 Dissolution rates of various materials by moving water have been the focus of many researchers. Indeed, the ability of aggressive water to dissolve copper ‘coupons’ has been a standard for estimating the corrosiveness of potable water for a number of years. These coupons have also been deployed on natural coral reefs for study of long-term water motion). Obviously, dissolving copper in a small body of water (such as an aquarium) is a poor choice when long-term maintenance of invertebrates is the goal. Other materials, such as salt tablets and even candy (LifeSavers™), have been used. Certainly, these could of use in aquaria, but this article will focus on the use of gypsum blocks (calcium sulfate, commonly known as Plaster of Paris) to determine a totalized amount of water motion in reef aquaria. The advantages are many – the material is readily available and inexpensive. In addition, there is a good deal of previous research available for comparative purposes.
Dissolution rates of various materials by moving water have been the focus of many researchers. Indeed, the ability of aggressive water to dissolve copper ‘coupons’ has been a standard for estimating the corrosiveness of potable water for a number of years. These coupons have also been deployed on natural coral reefs for study of long-term water motion). Obviously, dissolving copper in a small body of water (such as an aquarium) is a poor choice when long-term maintenance of invertebrates is the goal. Other materials, such as salt tablets and even candy (LifeSavers™), have been used. Certainly, these could of use in aquaria, but this article will focus on the use of gypsum blocks (calcium sulfate, commonly known as Plaster of Paris) to determine a totalized amount of water motion in reef aquaria. The advantages are many – the material is readily available and inexpensive. In addition, there is a good deal of previous research available for comparative purposes.
In order to evaluate water motion, at least two clods must be used. The first is placed in the aquarium at the point of interest while the second (the ‘control’) is placed in a 5-gallon bucket of similar water. After a pre-determined amount of time, the diffusion cards are removed and dried. They are then weighed, and the weight loss of the ‘aquarium’ card is divided by the weight loss of the control. The result is the Diffusion Factor (or DF).
Making the Diffusion Cards
Making the diffusion cards is simple but requires some preparation. Gather these materials (see Figure 1):

Figure 1. With the exception of the relatively inexpensive mail order scale, all other supplies are readily available. The result (the ‘clod’) is in the forefront.
- Plaster of Paris (at least 1 pound).
Caution: Some glorified brands of Plaster of Paris contain additives to increase strength or decrease set times. Avoid these – they are much more expensive. Furthermore, the results obtained when using blocks made from these materials may not be useful for comparative purposes (see below). Garden variety Plaster of Paris is what you want. It is available at any craft store (Wal-Mart has it in their craft/hobby section) or home improvement center (Lowe’s sells it as wall plaster).
- Measuring cup (marked in US cups and milliliters)
- Mixing spoon
- Mixing bowl
- Contact cement (Elmer’s Contact Cement)
- Ice cube tray (full size)
- Plastic material for the cards’ bases
- 5-gallon bucket
- Means to weigh the cards (see below for details)
The Scale
The greatest challenge for hobbyists will be that of accurately measuring the amount of gypsum, as well as ‘before’ and ‘after’ weights of the cards. Postal scales do not offer the resolution needed, so I looked for relatively inexpensive options. After some research, I decided to try a digital ‘pocket scale’ manufactured by My Weigh. The particular model, Durascale 50, is capable of weighing 50 grams with a resolution of 0.01 gram. It offers a 12 mm (1/2″) back-lit LCD display, a tare capability, a stainless steel weighing platform (the plastic protective cover doubles as a larger weigh boat), an auto-off feature, and comes with a 50 gram calibration weight, 4 AAA batteries and a carrying pouch. This device retails for $58.90 (US) at the time of this writing. Shipping (USPS Priority Mail) for an additional $8.95, for a total of $67.85 US. I obtained this unit from Precision Weighing Balances in Bradford, Massachusetts (www.balances.com). I ordered on a Sunday, received electronic confirmation of the order and a Priority Mail tracking number that afternoon. The scale was delivered the following Thursday. Considering this package traveled almost 6,000 miles in 4 days, I can truly say I am most impressed with services of the vendor and the postal service. For a photo of the scale, see Figure 2.
I decided to check the performance of the Durascale 50 against full-blown analytical balances in my lab (a Sartorius Basic and an Ohaus Adventurer).
These are the results:
| Milligrams | Analytical Balance (grams) | DuraScale (grams) |
|---|---|---|
| 10mg | 0.0104 | 0.00 |
| 20mg | 0.0222 | 0.00 |
| 30mg | 0.0332 | 0.03 |
| 50mg | 0.0521 | 0.05 |
| 100mg | 0.1000 | 0.10 |
| 500mg | 0.5063 | 0.51 |
| 1000mg | 1.0000 | 1.00 |
| 2000mg | 1.9999 | 2.01 |
| 5000mg | 5.0000 | 5.00 |
| 10,00 mg | 10.0004 | 9.99 |
| 20,000mg | 19.9918 | 19.99 |
| 40,000mg | 39.9885 | 39.96 |
| 50,000mg | 49.9883 | 49.92 |
As Table 1 shows, the DuraScale is useless for making measurements below about 30 milligrams, but compares favorably with weights above that threshold. Its upper limit is 50 grams (some instruments report results slightly higher than advertised – the DuraScale does not). Even with these limitations, the DuraScale is a fine instrument for the money and I highly recommend it for fine-scale measurements.
Procedure for Making the Clods
Find a sheet of suitable inert material, such as acrylic or plastic (bases cut from plastic lids from bottles containing peanut butter, kalkwasser, or whatever are OK if you don’t want to buy a thin sheet of acrylic. In any case, the material should be rigid and not flex in the water currents when that time comes). This will serve as the base to which the plaster clod is glued. Each base should be about 2″ x 3″ (0.5 x 1.2 cm). Drill at least two small holes in the base in order to later attach the clod to a weight if measuring high velocity flows. I use a 3 pound plastic-coated lead dive weight.
Pick an area to work where making a mess will not bother anyone – Plaster of Paris is a very fine powder and tends to find its way to places other than where you’re working.
Since Plaster of Paris absorbs moisture over time, it should be ‘activated’ before use by baking at about 176°F (80°C) for 2 hours. Allow the material to cool. I would suggest making a full tray of clods (usually 16) – this will require about 500 grams (1.1 pounds) of plaster.
There are two methods for mixing – both will deliver clods of almost equal quality. However, choose one method and use it consistently – do not mix clods made from different methods in order to assure consistent results.
Meticulous Method: Since you should have a scale available, I would suggest weighing out the material for best results. After weighing 500 grams of plaster, add it to a container holding 450 milliliters of cool (room temperature) water, while stirring with a spoon. Work rapidly, as the mixture begins to set quickly. Pour the liquid into the tray. Once the tray is full, tap the tray sharply on the counter for two minutes to remove entrapped bubbles. Using a toothpick to probe the tray for air bubbles might also help ensure clods with smooth, uniform surfaces
Quick & Easier Method: 1 level tablespoon measures out about 10 grams of plaster – this is OK for small batches or if you’ve got a few minutes to scoop plaster. To each 10 grams, add 9 grams (milliliters) of water. If mixing a full tray, add 3 cups of Plaster of Paris to 1.85 cups (440 ml) of cool, clean water. Mix and pour as above.
Let the mixture dry for an hour or so and then carefully pop each cube from the tray. The clods should be hard (not crumbly), well-formed with smooth surfaces and without cracks.
DO NOT ATTEMPT TO DISCARD UNUSED MATERIAL DOWN A DRAIN. It is possible that the plaster will set up in the pipe, and clog the drain!
Sand the bottom of the clods to bring the weight to within 0.4 grams of each other. Glue the clods to the plastic bases using the rubber contact cement and allow everything to air-dry for two weeks.
Optional: Just before use, I prefer to again bake the clods in an oven at 176°F (80°C) for 2 hours. Place the cards on a piece of glass to keep the bottoms smooth.
No matter which drying method is used, once the clods are acceptably dry, carefully weigh them on the scale and record the weight on the bottom of the plastic base with an indelible marker, such as a Sharpie™.
Helpful References
- The Classic Formula: Mix 45 milliliters water to 50 grams calcium sulfate
- 1 level tablespoon of Plaster of Paris = ~10 grams
- Multiply grams of dry Plaster of Paris by 0.9 to find number of milliliters for mixing
- 1 U.S. Cup = 236.588 milliliters
- 1 cubic centimeter = 1 milliliter = 1 gram (at about room temperature)
- ‘Standard’ ice cube tray (16 cubes 2″ x 1″ x 1″) holds 680 ml water
- 1 U.S. Cup = ~163 grams Plaster of Paris (dry)
- 1 pound (454 grams) of Plaster of Paris will make about 14 clods
Factors Affecting Dissolution
Although the diffusion card method is relatively easy, reasonable care must be taken in order to obtain reliable results. Several variables should be controlled as closely as possible.
Water Volume of Calibration Chamber
The ‘calibration chamber’ is simply a 5-gallon bucket filled with water of the same properties as the aquarium. Five gallons has been determined to be the proper volume since smaller volumes might become saturated with gypsum. Placement of the clod within the calibration chamber is important as well – it should be elevated off the bottom to prevent it from resting in the elevated concentration of gypsum solute. Once the control is begun, do not move the bucket – it must remain motionless for the period of the test.
Temperature
Temperature affects dissolution rate, hence control and test clods should be in water of the same temperature. For aquaria, placing the 5-gallon bucket in the sump is the easiest answer. If not, you’ll have to devise a method to keep the control within a degree or two of the aquarium.
Salinity
Salinity should be the same for all test and control clods.
Clod Variability
Even when quality control while making the clods is closely observed, there will be some variability among them. Hence, use only clods from the same batch.
Surface Area
Jokiel and Morissey (1993) determined that weight loss of the clod is linear until the surface area falls below about 30%. The size of the clod is hence an important factor. When testing high water velocities, use clods made in a standard ice tray (cubes about 2″ long x 1″ wide x 1″ high – 2cm x 1 cm x 1cm).
Procedure
Calcium and sulfur (and various contaminants) will be added to the aquarium water. Although this procedure has been used safely many times in aquaria, it is best to perform water flow measurements just before a water change.

Figure 3. The control clod (left) will lose only a slight amount of weight while the experimental clod (right) will dissolve at a rate proportional to water motion.
Place the control card and test card in their respective places for 24 hours. Remove, gently rinse with fresh water, and allow drying for three weeks (I prefer to bake the cards as previously described at 176°F (80°C) for 2 hours to expedite getting results. The cards should have an appearance similar to those shown in Figure 3).
Weigh the dry clods and record the weight. Calculate the Diffusion Factor using this formula: Dry weight of the card after immersion divided by the initial dry weight
Example:
- Weight loss of control card = 0.7324 grams
- Weight loss of test card = 15.0689 grams
- 15.0689 divided by 0.7324 = 20.57 Diffusion Factor
| Control | Test | |
|---|---|---|
| Initial Weight (grams) | 29.465 | 31.2401 |
| Final Weight (grams) | 28.7326 | 16.1712 |
| Difference | 0.7324 | 15.0689 |
| Diffusion Factor = 20.57 |
If using a scale (such as the DuraScale), the answer, using the above numbers two places to the right of the decimal and rounding off, would be: 20.68 Diffusion Factor – close enough for our purposes!
Interpreting the Results
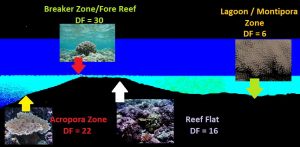
Chances are you’ll arrive at a result in a range of 2 to 25. Without references, this number is meaningless. Fortunately, a number of researchers have used the materials and methods previously described, and arrived at Diffusion Factors on various parts of natural reefs. See Figure 4.
Acropora Zone
Jokiel and Tyler (1992) measured diffusion rates at Johnston Atoll. He found the reef seaward of the reef crest there dominated by growths of Acropora cytheria (a ‘table top’ Acropora species). They describe the water motion as ‘moderate’ with a Diffusion Rate of 22. ‘Moderate’ means the flow is sufficient to bathe the animal with saturating flow, but is usually not violent enough to break the coral.
Breaker Zone / Fore Reef
This area receives the full brunt of breaking waves. At Johnston Atoll, this portion of the reef is sparsely inhabited by only a few coral taxa, including the Lobe coral (Porites lobata) and the Cauliflower coral (Pocillopora meandrina). At first glance, it would seem these tortured corals require violent water flow but further investigation finds they only tolerate the fury of the ocean as their skeletons are exceptionally strong and resistant to breakage (Rodgers, 2001). Jokiel and Tyler (1992) found a Diffusion Factor of about 30 in the breaker zone.
Note: Yates and Carlson (1992) noted than husbandry techniques for Pocillopora meandrina were unsuccessful in early attempts at the Waikiki Aquarium. This coral is known to prefer areas of strong water motion, and we can speculate that insufficient water movement was the culprit.
The Reef Flat
A reef flat is generally that area of fairly shallow water between the breaker zone and the beach. Once the breaker/surf zone dissipates much of waves’ energies, the reef flat receives good amounts of water movement. Since this area is fairly well protected, coral growths begin to flourish as they should – energy from the sun and waves makes an ideal spot for corals. Jokiel and Tyler found this portion of Johnston Atoll reefs was characterized by a diverse coral community, including Acropora valida, Pocillopora meandrina and Millepora tenera. Rodgers (2001) found a Diffusion Factor of 16 on the reef flat at Kahalu’u Beach Park, Big Island of Hawaii. Interestingly, this beach is less than a mile from my lab and is a favorite spot for coral observations. In fact, 3 of the 4 photos in Figure 5 were taken there. Kahalu’u’s reef flat is inhabited by almost every Hawaiian shallow-water scleractinian coral including Porites lobata, Porites evermanni (lutea), Pocillopora meandrina, Pocillopora eydouxi, Pavona varians, Leptastrea bewickensis, and the soft coral Sarcothelia (formally Anthelia). Notably very scarce or absent are Porites compressa and any of the fungiids including Fungia and Cycloseris species. Porites compressa favors water with much less water motion, including sheltered areas (such as Honaunau, Big Island of Hawai’i) or deeper waters (greater than 40 feet, personal observations). In fact, Porites compressa is easily broken by strong natural water motion.
The free-living Fungia and Cycloseris would not last long as Kahalu’u’s water motion would keep them rolling on and being abraded by the substrate.
The Lagoon / Montipora Zone

Figure 5. Force required to cause deformation of various coral skeletons, reported in units of megapascals. See text for details and conversion information. From Rodgers, 2001, and Denny, 1988.
Here, in the lagoon, water motion has dropped substantially and suspended particles once held in suspension began to settle. Water clarity falls as well, and corals preferring lower light levels and calm water begin to become predominant. The Diffusion Factor falls to 6.5 here. Montipora species include Montipora capitata (verrucosa) and M. verrilli.
If there is any doubt that we do not want to truly replicate the power of the ocean in our aquaria (even if we could), let the information shown in Figure 5 to persuade you.
These researchers examined tensile strength of various coral skeletons, and determined the amount of force per unit area to deform (cause ‘necking’) them. The tensile strength is reported in units of megapascals (1 megapascal is equivalent to 145.0377 pounds per square inch). Hence, a Montipora capitata (plating form) is distorted (or broken) at about 392 psi, when the surf-loving Pocillopora meandrina is broken by about 1,000 psi.
This article has presented an inexpensive method for determining water motion. It is simple and inexpensive. When proper attention is given to quality control while making the clods, the results are useful for comparative purposes.
Questions? Comments? Please leave them below, or contact me at [email protected].
References
- Denny, M., 1988. Biology and Mechanics of the Wave-Swept Environment. Princeton University Press, Princeton, New Jersey. 329 pp.
- Doty, M., 1971. Measurement of water movement in reference to benthic algal growth. Botanica Marina, 14: 32-35.
- Jokiel, P. and W. Tyler, III, 1992. Distribution of stony corals in Johnston Island atoll. Proc. 7th Int. Coral Reef Symp., Guam. 2: 683-692.
- Jokiel, P., and J. Morrissey, 1993. Water motion on coral reefs: evaluation of the ‘clod card’ technique. Mar. Ecol. Prog. Ser., 93: 175-181.
- Rodgers, K., 2001. A quantitative evaluation of trampling effects on Hawai’i’s coral reefs. Master’s Thesis, University of Hawai’i.
- Yates, K. and B. Carlson, 1992. Corals in aquariums: How to use selective collecting and innovative husbandry to promote reef conservation. Proc. 7th Int. Coral Reef Symp., Guam, 2: 1091-1095.




0 Comments