
Photosynthesis is the link between inorganic and organic worlds, and the importance of contributions made by zooxanthellae in maintaining health of photosynthetic corals is well known. We also know that rates of photosynthesis are dependent upon the amount of light, its spectral quality, as well as a supply of nutrients (for terrestrial plants, ‘nutrient’ is generally defined as nitrogen, potassium and phosphorus with ‘micronutrients’ described as iron, copper, and a host of others.) Though not listed as such, inorganic carbon (in the form of carbon dioxide) is an important nutrient in that it is necessary for plant growth and production of simple sugar. In marine aquaria, nutrients such as nitrogen and phosphorus are usually in such supply that means of removing them are often necessary. Potassium, with a concentration of about 400 mg/L, is also in good supply, and micronutrients might be added or replenished through feeding or water changes. But what of carbon? While it is true that some carbon dioxide may be present in an aquarium, and corals’ respired CO2 can meet some of photosynthesis’ carbon demand, some of the carbon used by at least some zooxanthellae will be provided by bicarbonate dissolved in the aquarium water.
Glossary
- Carbon, Inorganic: There are several forms of inorganic carbon including carbon dioxide, carbonic acid, bicarbonate, and carbonate.
- Carbonic Anhydrase (CA): An enzyme that reversibly converts carbon dioxide to bicarbonate. Carbonic anhydrase enzymes can be attached to the outside of a cell (thus called external CA). If inside the cell, it is an internal CA.
- Clade: For our purposes, a type of zooxanthella that has a common ancestor but can different significantly from other types (clades.) Some corals have co-evolved with a specific zooxanthella clade, and show a fidelity to that type.
- Alkalinity: The ability of a solution to neutralize acids. Alkalinity can be reported in several ways (meq/L, ppm, mg/L as calcium carbonate – CaCO3) but Degree Carbonate Hardness (dKH) is often used by reef hobbyists.
- mg/L: Milligrams per liter, which is roughly equivalent to parts per million (ppm.)
- Photosynthesis: Conversion of inorganic carbon to simple sugar (organic carbon.) Carbon dioxide or bicarbonate can be the source of inorganic carbon. A by-product of photosynthesis is nascent oxygen.
- Electron Transport Rate (ETR): A measure of the rate of photosynthesis. Light particles (photons) once absorbed by photopigments (chlorophylls, accessory pigments) become electrons and flow (normally) between photosystems. A low ETR means a low rate of photosynthesis; a high ETR means a high rate. If light absorption is measured, the electron flow is called ETR. If not, it is referred to as the Relative Electron Transport Rate or rETR.
Liebig’s Law states that photosynthesis is controlled by not the concentration of the most abundant nutrient, but by the supply of the least – the weakest link will define the rate of photosynthesis. This fact is well recognized by freshwater planted enthusiasts and nutrient additions are routinely made to prevent the general plant ailment known as chlorosis. As such, we are the master of the microenvironment we have created.
Reef aquarists should routinely monitor the alkalinity content of their aquaria as it is important for a number of reasons. Alkalinity acts as a buffer against downward shifts in pH which is important since the nitrification process destroys it. Carbonates and calcium are essential in formation of corals’ skeletons. But what of its importance in acting as an inorganic carbon source for photosynthesis?
To answer this question, experiments were conducted to test the hypothesis that alkalinity concentrations could affect the rates of photosynthesis.
Methods and Materials
Natural seawater in a 10-gallon (38 liter) aquarium was spiked with ammonium hydroxide, and the nitrification process (conversion of ammonia to nitrate) reduced alkalinity to an unnaturally low concentration over the course of a few weeks. Once fully nitrified, half of this water was transferred to a five-gallon (19 liter) aquarium which contained a rack fashioned of plastic egg crate material. Fragments of the stony coral Porites lobata (glued to ‘reef plugs’) were then placed on this rack. Lighting was provided by a LED luminaire (dimmable) made by BuildMyLED (Austin, Texas, USA) and maintained at a sub-saturating intensity of 100 µmol·m²·sec as measured by a LiCor quantum meter and submersible LI-192 2 sensor (LiCor Biosciences, Lincoln, Nebraska, USA.) A tunze 6040 (tunze Aquarientechnik GmbH, Penzberg, Germany) variable speed propeller pump moved water in this aquarium.
A Pulse Amplitude Modulated (PAM) fluorometer (Junior PAM, Heinz Walz GmbH, Effeltrich, Germany) with a fiber optic cord measured the relative rate of photosynthesis (relative electron transport rate, or rETR) of the coral’s zooxanthellae under conditions of low, natural, slightly elevated, and high alkalinity. Alkalinity concentrations were adjusted upwards with a commercially available buffer (Reef Builder, Seachem Laboratories, Madison, Georgia, USA.) Alkalinity was measured with a colorimeter (Alkalinity Checker HI755, Hanna Instruments, Woonsocket, Rhode Island, USA.) and doubled checked initially by titration to a pH of 4.2 through use of a commercially available titrant (1.6N sulfuric acid) and a digital titrator (Hach, Loveland, Colorado, USA.)
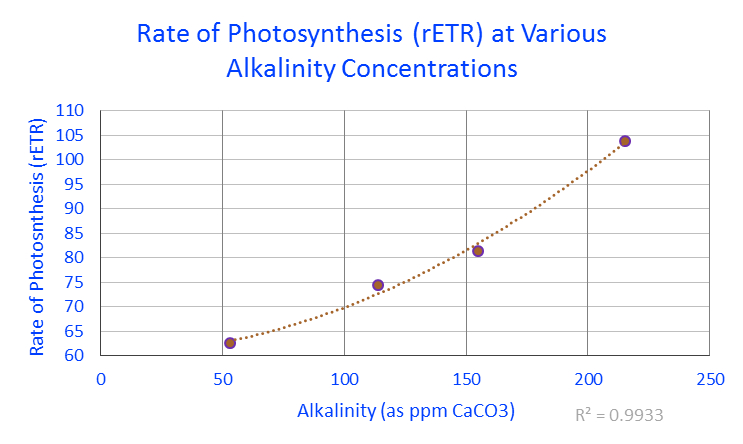
Figure 1. Rates of photosynthesis (expressed as relative electron transport rates) of zooxanthellae within a Porites lobata coral under various alkalinity concentrations. For those preferring alkalinity reported as dKH, 53 ppm = 2.968 dKH; 114 = 6.384; 155 = 8.68 and 215 = 12.04.
Results
PAM fluorometry confirmed rates of photosynthesis increased along with alkalinity concentrations. See Figure 1.
Discussion
The result of these experiments demonstrated that the rate of photosynthesis could be boosted by about 29% by increasing alkalinity from a ‘normal’ level (6.4 dKH) to an elevated level (~12 dKH.)
Use of bicarbonate as a source of inorganic carbon for photosynthesis might come as a surprise to some hobbyists. Although we think of corals as relatively simple creatures, the biochemistry involved in their life processes can be anything but uncomplicated. Some of the steps involved in the use of bicarbonate in photosynthesis are as follows:
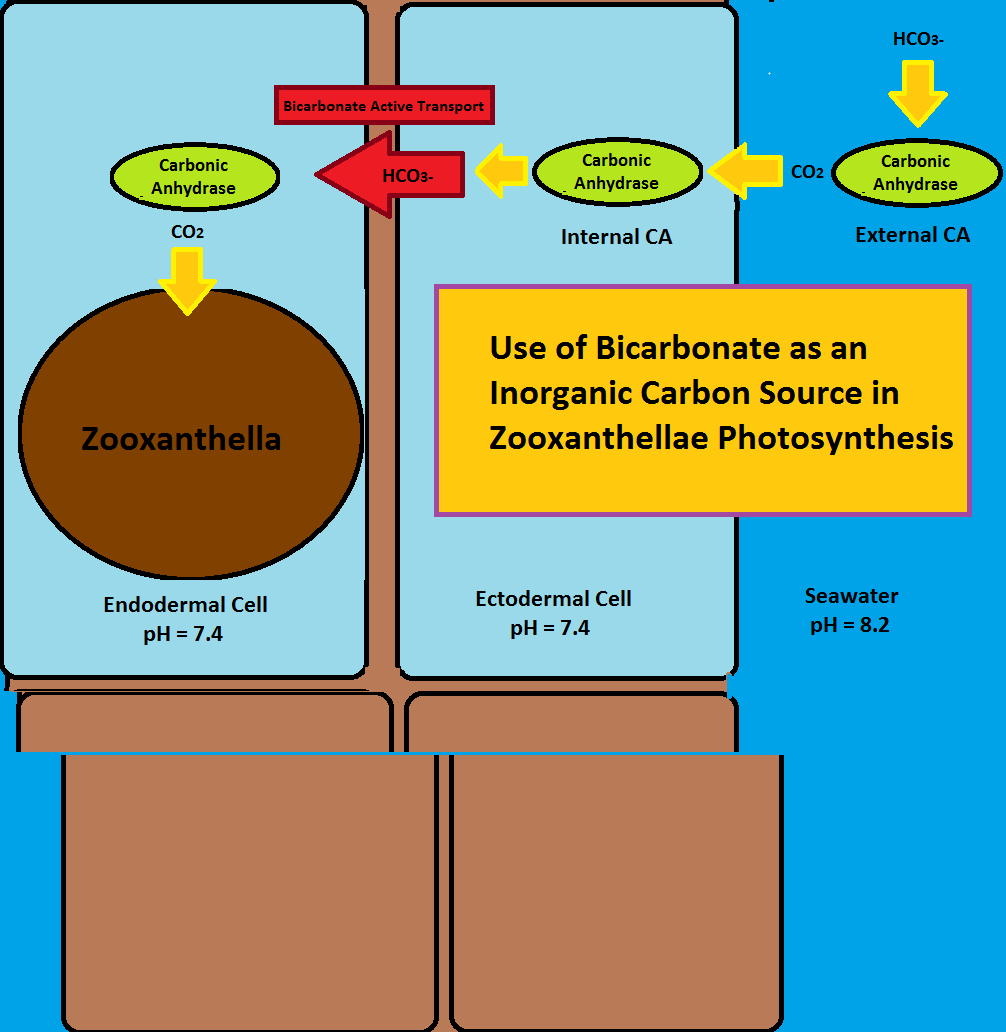
Figure 2. Use of bicarbonate (HCO3-) as an inorganic carbon source for photosynthesis. This involves the enzyme carbonic anhydrase (internal and external) and ‘bicarbonate active transport.’ After Zoccola et al., 2015. The ‘external carbonic anhydrase’ enzyme (top right) is actually attached to the coral’s ectoderm cell, something this drawing does not accurately reflect.
Carbonic Anhydrase (CA): Carbonic anhydrase is an enzyme that can convert bicarbonate to carbon dioxide or carbon dioxide to bicarbonate:
HCO3– + H+ ↔ CO2 + H2O
As we can see, this enzyme could be useful in both zooxanthellae photosynthetic processes and coral skeleton calcification. Bicarbonate does not pass easily through cell walls but carbon dioxide does, hence an external CA could convert bicarbonate to carbon dioxide to allow easy passage. Once inside the cell, CA converts carbon dioxide back to bicarbonate to prevent back-diffusion. Bicarbonate Active Transport (BAT; see below) carries bicarbonate through the mesoglea where it once again is converted to carbon dioxide by CA. CO2 is then utilized by zooxanthellae. See Figure 2.
Most carbonic anhydrase enzymes contain zinc, although it has been recently discovered that some of these enzymes found in marine diatoms contain cadmium in lieu of zinc. This is the first helpful biological function known to utilize cadmium.
CA can be external (to the cell wall) or internal.
Bicarbonate Active Transport (BAT): Bicarbonate Active Transport (BAT) is a process that selectively moves bicarbonate across a cellular membrane that would normally be semi-permeable or perhaps impermeable. Sometimes called a bicarbonate pump, this process can move bicarbonate ions against a gradient. See Figure 3.
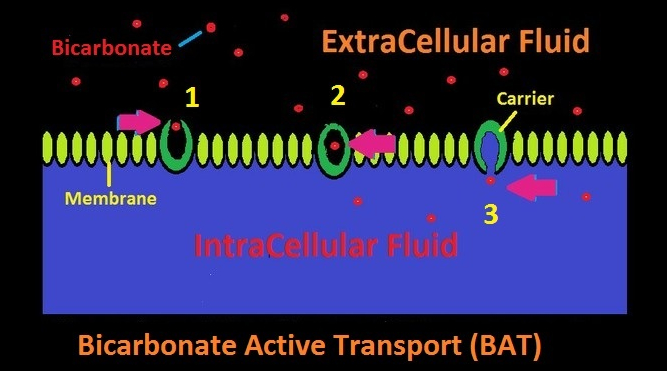
Figure 3. Active and selective transport of bicarbonate across a cell membrane by Bicarbonate Active Transport (BAT.) A carrier accepts the bicarbonate ion in Step 1. In Step 2, the bicarbonate ion is enclosed in the carrier, and in Step 3, it is released into the cell’s intracellular fluid.
While this experiment found that Porites lobata’s rate of photosynthesis could be influenced by the concentration of bicarbonate ions, there is evidence that such is not the case with all corals’ zooxanthellae.
Brading et al. (2013) researched the inorganic carbon preferences of two zooxanthellae clades and found they differed. They found that zooxanthellae clade A13 preferred to uptake and fix carbon dioxide, while clade A20 could utilize bicarbonate as well. (Clade A13 is known to infect the jellyfish Cassiopeia frondosa, the anemone Condylactis gigantea, and the Atlantic stony corals Porites astreoides and Montastrea annularis, while A20 is considered to be free-living and is not known to associate with any invertebrates.) Hence, blanket statements concerning the degree of utilization of bicarbonate by zooxanthellae cannot be made. Although not tested for this experiment, it is generally accepted that Hawaiian Porites lobata corals contain the zooxanthellae clade C15.
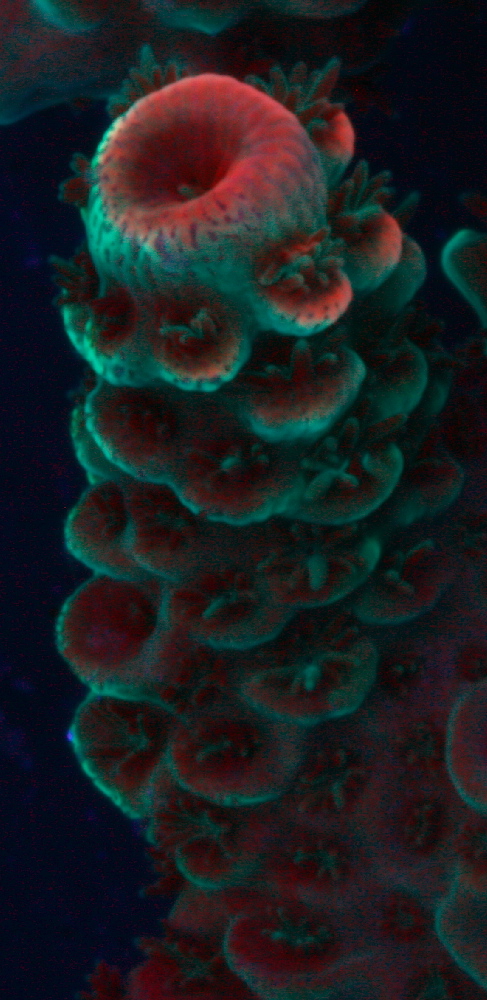
Figure 4. When viewed under a black light, chlorophyll’s red fluorescence is apparent in this Acropora coral. A blue-green fluorescent protein is seen as well. Compare the areas of red fluorescence to those showing tissue loss in Figure 5. Photo by the author.
An interesting thought runs as follows: Some corals might not possess many (or any) external carbonic anhydrase enzymes. Hence, their dependence upon respired carbon dioxide might be much greater than those with external CA. Hence, coral growth rates might be slower than those with the ability to utilize relatively easily obtained bicarbonate.
‘Alkalinity Burn’ – Is an Increased Rate of Photosynthesis Responsible?
‘Alkalinity Burn’ is a term coined by reef aquarists about tissue loss (usually in the ‘tip’ or, in the case of Acroporids, the apical and near-by axial polyps.) Is it possible that chlorophyll concentrations in certain areas and the by-products of photosynthesis such as hydrogen peroxide and oxygen radicals overwhelm the natural defenses offered by enzymes such as peroxidases and catalases?
Figure 4 shows the chlorophyll concentrations of chlorophyll as indicated by chlorophyll fluorescence.
Compare the areas of chlorophyll concentrations in Figure 4 to those areas of an Acropora species suffering from ‘alkalinity burn’ in Figure 5.
As Figure 5 shows, tip burn is prevalent in areas receiving the most light, hence an elevated level of bicarbonate could turbocharge photosynthesis resulting in production of detrimental oxygen species.
Indeed, Finelli et al. (2006) discuss the link between coral tissue thickness and damage by ‘harmful levels of oxygen.’ Their discussion concerned water motion and photosynthesis – that will be our topic for next time, along with synergistic effect of bicarbonate.
References and Further Reading
- Al-Moghrabi, S., C. Goiran, D. Allemand, N. Speziale, and J. Jaubert, 1996. Inorganic carbon uptake for photosynthesis by the symbiotic coral-dinoflagellate association. II. Mechanisms for bicarbonate uptake. J. Exp. Mar. Biol. Ecol., 199(2):227-248.
- Brading, P., M. Warner, D. Smith and D. Suggett, 2013. Contrasting modes of inorganic carbon acquisition amongst Symbiodinium (Dinophyceae) phylotypes. New Phytologist, 200(2):432-442.
- Comeau, S., P. J. Edmunds, N. B. Spindel, and R. C. Carpenter 2013b. The responses of eight coral reef calciers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58: 388-398.
- Finelli, C., B. Helmuth, N. Pentcheff, and D. Wethey, 2006. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs, 25: 47-57.
- Herfort, L., B. Thake, and I. Taubner. 2008. Bicarbonate stimulation of calcication and photosynthesis in two hermatypic corals. J. Phycol. 44: 91-98.
- Jury, C., R. Whitehead, and A. Szmant. 2010. Effects of variations in carbonate chemistry on the calcication rates of Madracis auretenra (Madracis mirabilis sensu Wells, 1973): Bicarbonate concentrations best predict calcication rates. Glob. Chang. Biol. 16: 1632-1644.
- Marubini, F. and B. Thake, 1999. Bicarbonate addition promotes coral growth. Limnol. Oceanogr., 44(3):716-720.
- Marubini, F. and M.J. Atkinson, 1999. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser., 188:117-121.
- Wall, C. and P. Edmunds, 2013. In situ effects of low pH and elevated HCO3- on juvenile massive Porites spp. in Moorea, French Polynesia. Biol. Bull. 225: 92-101.
- Zoccola, D., P. Ganot, A. Betucci, N. Caminiti-Segonds, N. Techer, C. Voolstra, M. Aranda, E. Tambutté, D. Allemand, J. Casey, and S. Tambutté, 2015. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Scientific Reports 5, Article number: 9983.




0 Comments