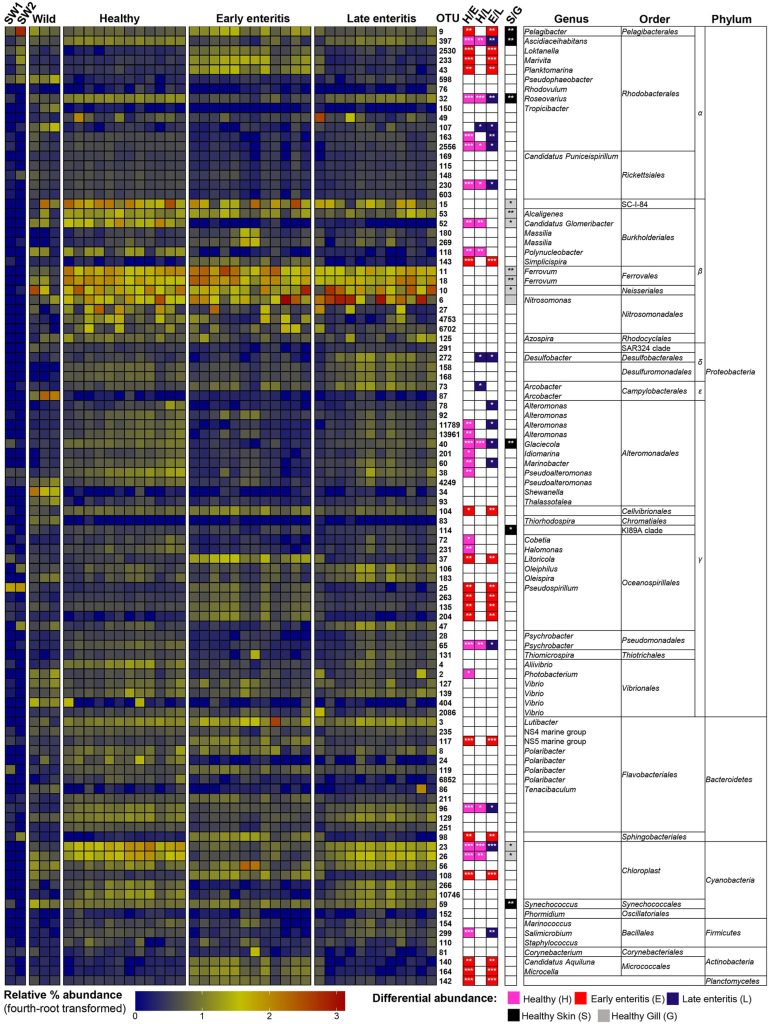
Fig 9. Heatmap analyses of the microbial operational taxonomic units (OTUs; ASVs; phylotypes) found in seawater (SW1/SW2), the gill mucosae of healthy wild and cultured yellowtail kingfish (Seriola lalandi), and throughout the onset and progression of enteritis. Heatmap and extensive analyses courtesy of Legrand et al. 2018 and the Creative Commons Attribution Licence (CC BY). http://creativecommons.org/licenses/by/4.0/
References
Adams, J. (2018) Interesting Ecology Shift of Blacktail and Orangeface Butterflyfish. ReefBuilders.com. https://reefbuilders.com/2018/12/18/blacktail-and-redface-butterflyfish/
Akhter, N., Wu, B., Memon, A., M. & Mohsin, M. (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish Shellfish Immunol. 45, 733-741. https://www.doi.org/10.1016/j.fsi.2015.05.038
Amann, R., I., Ludwig, W. & Schleifer, K., H. (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59, 143-169.
Amillano-Cisneros, J., M., Fuentes-Valencia, A., M., Leyva-Morales, J., B., Davizón, Y., A., Marquéz-Pacheco, H., Valencia-Castañeda, G., Maldonado-Coyac, J., A., Ontiveros-García,L., A. & Badilla-Medina, C., N. (2023) Prebiotics in Global and Mexican Fish Aquaculture: A Review. Animals. 13, 3607. https://doi.org/10.3390/ani13233607
Andreoni, F. & Magnani, M. (2014) Photobacteriosis: Prevention and Diagnosis. Journal of immunology research. 2014,.
Aslett, C., G. (2023a) Coral Immunity Part I. https://www.reefranch.co.uk/
Aslett, C., G. (2023b) Holosystemics Part IV: Dysbiosis and the Microscopic Coral Alliance. https://www.reefranch.co.uk/
Aslett, C., G. (2023c) Fish Immunostimulation. https://www.reefranch.co.uk/
Aslett, C., G. (2024) The Complete Reef Aquarist, for hobbyists, the trade and academics – A Conservation Manual. Reef Ranch Publishing Ltd, Leeds, West Yorkshire, UK. https://www.reefranch.co.uk
Austin, B. & Austin, D., A. (2016) Bacterial fish pathogens, 6th ed. Springer, Stirling, UK
Austin, B. (1982) Taxonomy of bacteria isolated from a coastal, marine fish-rearing unit. J. Appl. Bacteriol. 53, 253-268. https://www.doi.org/10.1111/j.1365-2672.1982.tb04684.x
Bartelme, T., D. (2004) Aquarium Fish: News from the Warfront with Cryptocaryon irritans: Part Four of Five. Advanced Aquarist.com. https://www.advancedaquarist.com/2004/3/mini
Bass, D., Stentiford. G., D., Wang, H-C., Koskella, B. & Tyler, C., R. (2019) The pathobiome in animal and plant diseases. Trends Ecol Evol. 34(11), 996-1008.
Bell, G., R., Hoskins, G., E. & Hodgkiss, W. (1971) Aspects of the characterization, identification, and ecology of the bacterial flora associated with the surface of stream-incubating Pacific salmon (Oncorhynchus) eggs. J. Fish. Board Can. 28, 1511-1525. https://www.doi.org/10.1139/f71-232
Birrell, C., Mccook, L., Willis, B. & Diaz-Pulido, G. (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanography and marine biology. 46,.
Blazer, V., Phillips, S. & Pendleton, E. (2016) Fish Health, Fungal Infections, and Pfiesteria: The Role of the U.S. Geological Survey. U.S. Geological Survey Fact Sheet. pp 114-198.
Boilard, A., Dubé, C., E., Gruet, C., Mercière, A., Hernandez-Agreda, A. & Derome, N. (2020) Defining Coral Bleaching as a Microbial Dysbiosis within the Coral Holobiont. Microorganisms. 8, 1682. https://doi.org/10.3390/microorganisms8111682
Bone, Q., Marshall, N., B. & Blaxter, J., H., S. (1995) Biology of Fishes. Glasgow: Blackie Academic & Professional. https://www.doi.org/10.1007/978-1-4615-2664-3
Bozzi, D., Rasmussen, J., A., Carøe, C., et al. (2021) Salmon gut microbiota correlates with disease infection status: potential for monitoring health in farmed animals. Anim Microbiome. 3(1), 1-17.
Brandley, B., K. & Schnaar, R., L. (1986) Cell-Surface Carbohydrates in Cell Recognition and Response. J Leukoc Biol 40, 97–111. https://doi.org/10.1002/jlb.40.1.97
Brown, R., Moore, L., Mani, A., Patel, S., Salinas, I. (2021) Effects of ploidy and salmonid alphavirus infection on the skin and gill microbiome of Atlantic salmon (Salmo salar). PLoS One. 16(2), e0243684.
Cámara-Ruiz, M., Cerezo, I., M., Guardiola, F., A. et al. (2021) Alteration of the immune response and the microbiota of the skin during a natural infection by Vibrio harveyi in European seabass (Dicentrarchus labrax). Microorganisms. 9(5), 964.
Carnevali, O., Maradonna, F. & Gioacchini, G. (2017) Integrated control of fish metabolism, wellbeing and reproduction: the role of probiotic. Aquaculture 472, 144-155. https://www.doi.org/10.1016/j.aquaculture.2016.03.037
Cerezuela, R., Meseguer, J. & Esteban, M. (2011) Current knowledge in synbiotic use for fish aquaculture: a review. J. Aquac. Res. Dev. 1, 1-7.
Cherrak, Y., Flaugnatti, N., Durand, E., Journet, L. & Cascales, E. (2019) Structure and Activity of the Type VI Secretion System. Microbiology spectrum, 7(4), 1.
Colorni, A., Avtalion, R., Knibb, W., Berger, E., J., Colorni, B. & Timan, B. (1998) Histopathology of sea bass (Dicentrarchus labrax) experimentally infected with Mycobacterium marinum and treated with streptomycin and garlic (Allium sativum) extract. Aquaculture. 160, 1-17.
Cordero, H., Guardiola, F., A., Tapia-Paniagua, S., T., Cuesta, A., Meseguer, J., Balebona, M., C., et al. (2015) Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 45, 608-618. https://www.doi.org/10.1016/j.fsi.2015.05.010
Das, P., Mandal, S., Khan, A., Manna, S., K. & Ghosh, K. (2014) Distribution of extracellular enzyme-producing bacteria in the digestive tracts of 4 brackish water fish species. Turk. J. Zool. 38, 79-88. https://www.doi.org/10.3906/zoo-1205-3
Delcroix, J., Gatesoupe, F., J., Desbruyères, E., Huelvan, C., Le Delliou, H., Le Gall, M., M., Quazuguel, P., et al. (2015) The effects of dietary marine protein hydrolysates on the development of sea bass larvae, Dicentrarchus labrax, and associated microbiota. Aquac. Nutr. 21, 98-104. https://www.doi.org/10.1111/anu.12139
Duperron, S., Halary, S., Habiballah, M., Gallet, A., Huet, H., Duval, C. et al. (2019) Response of fish gut microbiota to toxin-containing cyanobacterial extracts: a microcosm study on the Medaka (Oryzias latipes). Environ SciTechnol Lett. 6, 341–7.
Egerton, S., Culloty, S., Whooley, J., Stanton, C. & Ross, R., P. (2018) The Gut Microbiota of Marine Fish. Frontiers in Microbiology. 9(873),. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2018.00873/full
Elhassan, Y., Philp, A. & Lavery, G. (2017) Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule. Journal of the Endocrine Society. 1(7), 816-835.
Estruch, G., Collado, M., Peñaranda, D., Vidal, A., T., Cerdá, M., J., Martínez, G., P., et al. (2015) Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA gene. PLoS One 10, e0136389. https://www.doi.org/10.1371/journal.pone.0136389
Flerova, E. & Balabanova, L. (2013) Ultrastructure of granulocytes of teleost fish (Salmoniformes, Cypriniformes, Perciformes). Journal of Evolutionary Biochemistry and Physiology. 49(2), 223-233.
Fournier, V., Huelvan, C. & Desbruyeres, E. (2004) Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psetta maxima). Aquaculture 236, 451-465. https://www.doi.org/10.1016/j.aquaculture.2004. 01.035
Gallet, A., Halary, S., Duval, C., Huet, H., Duperron, S. & Marie, B. (2023) Disruption of fish gut microbiota composition and holobiont’s metabolome during a simulated Microcystis aeruginosa (Cyanobacteria) bloom. Microbiome. 11, 108. https://doi.org/10.1186/s40168-023-01558-2
Ghanbari, M., Kneifel, W. & Domig, K., J. (2015) A new view of the fish gut microbiome: advances from next-generation sequencing. Aquaculture 448, 464-475. https://www.doi.org/10.1016/j.aquaculture.2015.06.033
Giatsis, C., Sipkema, D., Smidt, H. et al. (2015) The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci Rep. 5(1), 1-15.
Green, T., J., Smullen, R. & Barnes, A., C. (2013) Dietary soybean protein concentrate-induced intestinal disorder in marine farmed Atlantic salmon, Salmo salar is associated with alterations in gut microbiota. Vet. Microbiol. 166, 286-292. https://www.doi.org/10.1016/j.vetmic.2013.05.009
Hansen, G. & Olafsen, J. (1999) Bacterial interactions in early life stages of marine cold water fish. Microb. Ecol. 38, 1-26. https://www.doi.org/10.1007/s002489900158
Hlongwane, P., Mungra, N., Madheswaran, S., Akinrinmade, O., A., Chetty, S. & Barth, S. (2018) Human Granzyme B Based Targeted Cytolytic Fusion Proteins. Biomedicines. 6(2), 72.
Ho, J. & Kim, I. (2001) New species of Hatschekia Poche, 1902 (Copepoda: Hatschekiidae) parasitic on marine fishes of Kuwait. Syst Parasitol. 49, 73-79.
Hooper, L., V. & Gordon, J., I. (2001) Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology 11, 1R-10R. https://doi.org/10.1093/glycob/11.2.1R
Hovanec, T. (2019) Dr. How to harness bacteria to cycle your saltwater tank quickly! | MACNA 2019. BrsTV. https://www.youtube.com/watch?v=zDI7sxqC-ss
James, A., G. (1988) Are clupeid microphagists herbivorous or omnivorous? A review of the diets of some commercially important clupeids. S. Afr. J. Mar. Sci. 7, 161-177. https://www.doi.org/10.2989/025776188784379017
Kapoor, B. & Khawna, B. (1993) The potential spectrum of the gut in teleost fishes. Adv. Fish Res. 1, 221-226.
Karachle, P., K. & Stergiou, K., I. (2010) Gut length for several marine fish: relationships with body length and trophic implications. Mar. Biodivers. Rec. 3, e106. https:///www.doi.org/10.1017/S1755267210000904
Kelly, C. & Salinas, I. (2017) Under pressure: Interactions between commensal microbiota and the teleost immune system. Front. Immunol. 8, 1.
Kim, B.,-R., Shin. J., Guevarra, R., B., et al. (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 27(12), 2089-2093.
Kim, J. & Lee, J., L. (2017) Correlation of Total Bacterial and Vibrio spp. Populations between Fish and Water in the Aquaculture System. Frontiers in Marine Science. 4,.
Kiron, V. (2012) Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 173, 111-133. https://www.doi.org/10.1016/j.anifeedsci.2011.12.015
Kline, D., I., Kuntz, N., M., Breitbart, M., Knowlton, N. & Rohwer, F. (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Marine Ecology Progress Series. 314, 119-125.
Lalloo, R., Ramchuran, S., Ramduth, D., Gorgens, J., Gardiner, N., (2007) Isolation and selection of Bacillus spp. as potential biological agents for enhancement of water quality in culture of ornamental fish. J. Appl. Microbiol. 103, 1471-1479.
Langille, M., G., Zaneveld, J., Caporaso, J., G. et al. (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 31(9), 814-821.
Larsen, A., M. (2014) Studies on the Microbiota of Fishes and the Factors Influencing Their Composition. Auburn, AL: Auburn University.
Lasica, A., Ksiazek, M., Madej, M. & Potempa, J. (2017) The Type IX Secretion System (T9SS): Highlights and Recent Insights into Its Structure and Function. Frontiers in Cellular and Infection Microbiology. 7,.
Legrand, T., P., R., A., Catalano, S., R., Wos-Oxley, M., L., Stephens, F., Landos, M., Bansemer, M., S., Stone, D., A., J., Qin, J., G. & Oxley, A., P., A. (2018) The Inner Workings of the Outer Surface: Skin and Gill Microbiota as Indicators of Changing Gut Health in Yellowtail Kingfish. Front. Microbiol. 8:2664. https://www.doi.org/10.3389/fmicb.2017.02664
Lesel, R., De La Noüe, J. & Choubert, G. (1989) Fecal bacterial flora of rainbow trout under antibiotic treatment: effect of the number of pyloric caeca and the lipid content of food. Aquaculture: A Biotechnology in Progress, Vol. 1. De Pauw, N., Jaspers, E., Ackefors, H. & Wilkins, N. (eds.). Bredene: European Aquaculture Society, 592.
Li, P. & Gatlin, D., M. (2003) Evaluation of brewers yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysopsX—M. saxatilis). Aquaculture. 219(1), 681-692.
Li, T., Li, H., Gatesoupe, F-J. et al. (2017) Bacterial signatures of ‘red-operculum’ disease in the gut of crucian carp (Carassius auratus). Microb Ecol. 74(3), 510-521.
Liu, Q., Lai, Z., Gao, Y. et al. (2021) Connection between the gut microbiota of largemouth bass (Micropterus salmoides) and microbiota of the pond culture environment. Microorganisms. 9(8), 1770.
Llewellyn, M., Leadbeater, S., Garcia, C. et al. (2017) Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci Rep. 7(1), 1-10.
Llewellyn, M., S., Boutin, S., Hoseinifar, S., H. & Derome, N. (2018) Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front Microbiol. 2014, 5.
http://journal.frontiersin.org/article/10.3389/fmicb.2014.00207/abstract
Lobo, C., Moreno-Ventas, X., Tapia-Paniagua, S., Rodríguez, C., Moriñigo, M., A. & de La Banda, I., G. (2014) Dietary probiotic supplementation (Shewanella putrefaciens Pdp11) modulates gut microbiota and promotes growth and condition in Senegalese sole larviculture. Fish Physiol. Biochem. 40, 295-309. https://www.doi.org/10.1007/s10695-013-9844-0
Lom, J. & Corliss, J. (1971) Morphogenesis and Cortical Ultrastructure of Brooklynella hostilis, a Dysteriid Ciliate Ectoparasitic on Marine Fishes. The Journal of Eukaryotic Microbiology. 18(2), 261-281.
López Nadal, A., Peggs, D., Wiegertjes, G., F. & Brugman, S. (2018) Exposure to Antibiotics Affects Saponin ImmersionInduced Immune Stimulation and Shift in Microbial Composition in Zebrash Larvae. Front Microbiol 9, 2588. https://doi.org/10.3389/fmicb.2018.02588
López-Dóriga, M., Barnes, A., dos Santos, N. & Ellis, A. (2000) Invasion of fish epithelial cells by Photobacterium damselae subsp. piscicida: evidence for receptor specificity, and effect of capsule and serum. Microbiology. 146(1), 21-30.
Lorgen-Ritchie, M., Clarkson, M., Chalmers, L., Taylor, J., F., Migaud, H. & Martin, S., A. M. (2021) A temporally dynamic gut microbiome in Atlantic salmon during freshwater recirculating aquaculture system (RAS) production and post-seawater transfer. Front. Mar. Sci. 8, 869. https://doi.org/10.3389/fmars.2021.711797
Lorgen-Ritchie, M., Webster, T., U., McMurtrie, J., Bass, D., Tyler, C., R., Rowley, A. & Martin, S., A., M. (2023) Microbiomes in the context of developing sustainable intensified aquaculture. Frontiers in Microbiology. 14, 1200997.
Ma, C., Chen, C., Jia, L., He, X. & Zhang, B. (2019) Comparison of the intestinal microbiota composition and function in healthy and diseased Yunlong Grouper. AMB Express. 9(1), 1-11.
Martins, J. & Vasconcelos, V. (2015) Cyanobactins from cyanobacteria: current genetic and chemical state of knowledge. Mar Drugs. 13, 6910-6946.
Mashoof, S. & Criscitiello, M., F. (2016) Fish Immunoglobulins. Biology. 5(4), 45.
McBride, M., J. & Nakane, D. (2015) Flavobacterium gliding motility and the type IX secretion system. Curr. Opin. Microbiol. 28, 72-77.
McCauley, M., Goulet, T., L., Jackson, C., R. & Loesgen, S. (2022) Systematic review of cnidarian microbiomes reveals insights into the structure, specificity, and fidelity of marine associations. Nature Communications. 14, 4899. https://doi.org/10.1038/s41467-023-39876-6
Merrifield, D., L. & Carnevali, O. (2014) Probiotic modulation of the gut microbiota of fish. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics (Merrifield, D. & Ringø, E. (eds.). Wiley-Blackwell Publishing, Oxford, UK. pp. 185-222.
Merrifield, D., L. & Rodiles, A. (2015) The fish microbiome and its interactions with mucosal tissues. Mucosal Health in Aquaculture. Peatman, E. (ed.) Academic Press, San Diego, CA. pp 273-295.
Michael, S., Burian, A. & Bulling, M. (2021) Corals as canaries in the coalmine: towards the incorporation of marine ecosystems into the “One Health” concept. Journal of Invertebrate Pathology. 2021,.
Mihalitsis, M. & Bellwood, D. (2017) A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE. 12(9),.
Miyake, S., Ngugi, D. K. & Stingl, U. (2015) Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 24, 656-672. https://www.doi.org/10.1111/mec.13050
Miyake, S., Soh, M., Azman, M., N., Ngoh, S., Y., Orbán, L., Seedorf, H. (2020) Insights into the microbiome of farmed Asian sea bass (Lates cal-carifer) with symptoms of tenacibaculosis and description of Tenacibaculum singaporense sp. nov. Antonie Van Leeuwenhoek. 113(6), 737-752.
Monroig, Ó., Tocher, D., R. & Navarro, J., C. (2013) Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar. Drugs 11, 3998-4018. https://www.doi.org/10.3390/md11103998
Moon, D. (2021) Boosting NAD+ to Reverse Aging? Overview of NR and NMN. GeneticLifeHacks.com. https://www.geneticlifehacks.com/nad-reversing-aging-overview-of-nr-and-nmn/
Mouchet, M., A., Bouvier, C., Bouvier, T., Troussellier, M., Escalas, A. & Mouillot, D. (2012) Genetic difference but functional similarity among fish gut bacterial communities through molecular and biochemical fingerprints. FEMS Microbiol. Ecol. 79, 568-580. https://www.doi.org/10.1111/j.1574-6941.2011. 01241.x
Mougin, J. & Joyce, A. (2022) Reviews in Aquaculture. Fish disease prevention via microbial dysbiosis-associated biomarkers in aquaculture. 15, 579-594. https://doi.org/10.1111/raq.12745
Moyle, P., B. & Cech, J., J., Jr. (2000) An Introduction to Ichthyology. Ryu, T., Tarabokjia, L., Schiaparelli, K. & Dellas, K. (eds.). Prectice Hall Inc., Upper Saddle river, NJ, U.S. pp 103-105.
Muñoz-Baquero, M., Lorenzo-Rebenaque, L., Garc´ıa-Va´zquez, F., A., Garc´ıa-Pa´rraga, D., Mart´ınez-Priego, L., De Marco-Romero, G., Gala´n-Vendrell, I., D’Auria, G. & Marco-Jime´nez, F. (2023) Unveiling Microbiome Signature In Inner Body Fluids: Comparison Between Wild And Aquarium Small-Spotted Catshark (Scyliorhinus canicular). Frontiers in Marine Science. https://www.doi.org/10.3389/fmars.2023.1151119
Murdoch, C., C. & Rawls, J., F. (2019) Commensal microbiota regulate vertebrate innate immunity-insights from the zebrafish. Front. Immunol. 10:2100. https://doi.org/10.3389/fimmu.2019.02100
Navarrete, P., Espejo, R. T. & Romero, J. (2009) Molecular analysis of microbiota along the digestive tract of juvenile Atlantic salmon (Salmo salar L.). Microb. Ecol. 57, 550-561. https://www.doi.org/10.1007/s00248-008-9448-x
Naya-Català, F., Piazzon, M., C., Calduch-Giner, J. A., Sitjà-Bobadilla, A. & Pérez-Sánchez, J. (2022) Diet and host genetics drive the bacterial and fungal intestinal metatranscriptome of Gilthead Sea bream. Front. Microbiol. 13, 883738. https://doi.org/10.3389/fmicb.2022.883738
Nayak, S., K. (2010) Role of gastrointestinal microbiota in fish. Aquac. Res. 41, 1553-1573. https://www.doi.org/10.1111/j.1365-2109.2010.02546.x
Neuman, C., Hatje, E., Zarkasi, K. Z., Smullen, R., Bowman, J., P. & Katouli, M. (2016) The effect of diet and environmental temperature on the faecal microbiota of farmed Tasmanian Atlantic Salmon (Salmo salar L.). Aquac. Res. 47, 660-672. https://www.doi.org/10.1111/are.12522
Noga, E., J. (2010a) Fish Disease. Diagnosis and Treatment. Second Edition. Wiley-Blackwell, John Wiley & Sons Inc. p 139.
Noga, E., J. (2010) Fish Disease: Diagnosis and Treatment, Second Edition. Noga, E., J. (ed.). John Wiley & Sons Inc. p 306.
Oliva-Teles, A. & Goncalves, P. (2001) Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture. 202(3), 269-278.
Orlandi, I., Alberghina, L. & Vai, M. (2020) Nicotinamide, Nicotinamide Riboside and Nicotinic Acid-Emerging Roles in Replicative and Chronological Aging in Yeast. Biomolecules. 10(4), 604.
Ortiz-Estrada, A., M., Gollas-Galván, T., Martínez-Cordova, L., R. & Martínez-Porchas, M. (2019) Predictive functional profiles using metagenomic 16S rRNA data: a novel approach to understanding the microbial ecology of aquaculture systems. Rev Aquacult. 11(1), 234-245.
Perdiguero, P., Martin-Martin, A., Benedicenti, O., Diaz-Rosales, P., Morel, E., Munoz-Atienza, E., Garcia-Flores, M., Simon, R., Soleto, I., Cerutti, A. & Tafalla, C. (2019) Teleost IgD+Ig- B Cells Mount Clonally Expanded and Mildly Mutated Intestinal IgD Responses in the Absence of Lymphoid Follicles. Cell Reports. 29(13), 4223-4235.
Pérez-Pascual, D., Lunazzi, A., Magdelenat, G., Rouy, Z., Roulet, A., Lopez-Roques, C., Larocque, R., Barbeyron, T., Gobet, A., Michel, G., Bernardet, J. & Duchaud, E. (2017) The Complete Genome Sequence of the Fish Pathogen Tenacibaculum maritimum Provides Insights into Virulence Mechanisms. Frontiers in Microbiology. 8,.
Pollock, F.,J., McMinds, R., Smith, S., Bourne, D., G., Willis, B., L., Medina, M., Thurber, R., V. & Zaneveld, J., R. (2018) Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat Commun. 9, 4921.
Ray, A., K. & Ringø, E. (2014) The gastrointestinal tract of fish. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics. Merrifield, D. & Ringø, E. (eds.). Wiley-Blackwell Publishing, Oxford, UK. pp. 1-13.
Reid, K., M., Patel, S., Robinson, A., J., et al. (2017) Salmonid alphavirus infection causes skin dysbiosis in Atlantic salmon (Salmo salar L.) post-smolts. PLoS One. 12(3):e0172856.
Richardson, L., L. (1998) Coral diseases: what is really known? Trends in Ecology and Evolution. 13, 438-443.
Richardson, L., L., Sekar, R., Myers, J., L., Gantar, M., Voss, J., D., Kaczmarsky, L., Remily, E., R., Boyer, G., L. & Zimba, P., V. (2007) The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS Microbiology Letters. 272(2), 182-187.
Riley, M., A. & Wertz, J., E. (2002) Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie. 84, 357-364.
Ringø, E., Lødemel, J., B., Myklebust, R., Jensen, L., Lund, V., Mayhew, T., M., et al. (2002) The effects of soybean, linseed and marine oils on aerobic gut microbiota of Arctic charr Salvelinus alpinus L. before and after challenge with Aeromonas salmonicida ssp. salmonicida. Aquac. Res. 33, 591-606. https://www.doi.org/10.1046/j.1365-2109.2002.00697.x
Ringø, E., Zhou, Z., Vecino, J., L., G., Wadsworth, S., Romero, J., Krogdahl, Å., et al. (2016) Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 22, 219-282. https://www.doi.oeg/10.1111/anu.12346
Ritchie, K., B. & Smith, G., W. (1995) Preferential carbon utilization by surface bacterial communities from water mass, normal, and white-band diseased Acropora cervicornis. Mol Mar Biol Biotechnol. 4, 345-354.
Roberts, R., J. (1989) Fish Pathology 2nd Edition. Bailliere Tidal, 24-28 Oval Road, London. pp 37-40.
Rohde, K. (1932) Ciliophora (ciliates). Marine Parasitology. Rohde, K. (ed.). CSIRO Publishing, Clayton, Australia.
Rombout, J., H., Abelli, L., Picchietti, S., Scapigliati, G. & Kiron, V. (2011) Teleost intestinal immunology. Fish Shellfish Immunol. 31, 616-626. https://www.doi.org/10.1016/j.fsi. 2010.09.001
Rosado, D., Pérez-Losada, M., Pereira, A., Severino, R. & Xavier, R. (2021) Effects of aging on the skin and gill microbiota of farmed seabass and seabream. Anim Microbiome. 3(1), 1-14.
Rosado, D., Pérez-Losada, M., Severino, R. & Xavier, R. (2022) Monitoring infection and antibiotic treatment in the skin microbiota of farmed European seabass (Dicentrarchus Labrax) fingerlings. Microb Ecol. 83(3), 789-797.
Rosado, D., Perez-Losada, M., Severino, R., Cable, J. & Xavier, R. (2019b) Characterization of the skin and gill microbiomes of the farmed seabass (Dicentrarchus labrax) and seabream (Sparus aurata). Aquaculture. 500, 57-64.
Rosado, D., Xavier, R., Severino, R., Tavares, F., Cable, J. & Pérez-Losada, M. (2019a) Effects of disease, antibiotic treatment and recovery trajectory on the microbiome of farmed seabass (Dicentrarchus labrax). Sci Rep. 9(1), 1-11.
Rozas-Serri, M. (2019) Gill diseases in marine salmon aquaculture with an emphasis on amoebic gill disease. CAB Reviews Perspectives in Agriculture Veterinary Science Nutrition and Natural Resources. 14, 1-15.
Santoro, E., P., Borges, R., M., Espinoza, J., L., Freire, M., Messias, C., S., M., A., Villela, H., D., M., Pereira, L., M., Vilela, C., L., S., Rosado, J., G., Cardoso, P., M., Rosado, P., M., Assis, J., M., Duarte, G., A., S., Perna, G., Rosado, A., S., Macrae, A., Dupont, C., L., Nelson, K., E., Sweet, M., J., Voolstra, C., R. & Peixoto, R., S. (2021) Coral microbiome manipulation elicits metabolic and genetic restructuring to mitigate heat stress and evade mortality. Science advances. 7(33), 3088.
Santos, F., F., Yamamoto, D., Abe, C., M., Bryant, J., A., Hernandes, R., T., Kitamura, F., C., Castro, F., S., Valiatti, T., B., Piazza, R., Elias, W., P., Henderson, I., R. & Gomes, T. (2019) The Type III Secretion System (T3SS)-Translocon of Atypical Enteropathogenic Escherichia coli (aEPEC) Can Mediate Adherence. Frontiers in microbiology. 10, 1527.
Scapigliati, G., Fausto, A., M. & Picchietti, S. (2018) Fish Lymphocytes: An Evolutionary Equivalent of Mammalian Innate-Like Lymphocytes? Frontiers in immunology. 9, 971.
Segata, N., Izard, J., Waldron, L., et al. (2011) Metagenomic biomarker discovery and explanation. Genome Biol. 12(6), 1-18.
Shabir, U., Ali, S., Magray, A., Ganai, B., Firdous, P., Hassan, T. & Nazir, R. (2018) Fish antimicrobial peptides (AMP’s) as essential and promising molecular therapeutic agents: A review. Microbial pathogenesis. 114, 50-56.
Sila, A., Nedjar-Arroume, N., Hedhili, K., Chataigné, G., Balti, R., Nasri, M., et al. (2014) Antibacterial peptides from barbel muscle protein hydrolysates: activity against some pathogenic bacteria. LWT Food Sci. Technol. 55, 183-188. https://www.doi.org/10.1016/j.lwt.2013.07.021
Siriyappagouder, P., Galindo-Villegas, J., Dhanasiri, A., K., S., Zhang, Q., Mulero, V., Kiron, V. et al. (2020) Pseudozyma priming influences expression of genes involved in metabolic pathways and immunity in zebrafish larvae. Front. Immunol. 11, 987. https://doi.org/10.3389/fimmu.200.00978
Siriyappagouder, P., Kiron, V., Lokesh, J., Rajeish, M., Kopp, M. & Fernandes, J. (2018) The intestinal mycobiota in wild zebrafish comprises mainly Dothideomycetes while Saccharomycetes predominate in their laboratory-reared counterparts. Front. Microbiol. 9, 387. https://doi.org/10.3389/fmicb.2018.00387
Sitjà-Bobadilla, A., Gil-Solsona, R., Estensoro, I., Piazzon, M., Martos-Sitcha, J., Picard-Sánchez, A., Fuentes, J., Sancho, J., Calduch-Giner, J., Hernández, F. & Pérez-Sánchez, J. (2019) Disruption of gut integrity and permeability contributes to enteritis in a fish-parasite model: a story told from serum metabolomics. Parasites & Vectors. 12,.
Skrodenyte-ArbaCIauskiene, V. (2007) Enzymatic activity of intestinal bacteria in roach Rutilus rutilus L. Fish. Sci. 73, 964-966. https://www.doi.org/10.1111/j.1444-2906.2007. 01421.x
Slinger, J., Adams, M., B., Stratford, C., N., Rigby, M. & Wynne, J., W. (2021) The effect of antimicrobial treatment upon the gill bacteriome of Atlantic salmon (Salmo salar L.) and progression of amoebic gill disease (AGD) in vivo. Microorganisms. 9, 987. https://doi.org/10.3390/microorganisms9050987
Spanova, M. & Daum, G. (2011) Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur J Lipid Sci Technol. 113(11), 1299-1320.
Spor, A., Koren, O. & Ley, R. (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 9, 279-290. https://doi.org/10.1038/nrmicro2540
Stevens, C., E. & Hume, I., D. (2004) Comparative Physiology of the Vertebrate Digestive System. Cambridge: Cambridge University Press.
Stevens, J., Jackson, R., Olson, J. (2016) Bacteria associated with lionsh (Pterois volitans/miles complex) exhibit antibacterial activity against known fish pathogens. Mar Ecol Prog Ser 558, 167–180. https://doi.org/10.3354/meps11789
Sullam, K., E., Essinger, S., D., Lozupone, C., A., O’Connor, M., P., Rosen, G., L., Knight, R., et al. (2012) Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol. Ecol. 21, 3363-3378. https://www.doi.org/10.1111/j.1365-294X.2012.05552.x
Swiatecka, D., Markiewicz, L., H. & Wróblewska, B. (2012) Pea protein hydrolysate as a factor modulating the adhesion of bacteria to enterocytes, epithelial proliferation and cytokine secretion–an in vitro study. Cent. Eur. J. Immunol. 37, 227-231. https://www.doi.org/10.5114/ceji.2012.3079
Tapia-Paniagua, S., T., Ceballos-Francisco, D., Balebona, M., C., Esteban, M., A. & Moriñigo, M., A. (2018) Mucus glycosylation, immunity and bacterial microbiota associated to the skin of experimentally ulcered gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 75, 381-390.
Thiéry, R., Cozien, J., Cabon, J., Lamour, F., Baud, M. & Schneemann, A. (2006) Induction of a Protective Immune Response against Viral Nervous Necrosis in the European Sea Bass Dicentrarchus labrax by Using Betanodavirus Virus-Like Particles. Journal of Virology. 80(20), 10201.
Torrecillas, S., Montero, D. & Izquierdo, M. (2014) Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish Shellfish Immunol. 36, 525-544. https://doi.org/10.1016/j.fsi.2013.12.029
Tretina, K., Park, E., S., Maminska, A. & MacMicking, J., D. (2019) Interferon-induced guanylate-binding proteins: Guardians of host defense in health and disease. The Journal of Experimental Medicine. 216(3), 482-500.
Valero, Y., Saraiva‐Fraga, M., Costas, B. & Guardiola, F., A. (2019) Antimicrobial peptides from fish: beyond the fight against pathogens. Reviews in Aquaculture. 12, 224-253.
Vayssier-Taussat, M., Albina, E., Citti, C., et al. (2014) Shifting the paradigm from pathogens to pathobiome: new concepts in the light of metaomics. Front Cell Infect Microbiol. 4, 29.
Vestrum, R., I., Forberg, T., Luef, B., Bakke, I., Winge, P., Olsen, Y., et al. (2022) Commensal and opportunistic bacteria present in the microbiota in Atlantic cod (Gadus morhua) larvae differentially alter the hosts’ innate immune responses. Microorganisms. 10, 24. https://doi.org/10.3390/microorganisms10010024
Walke, J., B., et al. (2017) Dominance-function relationships in the amphibian skin microbiome. Environ. Microbiol. 19, 3387–3397.
Wang, S. & Loreau, M. (2014) Ecosystem stability in space: α, β and γ variability. Ecol Lett. 17(8), 891-901.
Webster, T., M., U., Rodriguez-Barreto, D., Consuegra, S. & Garcia de Leaniz, C. (2019) Cortisol-induced signatures of stress in the fish microbiome. bioRxiv:826503. https://doi.org/10.1101/826503
Wilson, J., M. & Castro, L., F., C. (2010) Morphological diversity of the gastrointestinal tract in fishes. Fish Physiol. 30, 1-55. https://www.doi.org/10.1016/S1546-5098(10)03001-3
Wilson, M. World Feeds Limited, 3b Coulman Street Industrial Estate, Thorne, Doncaster, DN8 5JS, United Kingdom.
Wu, J., Mao, C., Deng, Y., et al. (2019) Diversity and abundance of antibiotic resistance of bacteria during the seedling period in marine sh cage-culture areas of Hainan, China. Mar Pollut Bull 141:343–349. https://doi.org/10.1016/j.marpolbul.2019.02.069
Xavier, R., Pereira, A., Pagan, A., Hendrick, G., C., Nicholson, M., D., Rosado, D., Soares, M., C., Pérez-Losada, M., Sikkel, P., C. (2020) The effects of environment and ontogeny on the skin microbiome of two Stegastes damselfishes (Pomacentridae) from the eastern Caribbean Sea. Mar Biol. 167(7),1-12.
Xu, Z., Takizawa, F., Casadei, E., Shibasaki, Y., Ding, Y., Sauters, T., J., C., et al. (2020) Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 5, 3254. https://doi.org/10.1126/sciimmunol.aay3254
Yatsunenko, T., Rey, F., E., Manary, M., J., Trehan, I., Dominguez-Bello, M., G., Contreras, M., et al. (2012) Human gut microbiome viewed across age and geography. Nature 486, 222-227. https://www.doi.org/10.1038/nature11053
Yoon, J., Matsuo, Y., Matsuda, S., Adachi, K., Kasai, H. & Yokota, A. (2018) Rubritalea sabuli sp. nov., a carotenoid-and squalene-producing member of the family Verrucomicrobiaceae, isolated from marine sediment. Int J Syst Evol Microbiol. 58(4), 992-997.
Yoshimizu, M., Kimura, T. & Sakai, M. (1980) Microflora of the embryo and the fry of salmonids. Bull. Jpn. Soc. Sci. Fish. 46, 967-975. https://www.doi.org/10.2331/suisan.46.967
Yu, Y., Wang, Q., Huang, Z., Ding, L. & Xu, Z. (2020) Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Frontiers in immunology. 11, 567941.
Zanefeld, J., R. et al. (2017) Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2, 17121.
Zarkasi, K., Z., Taylor, R., S., Abell, G., C., Tamplin, M., L., Glencross, B., D. & Bowman, J., P. (2016) Atlantic salmon (Salmo salar L.) gastrointestinal microbial community dynamics in relation to digesta properties and diet. Microb. Ecol. 71, 589-603. https://www.doi.org/10.1007/s00248-015-0728-y
Zeng, A., Tan, K., Gong, P. et al. (2020) Correlation of microbiota in the gut of fish species and water. 3 Biotech. 0(11), 1-10.
Zhang, L., Ni, C., Xu, W., Dai, T., Yang, D., Wang, Q., Zhang, Y. & Liu, Q. (2016) Intramacrophage Infection Reinforces the Virulence of Edwardsiella tarda. Journal of bacteriology. 198(10), 1534-1542.
Zhang, M., Shan, C., Tan, F., Limbu, S., M., Chen, L. & Du, Z. (2020) Gnotobiotic models: powerful tools for deeply understanding intestinal microbiota-host interactions in aquaculture. Aquaculture 517:734800. https://doi.org/10.1016/j.aquaculture.2019.734800
Zhang, X., Ding, L., Yu, Y. et al. (2018) The change of teleost skin commensal microbiota is associated with skin mucosal transcriptomic responses during parasitic infection by Ichthyophthirius multifillis. Front Immunol. 9, 2972.
Zhang, Y. & Gui, J. (2012) Molecular regulation of interferon antiviral response in fish. Developmental and comparative immunology. 38(2), 193-202.
Zhou, Z., Liu, Y., Shi, P., He, S., Yao, B. & Ringø, E. (2009) Molecular characterization of the autochthonous microbiota in the gastrointestinal tract of adult yellow grouper (Epinephelus awoara) cultured in cages. Aquaculture 286, 184-189. https://www.doi.org/10.1016/j.aquaculture.2008.10.002
Zhou, Z., Yao, B., Romero, J., Waines, P., Ringø, E., Emery, M., et al. (2014) Methodological approaches used to assess fish gastrointestinal communities. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, Merrifield, D. & Ringø, E. (eds.). John Wiley & Sons Ltd., Hoboken, NJ.




0 Comments