
All red fluorescent pigments from Acropora species (so far) are of the DsRed type, and probably Clade C3. Photo courtesy of Justin Miedwig and madfragsonline.com.
In Parts One (http://www.advancedaquarist.com/2009/1/aafeature1) and Two (http://www.advancedaquarist.com/2009/2/aafeature1) of this series, we reviewed information about cyan and green fluorescent pigments, and factors known to induce these colors in corals and anemones, as well as cases of photoconversion. It should be apparent that the various issues of pigmentation and their responses to various external stimuli are complex. However, great strides have recently been made in aiding our understanding of cnidarian coloration. This month, we’ll look at several orange and red pigments of the DsRed-type. If some of the terminology seems unfamiliar, see either of the links listed above for a glossary.
The goal of this article is to stitch together bits and pieces of information into a form usable by hobbyists to not only induce and maintain coloration, but to aid in our understanding of why these colors occur at all. Do all DsRed-type pigments react in the same way to external stimuli, such as light and pH, or are these necessary at all? If not, why not? What are the potential effects of ‘trace elements’‘? Read on if I have your attention and interest.
DsRed Pigments
DsRed (shorthand for Discosoma Red) is one of 5 major pigment categories. DsRed was originally isolated from the corallimorpharian Discosoma, we now know that it is found additionally in zoanthids, stony corals and anemones (See Table 1). Fluorescent emissions range from orange (561 nm) to red (620 nm); but note that one DsRed chromophore possessing a green coloration is also included. It is generally regarded that the ‘original’ DsRed has a fluorescence emission at 583nm (in the orange/red portion of the visible spectrum).
DsRed Mutations
DsFP483 (Ds for the host ‘coral’ Discosoma; FP for Fluorescent Protein; 483 for 483nm – the pigment’s maximum fluorescence in the blue-green portion of the spectrum) has only two amino acid substitutions (in a protein containing a total of hundreds of amino acids) within the fluorescent protein and thus differs chemically only slightly from DsRed with fluorescence peaking in the red spectrum. However the effects of these amino acid substitutions are profound and the cyan pigment FP483 never matures to red. Other slight modifications to the DsRed chromophore structure can lead to pigments where the emission is ‘redder’ than that of the unmodified wild-type DsRed. Emissions of modified DsRed proteins are known to have maximum emissions at 592nm, 594nm, 600nm and 602nm. Hence, we can easily see that even slight mutations exhibit very different spectral characteristics. We should keep this in mind as we examine the fluorescence of other DsRed proteins.
Note that a number of DsRed mutant pigments exist, each with a different excitation, emission, speed of conversion from green to red and a ratio of green to red in the fully-matured pigment. Table 1 lists a few of the DsRed pigments genetically- engineered in the laboratory (while there is a good chance that these pigments are not seen in nature, it does give us an idea of the effects of small changes in the chemical makeup).
| Mutation | Red Emission | Maturation Speed (after 2 days) |
|---|---|---|
| Wild-type DsRed (no mutation) | 583 | ~40-fold increase in red fluorescence |
| K83R | 582 | very little change |
| K83E | 584 | very little change |
| K83N | 592 | 2-5 fold increase |
| K83P | 594 | 2-5 fold increase |
| K83F | 594 | very little change |
| K83W | 594 | 2-5 fold increase |
| K83M | 602 | very little change |
| Y120H | 600 | 2-5 fold increase |
| 5197T | 584 | 5-20 fold increase |
| K70R | 585 | 2-5 fold increase |
Color Conversion Timeframe
Baird et al. (2000) report that the conversion of the green precursor pigment to DsRed is relatively slow (a couple of days) and does not need light energy to occur. However, other researchers have shown color shifts in this pigment upon exposure to various wavelengths. A conversion from red to ‘super red’ can occur as a result of exposure to light is also known to occur.
Effects of pH on DsRed Fluorescence and Apparent Color
Baird et al. (2000) state that the DsRed fluorescence emission (from Discosoma) is insensitive to pH over a range of 4.5 – 12, and has a high quantum yield (0.7) at or near a pH of ~8.8. However, pH shifts within the coral tissue could conceivably cause a change in perceived coloration since absorption (excitation) maxima are caused by pH modulations – the wavelength absorption maxima falls from 558nm to 526nm as pH becomes lower.
Fluorescence Quenching Effects of Metals on DsRed Fluorescence
Any seasoned hobbyist knows that copper is toxic to marine invertebrates and avoids its medicinal use for fishes in a reef aquarium. However, even trace amounts of copper (Cu2+ & Cu+ ions in the parts per billion range) can bind with the wild-type DsRed protein as well as some of the engineered mutants and ‘quench’ (inhibit or stop) fluorescence (Rahimi et al. 2008). Fortunately, the effect is reversible as an addition of a chelator can reverse the effect and restore fluorescence. This quenching effect becomes more pronounced with increasing pH.
Other researchers also found evidence of metal-induced fluorescence quenching by cobalt and nickel (though not to the extent of copper – Eli and Chakrabartty, 2006). Zinc has been noted to reduce fluorescence in some of the genetically-engineered green fluorescent proteins (but we don’t know which GFPs) but not in DsRed. See Figure 1 (note that this information is applicable to only the DsRed pigment and not any of its mutants).
Metals That Increased DsRed Fluorescence
Interestingly, some heavy metals have been found to marginally increase fluorescence of the protein found in Discosoma. Those with the most effect were (in descending order): Manganese, iron and chromium (Eli and Chakrabartty, 2006). See Figure 1.
Warning: These researchers were working with minute concentrations (mM) of metal concentrations. Resist the urge to dump a bucket of chemicals into your aquarium!
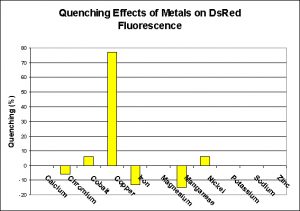
Figure 1. Some metals prevent (quench) fluorescence of the DsRed pigment, including copper, cobalt and nickel, while others (chromium, iron and manganese) increase fluorescence. Others have no effect (calcium, magnesium, potassium, sodium and zinc). After Eli and Chakrabartty, 2006.
Metals with No Effects on DsRed Fluorescence
Metals and their effects on DsRed fluorescence have recently become a subject of interest to researchers – not that they care about the pretty colors, instead they are most interested in quantifying ‘quenching’ of fluorescence by metals in order to study ailments such as Alzheimer’s and others. Eli and Chakrabartty, 2006 studied a number of metals and their effects on DsRed fluorescence. Those metal ions demonstrating no effects of were sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+). The latter is of particular interest, since there are internet reports from hobbyists I respect claiming that low magnesium levels can cause loss of color in some Montipora specimens. See Figure 1.
Color Mixing
A combination of fluorescence with maxima at different wavelengths can trick the eye into seeing a color that is not actually there. In the case of DsRed, researchers have noted an apparent (but not true) yellow coloration due to color mixing.
All pigment clades (A-D) are known to contain at least one DsRed-type color. See Table 2.
| Species | Pigment Name | Type | Suborder | Excit. | Emission | Clade |
|---|---|---|---|---|---|---|
| Echinopora forskaliana | eforCP/RFP | DsRed | Faviina | 589 | 609 | ? |
| Acropora millepora | amilRFP | DsRed | Astrocoeiina | 560 | 593 | C3 |
| Acropora millepora | amilFP597 | DsRed | Astrocoeiina | 558 | 597 | ? |
| Discosoma sp. | DsRed | DsRed | Corallimorpharia | 558 | 583 | B |
| Discosoma sp. | dis2RFP | DsRed | Corallimorpharia | 558 | 593 | D |
| Entacmaea quadricolor | eqFP611 | DsRed | Actinaria | 559 | 611 | A |
| Fungia concinna | Kusabira-Orange | DsRed | Fungiina | 548 | 561 | C1 |
| Montipora digitata | mdigFP572 | DsRed | Astrocoeiina | 556 | 572 | ? |
| Montipora efflorescens | meffRFP | DsRed | Astrocoeiina | 560 | 576 | C3 |
| Montipora sp. | Keima-Red | DsRed | Astrocoeiina | 440 | 620 | B |
| Porites porites | pPorRFP | DsRed | Faviina | 578 | 595 | B |
| Zoanthid sp. | Zoan2RFP | DsRed | Zoanthidae | 506 | 574 | C3 |
| Zoanthid sp. | Zoan2RFP | DsRed | Zoanthidae | 552 | 576 | C* |
| Zoanthus sp. | zoanGFP | DsRed | Zoanthidae | 496 | 506 | C3 |
We’ll begin our discussion with an examination of the widely-described red fluorescent pigment – DsRed.
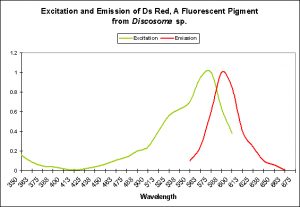
DsRed – The Red Fluorescent Pigment 583
- Host: Discosoma species
- Pigment Clade: B
- Pigment Type: DsRed
- Excitation/Emission: 558 nm/583 nm, respectively
- Fluorescence Induced by: Maturation of green to red is by chemical oxidation and no light is required. However, exposure to light can induce changes in fluorescence.
- Photoconversion Possible: Yes, from 583nm to 595nm upon exposure to green light (at ~570nm). Exposure to 750nm has been noted to reduce red fluorescence.
DsRed was one of the first red fluorescent pigments described by scientists. Interestingly, the pigment was cloned from a Discosoma specimen maintained in a Moscow reef aquarium.
DsRed pigments are found in all 4 known pigment clades (A-D) and in corals popular in aquaria (some Acropora, Montipora, Porites, Fungia, and Echinopora species), at least one anemone (the rose anemone), and various zoanthids.
The fluorophore structure is similar to that of many (if not all) of the non-fluorescent chromophores (we’ll examine the non-fluorescent chromoproteins in a separate article). We should note that not all DsRed fluorescence is due strictly to chemical oxidation – some of the DsRed pigments respond to light energy (particularly blue light, as we shall see below). Since DsRed is of Clade B (and this clade is also contained in some Porites porites and an unidentified Montipora) it is tempting to think that the pigments of at least some stony corals mature in the same manner.
Coloration shifts are due to a rearrangement of the fluorophores’ structures (a fluorophore is the ‘package’ containing the fluorescent materials). A transition from green to red is autocatalytic (although light energy can cause ‘reddening’). However, some green pigments fail to mature to red but the green coloration is not seen since there the emission of the green pigments is absorbed by the red pigment and fluoresced at 583nm. In some cases, the red fluorescence is lost, and since the green fluorescence is no longer absorbed by the red pigment, the coral appears green (see Figure 3).

Figure 4. The same Discosoma as in Figure 2 – it has lost its red coloration. The red coloration on the base rock is that of chlorophyll fluorescence. Excitation light was a ‘black light’.
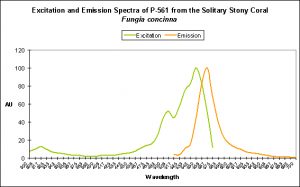
Red Fluorescent Pigment 561
- Host: Fungia concinna
- Pigment Name: Kusabira Orange
- Pigment Clade: C1
- Pigment Type: DsRed
- Excitation/Emission: 548 nm/561 nm, respectively
- Fluorescence Best Induced by: ?
- Light Intensity Required: ?
Kusabira-Orange is the only red C1 Clade described (there are a couple of green C1 pigments). This pigment’s fluorescence is easy to maintain in captivity and has been observed over a range of light intensities. Interestingly, this pigment is apparent on the bottom of the Fungia‘s disc.
Since this color is so easy to maintain (it is even apparent to a degree on the shaded underside of the coral), I am tempted to say that this pigment is a result of factors beyond the control of hobbyists (i.e., chemical oxidation).
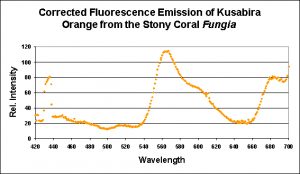
Figure 7. This pigment from a Fungia specimen (shown in Figure 5) has a fluorescent pigment that peaks in the orange portion of the spectrum, with a shoulder at ~600 nm. Fluorescence at ~680nm is caused by chlorophyll in symbiotic zooxanthellae. Apparent fluorescence at ~430nm is an artifact of the excitation source.
Red Fluorescent Pigment 572
- Host: Montipora digitata

Figure 8. The orange fluorescence of Montipora digitata. This pigment is apparently common in many Montipora species. Photo by the author.
- Pigment Clade: Unknown (probably C3)
- Pigment Type: DsRed
- Excitation/Emission: 556 nm/~572 nm, respectively
- Fluorescence Best Induced by: Blue light
- Light Intensity Required: Best fluorescence at 400 µmol·m²·sec
- Photoconversion Possible: Possible in some orange Montipora specimens
As with pigments previously described in this article, this pigment is remarkably easy to maintain in captivity (as are the several other Montipora pigments listed below). Researchers have discovered that this orange coloration is expressed when the coral is exposed to blue light, and blue light weighted towards the green portion of the spectrum. Red light also has an unusually high effect on pigment expression (although the red light effect is still very low when compared to ‘blue’ and ‘green’ light).

Figure 9. PAR and its effects on generation of fluorescent pigmentation. After D’Angelo et al., 2008.
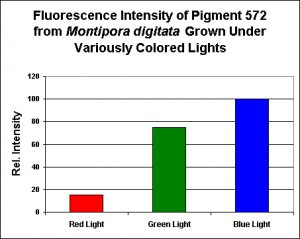
Figure 10. Effects of light color on pigmentation – notice this pigment’s production by the coral animal is unusually influenced by ‘green’ light. Each treatment was standardized to 200 µmol·m²·sec. After D’Angelo et al., 2008.
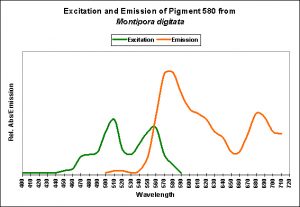
Figure 11. Excitation and emission characteristics of an orange pigment from the stony coral Montipora digitata. The spike at about 680 nm is chlorophyll fluorescence. After Mazel, unpublished data.

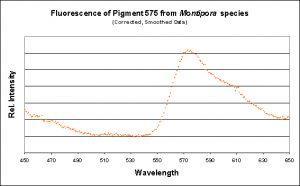
Figure 12. The fluorescence of a Montipora digitata from an aquarium (shown in Figure 8). Its spectral characteristics are virtually identical to those shown in Figures 11, 13, 15, 17, and 19.

Figure 13. A measurement of fluorescence from a second aquarium-grown Montipora digitata specimen. Although the emission shape is very similar to the others shown in this section, its emission peak is at 577nm (as opposed to 575nm). Does this really matter? Probably not.
Other Montipora Pigments
Other than non-fluorescent chromoproteins, there is presently no evidence suggesting that Montipora specimens contain any GFP-like proteins besides the DsRed type.

Figure 16. An unidentified Montipora species with an orange fluorescence typical of many in this genus. Photo by the author.

Figure 18. The ‘Sunset’ Montipora, showing its DsRed-type orange fluorescence, and, in its polyps, a green fluorescent color. Notice the nascent polyp growth – the tentacles are orange and the oral discs are blue-green. Does this pigment mature to orange from a GFP-type fluorescent protein? Photo by the author.
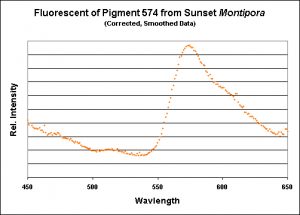
Figure 19. Again, the emission of this particular Montipora pigment is almost identical to those shown above.
A Case of Green-to-Orange Photoconversion in a Montipora species

Figure 20. The ‘Poker Star’ Montipora maintained under Ushio 150w DE ’20+K’ lamps. Photo courtesy of Steve Ruddy and www.coralreefecosystems.com.
Many thanks to Steve Ruddy (of www.coralreefecosystems.com) for sharing this information – he has provided photographic documentation of a color shift from green to orange in a Montipora specimen (traded under the descriptive name of ‘Pokerstar’ Montipora). My inclination is that light quality was responsible for the shift, but this needs to be confirmed. See details of the lighting setups in the captions for Figures 20 and 21.

Figure 21. A fragment of the same Montipora shown in Figure 19, but maintained under different lamps. We see a transition in polyp color from green to red. Photo courtesy of Steve Ruddy and www.coralreefecosystems.com. These lamps are: 250w Radium 20,000K, Iwasaki 150w 50,000K, 1-160w VHO UVL ‘Super Actinic’ and 1-160w UVL ‘Actinic White’.
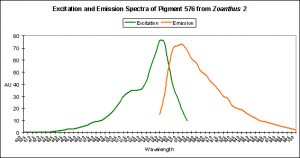
Red Fluorescent Pigment 576
- Host: Zoanthus species
- Pigment Clade: Clade C, possibly C3
- Pigment Type: DsRed
- Excitation/Emission: 552 nm/ 576 nm, respectively
- Fluorescence Best Induced by:?
- Light Intensity Required:?
- Photoconversion Possible:?
Red Fluorescent Pigment 593
- Host: Acropora millepora
- Pigment Clade: C3
- Pigment Type: DsRed
- Excitation/Emission: 560 nm/593 nm, respectively
- Fluorescence Best Induced by:?
- Light Intensity Required:?
- Photoconversion Possible: Probable
This pigment is listed to mesh together available information on Acropora pigments. We know this particular pigment is definitely of the DsRed type, and belongs to Clade C3.
There is another pigment described from Acropora millepora (FP-594) that transitions from red to green (535 nm) upon exposure to blue light at 488 nm. See additional data about another (and likely closely related) Acropora pigment below.

Figure 23. A nicely colored Acropora millepora. The initial appearance of a green fluorescence and its slow maturation to red fluorescence (plus color mixing of green and red to produce apparent yellow and orange coloration) would explain most of the coloration we see, however, this needs to be confirmed. Photo courtesy of Justin Miedwig and madfragsonline.com.
Red Fluorescent Pigment 597
- Host: Acropora millepora
- Pigment Clade: Probably C3
- Pigment Type: DsRed
- Excitation/Emission: 558 nm/597 nm, respectively
- Fluorescence Best Induced by: Blue light
- Light Intensity Required: Best fluorescence at 400 µmol·m²·sec
- Photoconversion Possible: Probable, based on aquaria specimens

Figure 24. PAR and its effects on generation of fluorescent pigmentation. After D’Angelo et al., 2008.
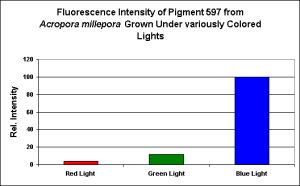
Figure 25. Effects of light color on pigmentation. Each treatment was standardized to 200 µmol·m²·sec. As with all pigments examined, blue light, by far, induced expression of fluorescent pigmentation. After D’Angelo et al., 2008.
Red Fluorescent Pigment 611
- Host: Entacmaea quadricolor
- Pigment Clade: A
- Pigment Type: DsRed
- Excitation/Emission: 611 nm, respectively
- Fluorescence Best Induced by: Chemical Oxidation
- Light Intensity Required: None
- Photoconversion Possible: No
Aquarists should note that this particular pigment is sensitive to temperature. Keep aquarium temperatures below 27C (~81F).
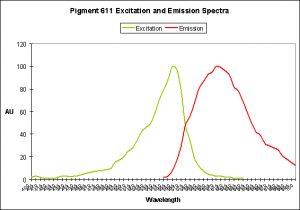
Figure 27. The bubble-tip anemone (Entacmaea quadricolor) contains a fluorescent pigment with a peak emission at 611 nm.
Other Probable DsRed-type Pigments
At this time, it seems that far-red fluorescent pigments are probably DsRed types (their spectral characteristics do not match the other red pigment type – Kaede. We’ll discuss Kaede-type pigments in a separate article).
Dove et al. (2001) report a pigment with an emission of 610nm (from Montipora monasteriata, clade unknown), 625nm (from Pocillopora damicornis, clade unknown), another pigment fluorescing at 625nm (Acropora horrida, clade C3?), and still a third at 625nm from Porites murrayensis (clade B?).
Red Fluorescent Pigment 620
- Host: Montipora species
- Pigment Clade: B
- Pigment Type: DsRed
- Pigment Name: Keima-Red
- Excitation/Emission: 440 nm/620 nm, respectively
- Fluorescence Best Induced by:?
- Light Intensity Required:?
- Photoconversion Possible: Unknown
Keima means ‘knight’ in Japanese – a reference by researchers to the emission shape of this protein and its similarity to the movement of a chess-piece knight. Interesting, the far-red shifted fluorescence of Montipora efflorescens and Porites porites are also of Clade B (this clade also contains, of course, the original red fluorescent pigment DsRed from the corallimorpharian Discosoma).
Unlike the Pigment 611 (found in the anemone E. quadricolor), Pigment 620 is stable at high temperatures (37 C, or 98.6 F).
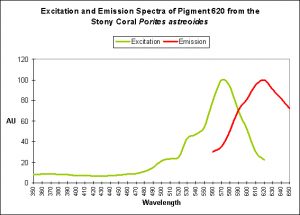
Red Fluorescent Pigment 620
- Host: Porites astreoides
- Pigment Clade: Unknown, but possibly B
- Pigment Type: DsRed
- Excitation/Emission: ~580 nm/620 nm, respectively
- Fluorescence Best Induced by:?
- Light Intensity Required:?
- Photoconversion Possible: Unknown
A pigment from Porites porites (excitation of 578nm, and an emission peaking at 595 nm) has been noted. It is a DsRed-type pigment of Clade B.
Red Fluorescent Pigment 630
- Host: Acropora aspera
- Pigment Clade: Unknown (possibly C3?)
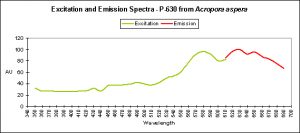
Figure 30. A ‘super red’ pigment from the stony coral Acropora aspera.
- Pigment Type: DsRed (?)
- Excitation/Emission: ~575 nm/620 nm, respectively
- Fluorescence Best Induced by:?
- Light Intensity Required:?
- Photoconversion Possible: Unknown
Fluorescent pigment 630 is the most red-shifted pigment of any described ‘coral’ pigment. All known red pigments from Acropora are of Clade C3, and therefore this pigment is tentatively attributed (by me) to this clade. The source of this information is from Salih et al. (2001).
Comments
In general, the fluorescence of DsRed pigments is not difficult to maintain in captivity. We have established that light (and blue light in particular) is important in the process of color promotion, although to varying degrees. Green light can promote coloration and in some cases, red light alone can make a coral weakly express pigmentation.
In some cases, temperature is critical (such as seen in the rose or bubble-tip anemone- E. quadricolor), where temperatures above 27C (~81F) inhibit coloration. On the other hand, the Keima pigment found in a Montipora species is stable at 37 C (98.6 F).
We have also learned metals can help promote or inhibit coloration. Metal binding to the protein definitely affects coral coloration, but it is unclear if those metals promoting fluorescence are required cofactors or simply a product of the binding.
As you’re probably aware by now, this subject has my interest and I’ll keep researching this subject. Next time, we’ll look at the ‘other’ red pigments – the ‘Kaede’ types.
Acknowledgements
Many thanks to Steve Ruddy (www.coralreefecosystems.com) and Justin Miedwig and (www.madfragsonline.com) for providing photos and information used in this article. Also, mahalo to Dr. Charles Mazel (www.nightsea.com) for remembering my fascination with coral coloration and providing me a copy of the D’Angelo paper.
Special thanks to hobbyist Sandy Shoup for stumping me (twice) with her questions about changes in coloration of her Montipora specimens. Sandy, I hope this article will shed some light on some of the issues surrounding coral coloration.
Questions? Comments? I am best reached at [email protected].
References
- Baird, G., D. Zacharias and R. Tsein, 2000. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci., 97, 22:11984-11989.
- D’Angelo, C., A. Denzel, A. Vogt, M. Matz, F. Oswald, A. Salih, G. Nienhaus, and J. Wiedenmann, 2008. Blue light regulation of host pigment in reef-building coral. Mar. Ecol. Prig. Ser. 364: 97-106.
- Eli, P. and A. Chakrabartty, 2006. Variants of DsRed fluorescent protein: Development of a copper sensor. Protein Sci. 15(10):2442-2447.
- Rahimi, Y., A. Goulding, S. Shrestha, S. Mirpuri and S. Deo, 2008. Mechanisms of copper induced fluorescence quenching of red fluorescent protein, DsRed. Biochem. Biophys. Res. Comm., 370(1):57-61.









0 Comments