

A species-rich mesophotic reef around Bunaken Island, Sulawesi, at a depth of 40 m (133 ft.). Photograph by Pim Bongaerts, Ph.D.
Recent scientific findings suggest that these so-called mesophotic reefs can act as refugia for many reef species, including fishes and corals, which may help repopulate shallow reefs after periods of environmental stress. This article will describe some of the mesophotic coral reefs found around the world, illustrated with photographs taken during deep dives.
Although coral reefs around the world have been studied since the time of Charles Darwin (1809-1882), it took many years before we developed a basic understanding of these fragile ecosystems. It is now agreed that the many forms of symbiosis between corals, sponges, zooxanthellae, bacteria and other species is the key to the success of coral reefs. Growing in the marine equivalent of a desert, with very low concentrations of inorganic nutrients, the constant exchange and recycling of matter allows reefs to persist (De Goeij et al. 2013).

A vertical rock wall, heavily encrusted with sponges. Note the Longnose Butterflyfish (Forcipiger flavissimus) in the center. Photograph by Pim Bongaerts, Ph.D.
Coral reefs are usually portrayed as colorful ecosystems growing in warm, clear and shallow waters, and this is how we usually enjoy them when diving or snorkelling. What is less known is that many reefs actually extend into deeper waters, with zooxanthellate corals found down to at least 165 meters (550 feet) in depth. These so-called mesophotic reefs (from the Greek words mésos, or middle, and phōt, or light) are currently defined as occurring between 30 and 200 meters in depth (Olson and Kellogg 2010). They are home to countless animal and plant species, and some of them only exist in these deeper waters.
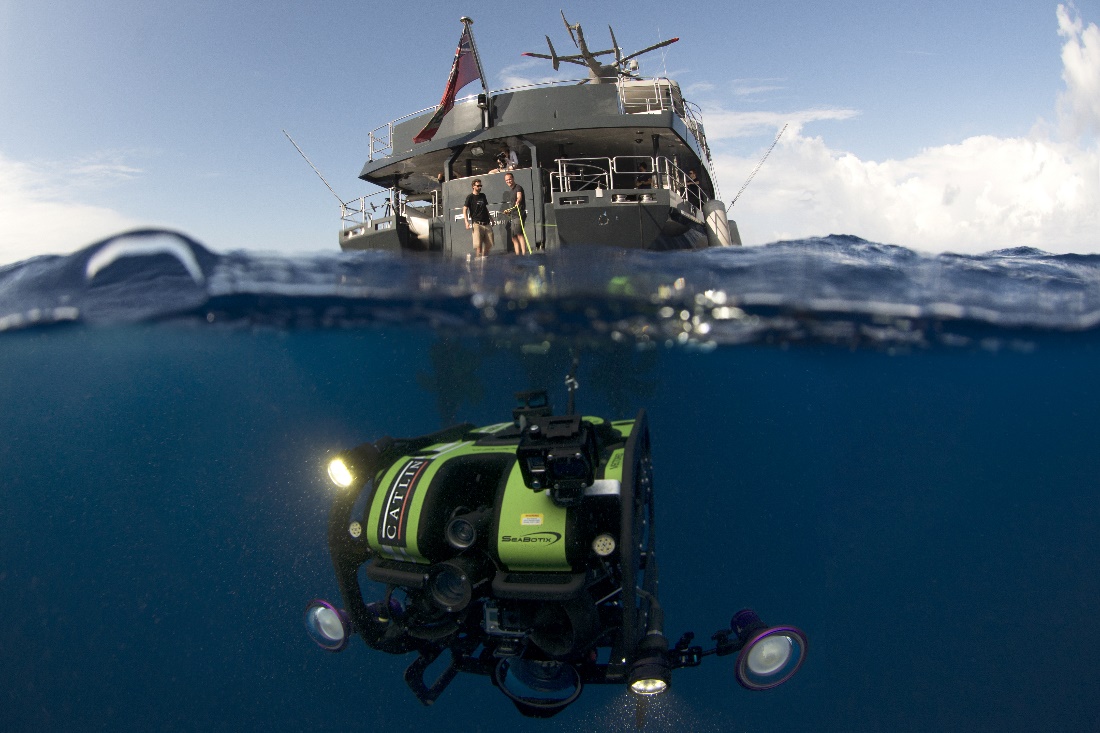
Due to their inaccessibility, mesophotic reefs have been the subject of limited scientific study. Today, modern technologies such as rebreathers, submersibles and ROV’s allow scientists to explore even the deepest reefs. Over the following years, marine biologists will uncover more about the ecology and biodiversity of mesophotic reefs, as well as the genetics and physiology of its key members, the stony corals. Here, Dr. Pim Bongaerts of the XL Catlin Seaview Survey team operates an ROV on the Great Barrier Reef. Image by Richard Vevers.

Plunging into the deep. In the abyss, lots of marine life awaits discovery. Note the various crinoids on the ridge. Photograph by Pim Bongaerts, Ph.D.
https://www.youtube.com/watch?v=AAVTynS4lIo&feature=player_embedded
Deep refugia

A deep Indonesian reef, densely populated by stony corals, gorgonians, soft corals, sponges, crinoids, coralline algae and damselfishes. Such reefs may act as a “safe house” for vulnerable species. Photograph by Pim Bongaerts, Ph.D.
What makes mesophotic reefs important is their high biodiversity, as well as their potential to serve as deep refugia, safe havens, for many species. This is because their depth renders them less susceptible to natural and human impacts (Bak et al. 2005; Bongaerts et al. 2010, 2011a,b). For example, there is evidence that deep reefs can escape damaging storms and coral bleaching events, although they are not completely immune to stressors.
Recent evidence shows that about 25% of all zooxanthellate coral species, i.e. corals hosting symbiotic dinoflagellates of the genus Symbiodinium, has a depth distribution from shallow waters down to mesophotic depths of 30-60 meters (van Oppen et al. 2011). In north-east Australia, Muir et al. (2015) found that of the 76 Acropora and Isopora species recorded in shallow waters, 21% extend to mesophotic depths. These so-called depth-generalist species may aid in reef recovery by releasing larvae and gametes from the deep, which eventually could lead to recruitment of new coral colonies on shallow reefs. Indeed, using DNA analysis, van Oppen et al. (2011) found that the brooding coral Seriatopora hystrix releases larvae in deeper waters which settle on shallow reefs. However, they also found that this phenomenon depends on the reef investigated. Thus, shallow reef recovery by coral larvae from the deep is limited by species number (depth-generalists) and does not necessarily occur everywhere.

A solitary Majestic Angelfish (Pomacanthus navarchus) forages on a mesophotic reef, with large stands of Pachyseris spp. Photograph by Pim Bongaerts, Ph.D.
Environment: light, temperature, water flow and nutrients
Mesophotic reefs extend far beyond their shallow counterparts, and they are worlds apart. Below 30 meters, warm, sunlit waters quickly fade into a gloomy coldness. For example, corals growing at 100 m depth (333 ft.) and below need to survive in an environment with less than 1% of maximum solar irradiance, temperatures of 20 degrees Celsius (68°F) and below, limited water flow and elevated nutrients. If one were to set up a dark, unheated aquarium with hardly any water flow and no filtration, and fill it with stony corals from the deep, it may raise a few eyebrows. However, this would come close to conditions experienced by corals from the deeper mesophotic zone. Below, I will discuss four major abiotic factors that define the mesophotic zone, with a depth between 30 and 200 meters (100-667 feet).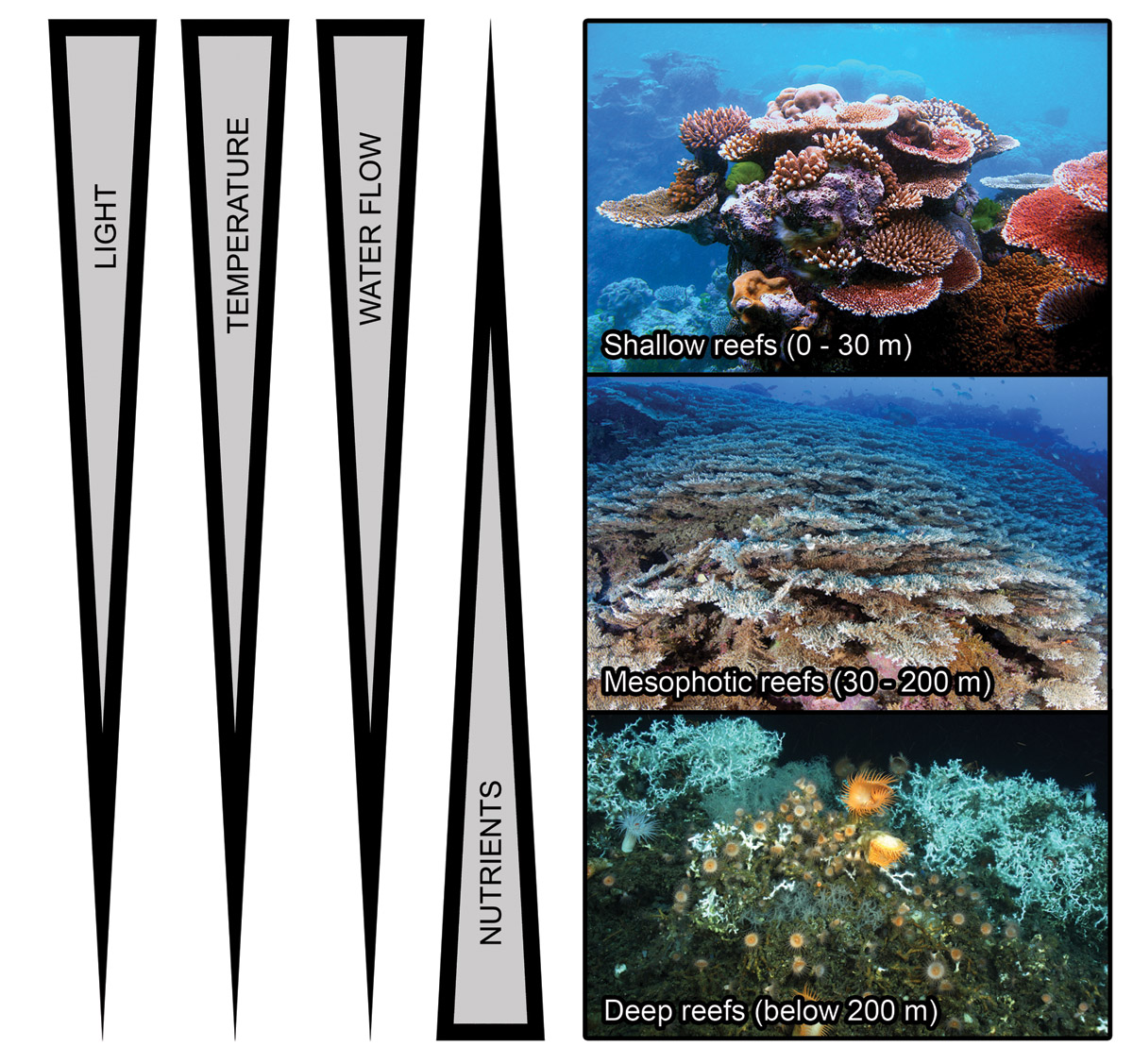
In terms of depth, coral reefs can be roughly divided into three major types; shallow reefs, with a depth range of 0-30 meters (0-100 feet), mesophotic reefs, occurring between 30 and 200 meters in depth (100-667 feet), and deep reefs, found below 200 meters (667 feet). Shallow reefs harbor a wide species range of light-dependent corals, sponges and (macro)algae. Mesophotic reefs are populated by fewer coral species, with often higher percentage cover. Here, corals have adapted their shape and physiology to this dimly lit environment, with flattened colonies and higher light-capturing efficiency. Both shallow and mesophotic reefs have a combination of zooxanthellate and azooxanthellate corals. Below 200 meters, virtually no light penetrates and so almost all corals found here lack zooxanthellae. In these deep waters, the scleractinian coral Lophelia pertusa is a major reef builder. Arrows indicate generalized gradients in terms of light intensity, temperature and water flow rate, which decrease with depth, and inorganic nutrients, which increase with depth. Image adapted from Lesser et al. (2009) and Olson and Kellogg (2010), with photographs by Wikimedia Commons/Lophelia II 2010 Expedition/NOAA-OER/BOEMRE/Muir et al. (2015).
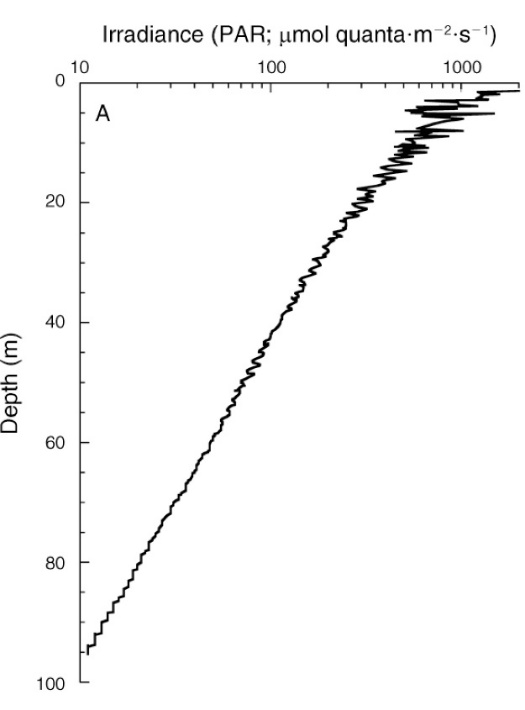
Irradiance depth profile between 0 and 95 m (0-317 ft.) at Bock Wall, Lee Stocking Island, Bahamas. Surface irradiance was 2,478 µmol photons m-2 s-1 (~400-700 nm). Note that the x-axis is plotted on a log scale, resulting in a straight curve, and that PAR levels are expressed as absolute values. From Lesser et al. (2010).
Light
When we dive into the ocean, both the intensity (irradiance) and spectral quality of light are affected. The deeper we go, the lower the light intensity, as light is absorbed and scattered by seawater and its constituents. On a sunny tropical day, around noon, light intensity at the surface is approximately 2,500 µmol photons m-2 s-1, measured as photosynthetically active radiation (or PAR, ~400-700 nm,Frade et al. 2008; Lesser et al. 2010). At 5 meters (17 feet) depth, this has already decreased to about 1,000 µmol photons m-2 s-1, and at a typical mesophotic depth of 60 meters (200 feet), irradiance has dropped significantly to only 50 µmol photons m-2 s-1.
To put these values into perspective: most reef aquaria experience light intensities in the range of 100 to 1,000 µmol photons m-2 s-1, corresponding to water depths of approximately 40 to 5 meters (133 to 17 feet). However, it is important to note that in the field, the irradiance profile is parabolic, i.e. light intensity is low in the morning and evening, and peaks at noon. In addition, irradiance levels in nature are significantly affected by season, latitude and weather. On a cloudy day, for example, significantly less light penetrates the water, which also shifts to the blue end of the light spectrum. Water clarity also has a major impact on coral depth distribution. In the Red Sea and around Hawaii, with very clear waters, the maximum depth of zooxanthellate corals is much lower at 145-165 m (483-550 ft.) compared to areas with more turbid waters such as Curaçao (80 m or 267 ft) and East-Florida (40 m or 133 ft, Kahng et al. 2010).
It is known that stony corals can adapt to low light levels in aquaria, although their growth rates and coloration are affected (Wijgerde and Laterveer 2013; Wijgerde and Tilstra 2014). In the field, similar observations have been made, with the record-holding zooxanthellate stony coral Leptoseris hawaiiensis found down to at least 165 meters (550 feet)in depth (Maragos and Jokiel 1986). At its maximum record depth, this coral species receives only 0.02% of surface solar irradiance, or between 0 and 0.5 µmol photons m-2 s-1. This is equivalent to a dimly lit hallway at night. To survive in this dark environment, deep water corals have evolved several adaptations. Firstly, their endosymbiotic zooxanthellae produce more chlorophyll and other photopigments to enhance light-use efficiency (Frade et al. 2008; Lesser et al. 2010). In addition, the corals themselves alter their morphology, with laterally flattened branches, plate-like growth forms and more widely spaced corallites, most likely to collect more light (Lesser et al. 2010; Muir et al. 2015). The coral skeleton acts as an efficient light collector, scattering light in such a way that the zooxanthellae can effectively use it (Enriquez et al. 2005).
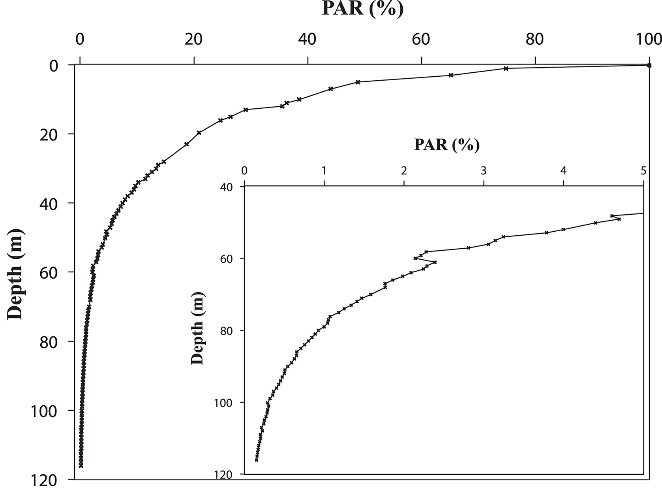
Irradiance depth profile between 0 and 115 m (0-383 ft.) in the Gulf of Aqaba off the Interuniversity Institute in Eilat in October, measured as photosynthetically active radiation (PAR, ~400-700 nm). Surface irradiance was relatively low during this measurement due to the time of year, at 1,400µmol photons m-2 s-1. Note that the x-axis is plotted on a linear scale, resulting in a hyperbolic curve, and that PAR levels are expressed as percentages. From Eyal et al. (2015).
Next to irradiance, light spectrum changes with water depth. The deeper we travel into the water column, the more natural sunlight is modified by the selective filtering properties of seawater. Red light, with the largest wavelength and lowest energy photons within the visible spectrum, is quickly attenuated by seawater. It penetrates no more than approximately 10 meters (33 feet) in tropical seawater, although this value varies with location and time (Mass et al. 2010). At 40 meters depth and below, light with wavelengths of 600 nm and beyond (i.e. orange, red and infrared) does not penetrate (Rivero-Calle et al. 2008; Mass et al. 2010). Thus, seawater acts as a chromatic filter, with blue and green becoming dominant in deeper waters.

Contrary to popular belief, Acropora spp. can grow under low light levels. In the deep water environment, corals and zooxanthellae adapt their physiology and morphology to efficiently capture the scarcely available blue light. Photograph by Pim Bongaerts, Ph.D.
Adaptation to a predominantly blue environment may explain why blue light alone seems sufficient to maintain healthy corals and zooxanthellae in aquaria, the latter both in hospite (growing naturally in the coral) as well as ex hospite (living outside the coral in culture flasks, Kinzie et al. 1984, 1987; Wang et al. 2008; Wijgerde et al. 2014). Corals from deeper waters also use blue light more effectively for photosynthesis when compared to shallow corals, suggesting a phenomenon known as chromatic adaptation, i.e. adaptation to a specific light spectrum or color (Mass et al. 2010). This adaptation may occur via increases in the synthesis of various photopigments by zooxanthellae, such as chlorophyll a and the carotenoid peridinin.

In deeper waters, corals often produce more photopigments such as chlorophylls to capture light more efficiently. Note the brown plate coral Pachyseris speciosa in the center, and the brownish pigmented tips of the Seriatopora hystrix colony in the lower right, both indicative of increased chlorophyll a synthesis. Photograph by Pim Bongaerts, Ph.D.
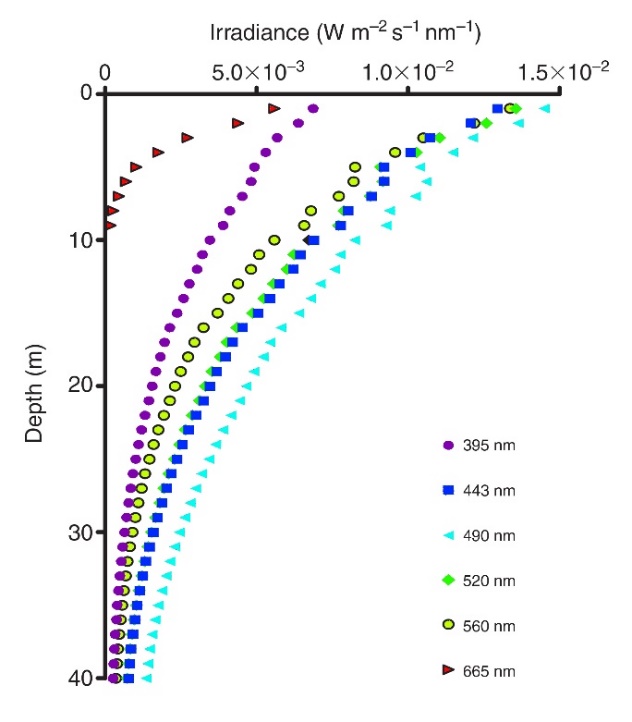
Irradiance profiles (395-665 nm) measured in coral reef waters in Eilat, Israel. Red and orange (not shown) wavelengths do not penetrate deep into seawater, resulting in blue-green water beyond 10 meters in depth. Adapted from Mass et al. (2010).
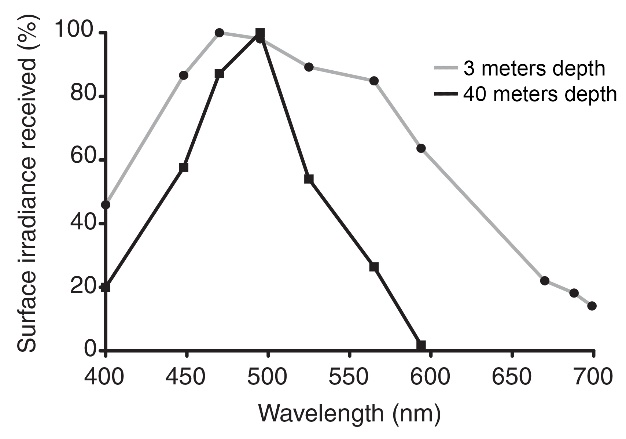
At 3 meters depth, all colors penetrate. At 40 meters depth, however, light with wavelengths above 600 nm (orange, red and infrared) is completely absent. Interestingly, cyan light (490 nm) remains its potency at 40 meters depth, although this depends on location and time of year. Adapted from Mass et al. (2010).
Temperature

By placing digital loggers in deep waters using submersibles, scientists can record fluctuations in temperature, light intensity and other abiotic factors on mesophotic reefs. Note the whip corals in the background. Location: Curaçao. Photograph by Pim Bongaerts, Ph.D.
Water temperature around coral reefs decreases with depth, although this relationship is strongly affected by location and time of year. Around the Florida Keys, for example, water temperature shows a steep decline of about 5 °C (9 °F) between 25 and 50 meters (83 to 167 feet). Beyond, it slowly drops to lower temperatures in deeper waters, with a chilly 12 °C (54 °F) at 120 meters (400 feet) depth. Although other locations and seasons show higher temperatures in deeper waters (Rooney et al. 2010), measurements reveal that mesophotic reefs can be exposed to a wide temperature range of roughly 12 to 29 °C (54 to 84 °F). This could (partially) explain the occurrence of different species across a depth gradient, as not all corals may be able to tolerate such temperature extremes. It also demonstrates that many tropical stony corals can adapt to low temperatures as well as low light levels. Interestingly, mesophotic reefs are less affected by high temperatures during summer periods, which allows them to thrive even during mass bleaching episodes on shallow reefs (Lesser al. 2009).

Stony corals such as Acropora spp. change their morphology with depth. (A) Deep-water specialists A. pichoni collected at 40 m and (B) A. tenella collected at 52 m, (C) depth generalist A. echinata collected at 40 m, (D) typical thick morphology for the shallow intertidal species A. humilis, collected at 1 m, (E) depth generalist A. granulosa from 46 m depth and (F) from 7 m depth. Staghorn corals collected at depths below 40 m generally have laterally flattened branches, a lighter and more fragile skeleton and increased spacing between corallites and branches. Scale bars: 1 cm. From Muir et al. (2015).
Water flow
Next to irradiance and temperature, water flow also diminishes with depth, as wind and tidal forces only act on the upper oceanic layer. An exception to this are hurricanes, which can mix oceanic waters to at least 200 meters deep (Lesser et al. 2009). Thus, mesphotic reefs generally inhabit a low energy environment, with reduced water flow around corals. Low water flow, in concert with low light levels, results in morphological adaptations by corals as described above. Next to forming plate-like morphologies, corals become more delicately branched, with thin and fragile colonies (Kahng et al. 2010; Done 2011). This may be due to the lack of a need for a robust skeleton in the absence of strong wave action.
Nutrients
Shallow reef waters are known to be exceptionally poor in dissolved nutrients, especially nitrate and phosphate (Lesser et al. 2009). In contrast, deeper waters show elevated nutrient levels. For example, the nitrate concentration around the Florida Keys is about 0.1 µmol L-1 (0.006 mg L-1 or ppm) at the surface, and gradually increases to approximately 24 µmol L-1 (1.49 mg L-1) below 200 meters depth (Lesser et al. 2009). This increase in nitrate with depth is caused by upwelling, a phenomenon during which nutrients are transported from the deep to surface waters. Upwelling stimulates the growth of phytoplankton (algae and cyanobacteria), which is measured by chlorophyll a, a photosynthetic pigment. In the Keys, nutrient upwelling results in a chlorophyll peak around 60 meters (200 feet).
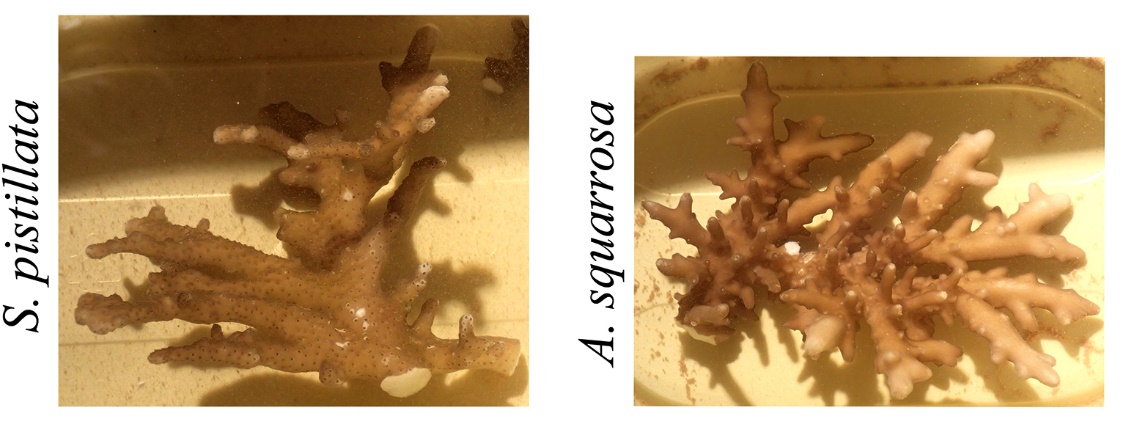
When corals adapt to very low light levels, their morphologies can be altered so dramatically that species identification can be challenging. These photographs show daylight appearances of Stylophora pistillata and Acropora squarrosa collected from ~60 m (200 ft) depth. Note the thin and flattened branches. Adapted from Eyal et al. (2015).
Although mesophotic corals may be adapted to eutrophic (nutrient-rich) waters, experiments by scientists and aquarists over the years has shown that most, if not all corals tolerate elevated nitrate levels. Even though coral growth is generally reduced at higher nitrate and phosphate levels (Marubini and Davies 1996; Ferrier-Pagès et al. 2000; Tanaka et al. 2007; Hylkema et al. 2014), mesophotic corals may ultimately be growth-limited by light availability and sedimentation.
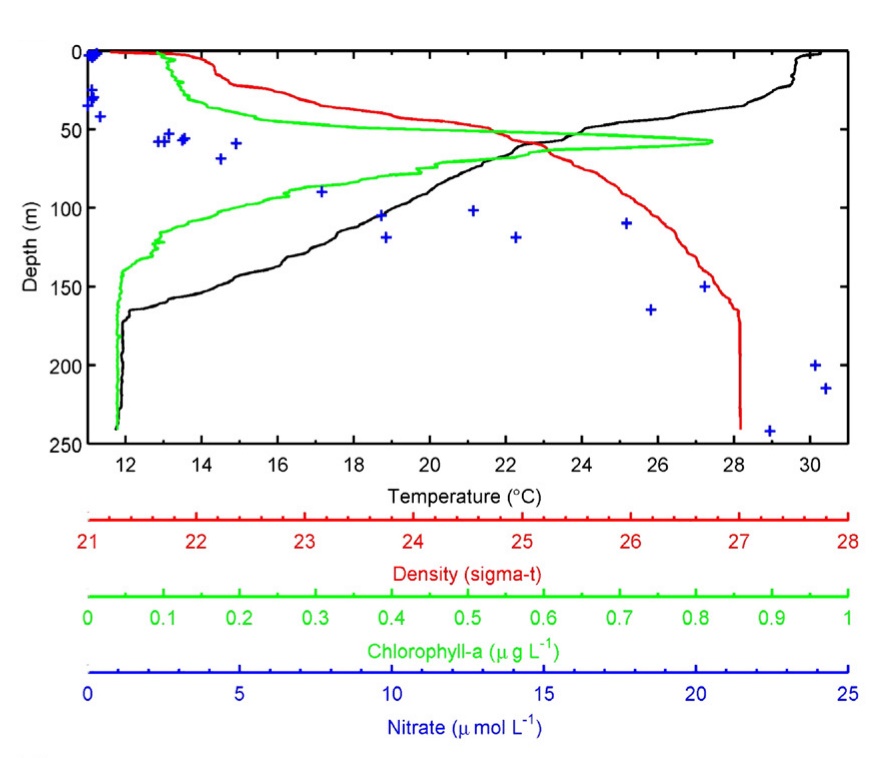
Profiles of temperature, water density, chlorophyll density and nitrate concentration with increasing depth collected off of Florida Keys. Note that these relationships are strongly affected by location and season, and are shown as an example. Based on Lesser et al. (2009) and references therein.
Coral and fish fluorescence in the mesophotic zone
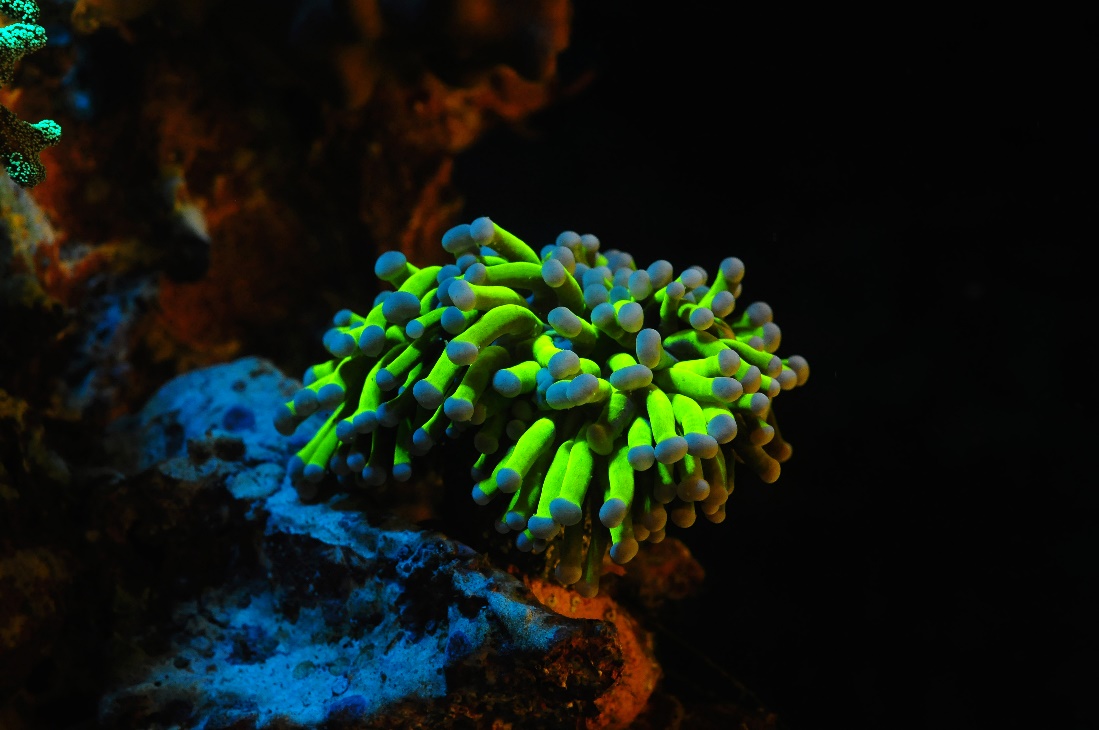
Yellow fluorescence is rare amongst shallow coral species, and is more frequently found in deep water specimens. Conversely, cyan fluorescence is uncommon in deep water corals and frequently found amongst shallow species. It is unclear why. This Euphyllia sp. was photographed using 400-450 nm excitation light and a Cokin yellow long pass filter. Image by Tim Wijgerde.
The fluorescent nature of many corals is well-known by scientists and aquarists alike, where one wavelength (color) of light is absorbed by fluorescent proteins and emitted as a different, but always longer wavelength with lower energy and frequency. For example, when excited by blue light, many corals fluoresce this light as longer wavelength light, in the form of green, yellow, orange and red colors. Although fluorescence is highly attractive, its function remains unclear. Several biological explanations for coral fluorescence have been proposed in the literature. Fluorescence may protect corals from harmful light in shallow water by fluorescing energetic blue light as less harmful green light, and by scavenging oxygen radicals as by-products from photosynthesis (Bou-Abdallah et al. 2006; D’Angelo et al. 2008, 2012; Gittins et al. 2015; Salih et al. 2000). In addition, it may help corals survive in deeper water by improving light harvesting (Eyal et al. 2015).
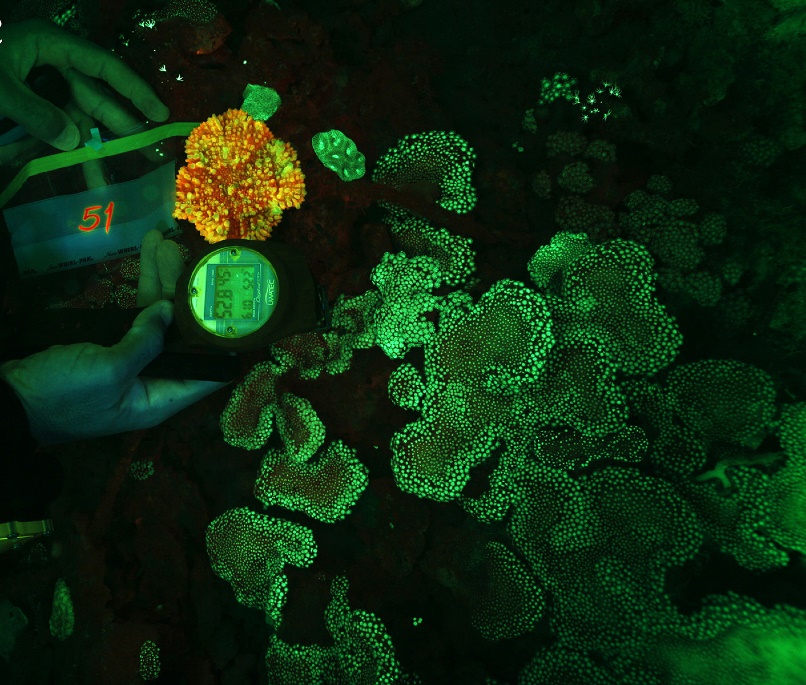
Orange-red fluorescence of Echinophyllia aspera and green fluorescence of Alveopora ocellata at 52.8 m (176 feet) in Eilat, Israel. Image adapted from Eyal et al. (2015).
Interestingly, shallow reef corals show different fluorescence patterns when compared to those from the mesophotic zone. For example, orange-red fluorescence is almost absent in corals from shallow reefs in Eilat, and much more common in specimens found below 40 meters (Eyal et al. 2015). In addition, deep water corals that exhibit green fluorescence maintain their fluorescence intensity irrespective of light levels, in contrast to shallow water corals which show stronger fluorescence under higher light intensity (Leutenegger et al. 2007; Oswald et al. 2007; Eyal et al. 2015). This suggests that fluorescence, in particular as orange-red light, has some as of yet unknown biological function in deeper waters. Possible roles for fluorescent proteins in deep and/or low light environments are enhancement of photosynthesis (Schlichter et al. 1986; Salih et al. 2000), and visual cues for symbiotic fish (Matz et al. 2006) and camouflage against certain fishes (Matz et al. 2006). Enhancement of photosynthesis by fluorescent proteins is substantiated by their localization in the coral’s endoderm (lower tissue layer), underneath zooxanthellae under low light conditions, allowing these proteins to reflect and fluoresce light back to the zooxanthellae (Salih et al. 2000). Conversely, under high light conditions, fluorescent proteins are located in the ectoderm (upper tissue layer), above the zooxanthellae, allowing these pigments to reduce the amount of light reaching the zooxanthellae (Salih et al. 2000). Cyan fluorescence, finally, is uncommon in deep water corals, which seems logical as its photoprotective function is not useful in a low light environment. Shallow corals may use this pigment to reflect excess visible light and fluoresce UV light as blue light, preventing their tissues and symbiotic zooxanthellae from light damage (D’Angelo et al. 2008; Salih et al. 2000).
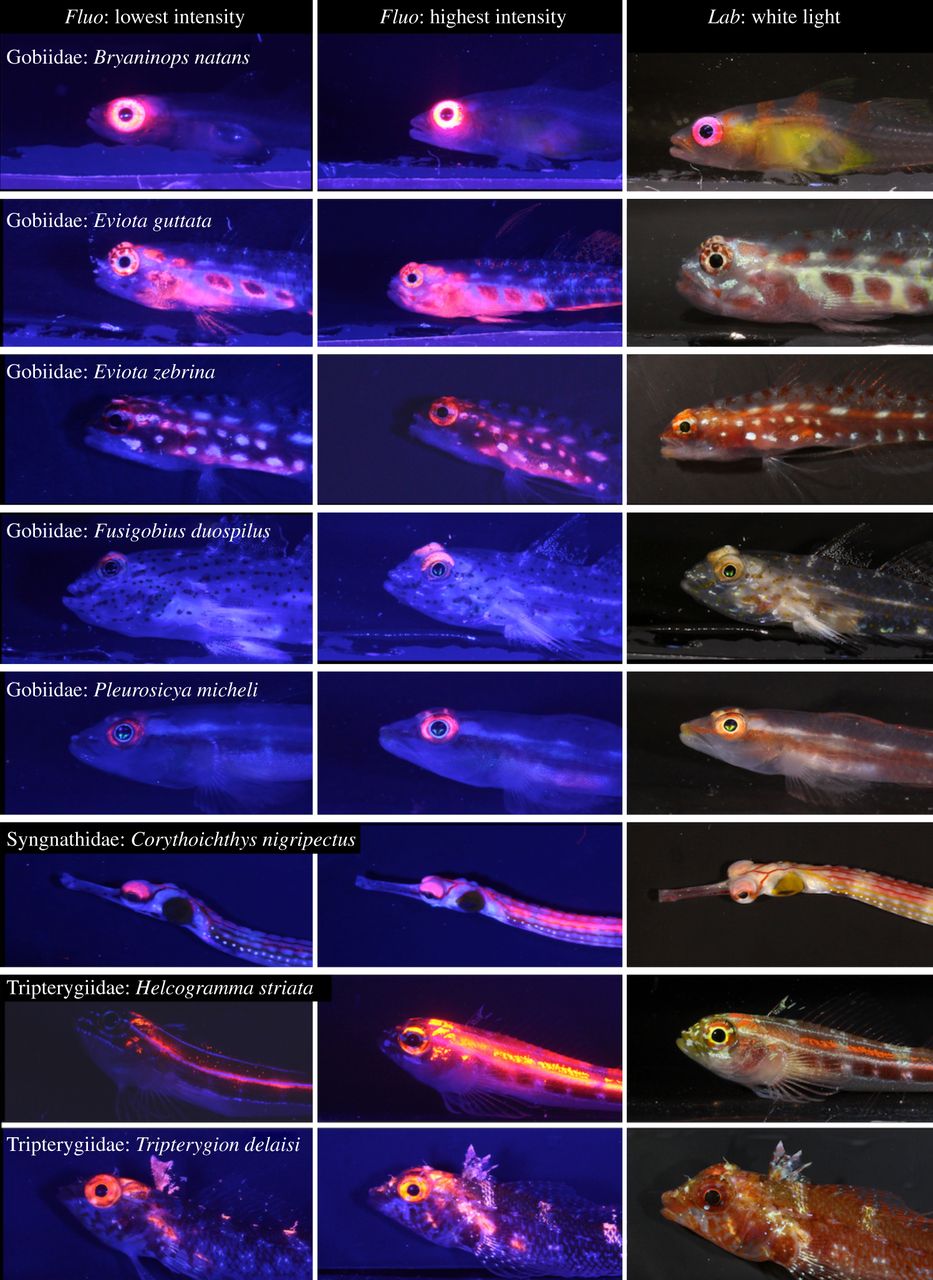
Many small reef fishes display red fluorescence around their eyes, with increased intensity at lower depths. In deep waters, the red glow around their eyes may allow fishes to better detect prey and signal mates. The left and middle columns show photographs taken in the laboratory under blue light, with minimum and maximum fluorescence brightness amongst sampled individuals. The right column shows fishes under standard white light conditions. All fishes are found on coral reefs, with the exception of the Mediterranean species Tripterygion delaisi. From Meadows et al. (2014).
Although corals are amongst the most fluorescent animals on the reef, many other organisms display this feature. These include many fishes, in particular small and cryptic species. Recently, biologists found that members of the Gobiidae, Syngnathidae and Tripterygiidae have striking red fluorescent eyes (Meadows et al. 2014). Interestingly, the intensity of eye fluorescence increases with depth, suggesting that it confers some benefit in gloomy, blue waters. The red glow around their eyes may allow these fishes to detect prey more easily, to signal potential mates and/or to warn rivals in mesophotic habitats.
Plankton and heterotrophic feeding in the mesophotic zone

In the gloomy depths off Curaçao, Dr. Norbert Englebert inspects a large sponge. Photograph by Pim Bongaerts, Ph.D.
It is well-known that corals feed on plankton, detrital matter and dissolved organic matter, a phenomenon known as heterotrophy (reviewed by Houlbrèque and Ferrier-Pagès 2009; Ferrier-Pagès et al. 2011; Wijgerde 2013). Heterotrophic feeding is an important strategy to acquire nutrients to supplement photosynthesis (autotrophy), and this would be particularly useful for survival in a low light habitat where photosynthesis is less effective. However, there is no conclusive evidence that stony corals with symbiotic zooxanthellae increase their feeding rates when growing in the mesophotic zone.
By analyzing stable isotopes of carbon and nitrogen, marine biologists are able to detect a specific chemical signature which reveals how corals obtain their food. For Montastraea cavernosa, isotope analysis of its tissue, skeleton and zooxanthellae has shown that photosynthesis decreases significantly in deeper waters. Although Lesser et al. (2010) suggested that at a depth between 45 and 61 m, feeding on plankton and other particles becomes the dominant feeding mode, a more in-depth isotopic analysis revealed that feeding rates remain similar while photosynthesis decreases (Crandall et al. 2016). In contrast, corals of the genus Agaricia use feeding as a primary mode of nutrient acquisition, regardless of depth (Crandall et al. 2016).

A pair of Raccoon Butterflyfish (Chaetodon lunula) hovers over a barrel sponge. Photograph by Pim Bongaerts, Ph.D.
As the phytoplankton density decreases with depth (Lesser et al. 2010), most likely due to decreased light availability, it would be more difficult for corals to feed on these particles. Similarly, the possible reduction of zooplankton, being largely dependent on phytoplankton, could further reduce feeding rates, even though zooplankton is present at depths of at least 150 meters, or 500 feet (Lesser et al. 2010). The availability of heterotrophic bacteria, protists and detritus (marine snow) in deeper waters may still allow corals to increase their feeding rates. In addition, nutrient upwelling, such as measured in the Florida Keys, can result in plankton blooms in deeper waters which could provide a food source for mesophotic corals.
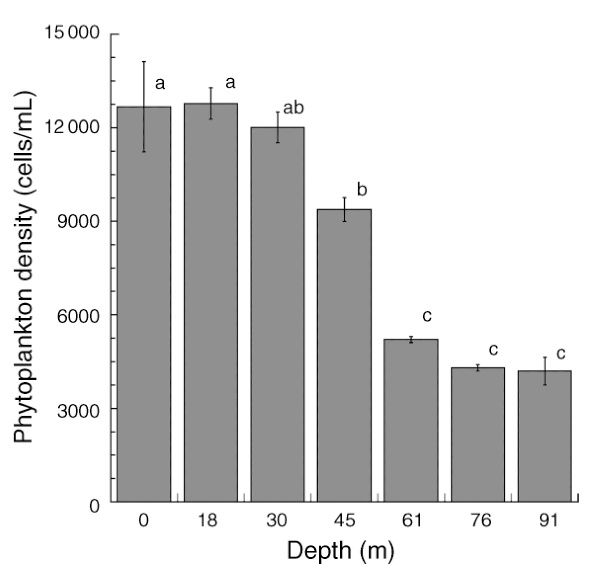
Total phytoplankton (algae and cyanobacteria) available from the surface to 91 m depth at Bock Wall, Lee Stocking Island, Bahamas. Between 30 and 61 meters, phytoplankton density significantly declines, with implications for coral feeding. Adapted from Lesser et al. (2010).
Species, adaptive divergence and speciation

A marine biologist inspects a mesophotic coral reef in Sulawesi (Celebes), which displays incredible biodiversity. Photograph by Pim Bongaerts, Ph.D.
As mentioned above, mesophotic reefs have different species compositions compared to their shallow counterparts. Growing in the gloomy depths requires significant morphological and physiological adapations, and it seems not all species are able to do this. Interestingly, coral species which occur across a broad depth range (depth generalists) could eventually evolve into multiple species due to DNA mutations and subsequent natural selection. Below, I will explain how this works.
The formation of new species is known as speciation, and it starts with random chemical changes known as mutations in DNA-which holds all hereditary information in living cells-that occur in each individual. Sometimes, such a mutation has a beneficial effect, as it will alter a gene-a DNA fragment encoding a protein with a specific function in an organism-in such a way that an individual becomes better adapted to its environment. For example, a genetic change resulting in a more efficient use of sunlight may allow a coral individual to survive in deeper water. Longer survival, in turn, will allow the coral to produce more offspring (sexual reproduction) or clones by fragmentation (asexual reproduction), resulting in more corals with this favorable gene (genotype) and associated physical attributes (phenotype). This process, where better adapted individuals show higher survival and reproduction rates, also known as higher fitness, is called natural selection. The combination of random DNA mutations and subsequent non–random natural selection on those mutations results in evolution, which is a gradual change in the form and function of organisms.
Eventually, a reproductively isolated coral population may arise, if planulae (coral larvae) of shallow-growing corals may have difficulty surviving in a deep, low light environment, and vice versa. If this occurs, at some point, the shallow population may no longer be able to interbreed with the deep population, as they have become too genetically and physically different. By then, when offspring of deep and shallow populations would prove to be principally infertile-such as the mule-these coral populations would be called different species, and their infertile offspring would be considered hybrids. The long genetic road to speciation, caused by random DNA mutations and the subsequent natural selection of those mutations which render individuals better adapted to a habitat, is called adaptive divergence. Indeed, there is evidence suggesting that this phenomenon occurs in corals, such as in the Indo-Pacific coral Seriatopora hystrix (Bongaerts et al. 2011a).
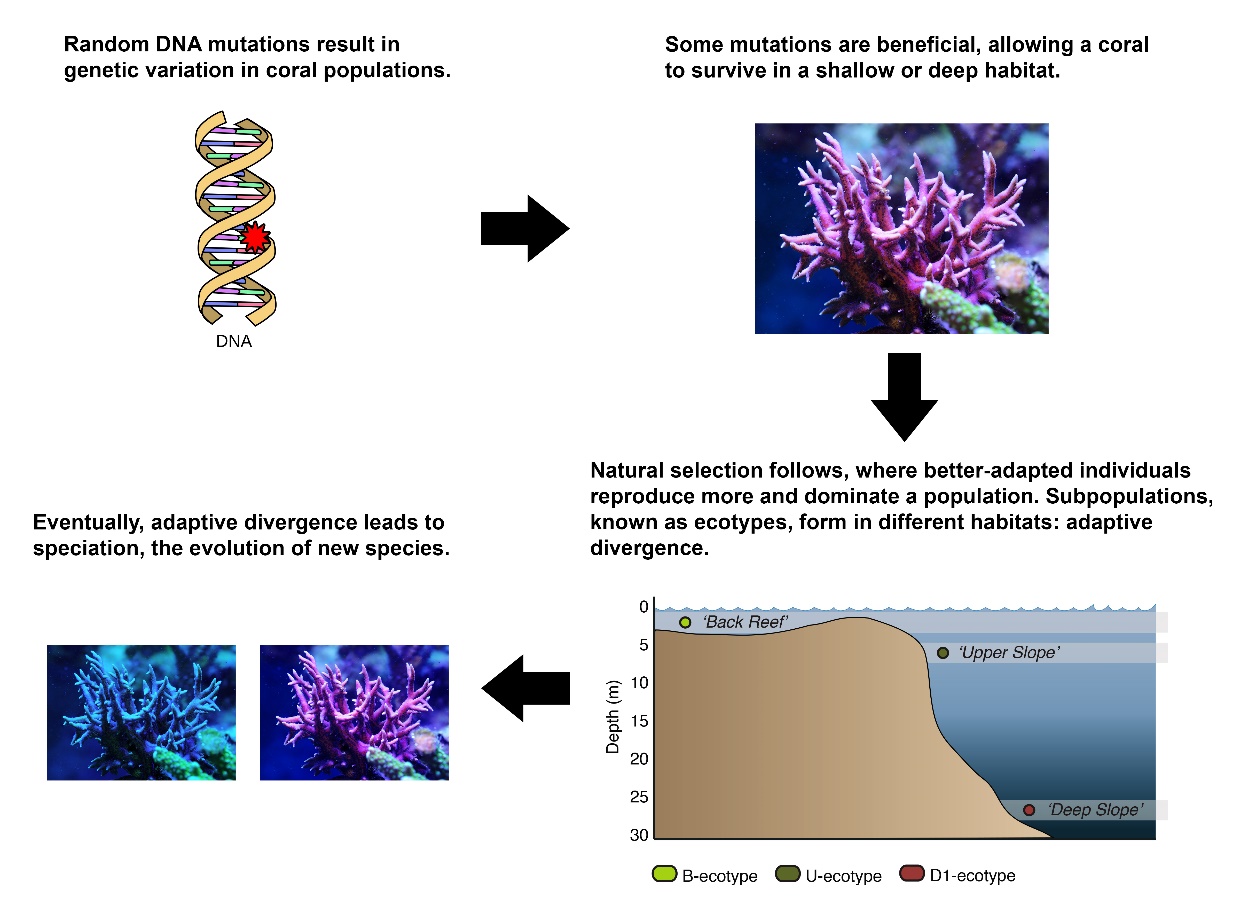
Schematic overview of how a coral such as Seriatopora hystrix could slowly evolve into shallow and deep species. Images by Tim Wijgerde, Forluvoft/Wikimedia Commons and Bongaerts et al. (2011a).
For Seriatopora hystrix, it has recently been demonstrated that various so-called ecotypes exist, populations from different depths with significant genetic and physical varations between them (Bongaerts et al. 2011a). These differences were also found between the symbiotic zooxanthellae of the various ecotypes, indicating natural selection at a so-called holobiont level, i.e. the coral and its associated symbiotic organisms. These ecotypes, most likely resulting from adaptive divergence, may be close to becoming actual different species. This was demonstrated by different survival rates after all three ecotypes were transplanted across various depth habitats. Most notably, the deep ecotype showed higher survival at 25 m (83 ft) compared to shallow ecotypes when the latter were transplanted to the same depth. Vice versa, the deep ecotype was unable to survive between 0 to 5 m (0-17 ft), in contrast to the shallow ecotypes. As the deep ecotype did not exhibit higher photosynthetic efficiency than the shallow ecotypes, its higher survival in deeper water could be due to an enhanced ability to feed on plankton (Bongaerts et al. 2011a). This remains to be determined, however. Even though deep water colonies did not survive in shallow waters during this experiment, there is evidence that on some reefs, S. hystrix larvae find their way back to the surface, and could help repopulate damaged reefs (van Oppen et al. 2011).

Research on mesophotic reefs is technically challenging, as it requires submarines, remotely operated vehicles (ROV’s) and special diving gear. Here, Dr. Pim Bongaerts and his colleagues are transplanting deep water corals for a scientific experiment. Photograph by Pim Bongaerts, Ph.D.
Morphological and physiological adaptation of a coral population to a specific environment, caused by natural selection which weeds out the weaker (less adapted) individuals, has remarkable effects. Most striking to aquarium hobbyists may be the maximum depth at which zooxanthellate corals are found. Acropora spp., regarded in hobbyist circles as “light-demanding”, are found to down to at least 73 m (243 ft) in depth. Here, maximum PAR levels are about 30 µmol photons m-2 s-1, or 1-2% of maximum solar irradiance (Lesser et al. 2010). This would constitute a low light level in an aquarium. Although their growth rates, morphology and coloration are all affected, it is interesting to observe such corals growing in deep waters.
Of all deep-dwelling zooxanthellate stony corals, Leptoseris hawaiiensis takes the cake, growing down to 165 meters (550 feet) in depth, where only 0.02% of sunlight penetrates! Other deep species are Agaricia grahamae at 119 m (397 ft) in the Caribbean and Leptoseris fragilis at 145 m (483 ft) in the Red Sea (Reed 1985; Maragos and Jokiel 1986; Fricke et al. 1987). Tables 1 to 4 below list many coral species, as well as macroalgae and seagrasses, with their maximum recorded depths.
| Species | Maximum depth (m) | Maximum depth (ft) | Location | % of surface irradiance | maximum estimated irradiance (µmol m-2 s-1) | Reference |
|---|---|---|---|---|---|---|
| Acanthastrea echinata | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Acanthastrea echinata | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Acropora abrolhosensis | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora aculeus | 60 | 200 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora austera | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora cardenae | 55 | 183 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora carduus | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora caroliniana | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora cerealis | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora cf. valida | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Acropora cf. valida | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Acropora cf. variabilis (now Acropora valida) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Acropora chesterfieldensis | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora clathrata | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora cytherea | 38 | 127 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora danai (now Acropora abrotanoides) | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Acropora donei | 45 | 150 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora echinata | 60 | 200 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora elegans | 55 | 183 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora elseyi | 34 | 113 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora exilis (now Acropora elseyi) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Acropora florida | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora granulosa | 73 | 243 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora granulosa | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Acropora granulosa | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Acropora horrida | 35 | 117 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora kimbeensis | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora latistella | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora lokani | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora loripes | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora lovelli | 30 | 100 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora microclados | 33 | 110 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora nasuta | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Acropora paniculata | 42 | 140 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora pichoni | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora rambleri (now Acropora speciosa) | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Acropora secale | 30 | 100 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora selago | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora solitaryensis | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora speciosa | 60 | 200 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora subglabra | 33 | 110 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora tenella | 56 | 187 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora torihalimeda | 63 | 210 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora tortuosa | 35 | 117 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora valenciennesi | 45 | 150 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora valida | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora vaughani | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Acropora willisae | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Agaricia fragilis | 107 | 357 | Belize | Busby et al. (1966) | ||
| Agaricia grahamae | 119 | 397 | Bahamas | 0.15 | 3.75 | Reed (1985) |
| Agaricia lamarcki | >40 | >133 | Florida, Gulf of Mexico, Puerto Rico, Curaçao, Barbados | Kangh et al. (2010) and references therein | ||
| Agaricia sp. | 74 | 247 | Barbados | MacIntyre et al. (1991) | ||
| Agaricia undata | 80 | 267 | Curaçao | 0.65 | 16.25 | Vermeij and Bak (2002) |
| Alveopora verrilliana | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Astreopora myriophthalma | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Astreopora myriophthalma | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Astreopora myriophthalma | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Colpophyllia sp. | >40 | >133 | Gulf of Mexico | Kangh et al. (2010) and references therein | ||
| Coscinaraea monile | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Coscinaraea wellsi (now Cycloseris wellsi) | 58 | 193 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Cycloseris sp. | >40 | >133 | Marshall Islands | Kangh et al. (2010) and references therein | ||
| Cynarina sp. | 70 | 233 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Cyphastrea microphthalma | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Cyphastrea serailia | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Cyphastrea sp. | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Dendrophyllia arbuscula | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Diaseris distorta | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Diaseris sp. | >40 | >133 | Chagos Archipelago, Maldives, Marshall Islands | Kangh et al. (2010) and references therein | ||
| Dichocoenia stokesi | >40 | >133 | Gulf of Mexico | Kangh et al. (2010) and references therein | ||
| Echinophyllia aspera | 90 | 300 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Echinophyllia aspera | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Echinophyllia aspera | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Echinophyllia aspera | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Echinopora cf. lamellosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Echinopora gemmacea | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Echinopora lamellosa | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Favia pallida | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Favia speciosa | >70 | >233 | Okinawa | Yamazato (1972) | ||
| Favia speciosa | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Favia speciosa | >40 | >133 | Ryukyu Islands | Kangh et al. (2010) and references therein | ||
| Favia speciosa (now Dipsastraea speciosa) | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Favia stelligera (now Goniastrea stelligera) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Favia stelligera (now Goniastrea stelligera) | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Favites abdita | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Favites halicora | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Favites pentagona | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Favites peresi | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Fungia cf. danae | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Fungia cf. granulosa (now Pleuractis granulosa) | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Fungia concinna (now Lithophyllon concinna) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Fungia concinna (now Lithophyllon concinna) | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Fungia fungites | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Fungia paumotensis (now Pleuractis paumotensis) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Fungia paumotensis (now Pleuractis paumotensis) | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Fungia repanda | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Fungia repanda | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Galaxea astreata | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Galaxea cf. astreata | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Galaxea fascicularis | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Gardineroseris planulata | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Gardineroseris planulata | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Gardineroseris planulata | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Goniastrea pectinata | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Goniopora djiboutiensis | 58 | 193 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Goniopora muscosa | >40 | >133 | Marshall Islands | Kangh et al. (2010) and references therein | ||
| Goniopora tenella | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Herpolitha limax | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Herpolitha limax | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Hydnophora exesa | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Isopora palifera | 40 | 133 | Great Barrier Reef, North-East Australia | Muir et al. (2015) | ||
| Leptastrea purpurea | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Leptastrea purpurea | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Leptastrea purpurea | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptastrea transversa | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Leptastrea transversa | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptoseris cailleti | >40 | >133 | Puerto Rico, Barbados | Kangh et al. (2010) and references therein | ||
| Leptoseris cucullata (now Helioseris cucullata) | >40 | >133 | Florida, Bahamas, Gulf of Mexico, Jamaica, Barbados | Kangh et al. (2010) and references therein | ||
| Leptoseris cullata | 84 | 280 | Northern Gulf of Mexico | Rezak et al. (1985) | ||
| Leptoseris explanata | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Leptoseris fragilis | 145 | 483 | Red Sea | 0.11 | 2.75 | Fricke et al. (1987) |
| Leptoseris fragilis | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Leptoseris fragilis | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptoseris hawaiiensis | 165 | 550 | Johnston Atoll | 0.02 | 0.5 | Maragos and Jokiel (1986) |
| Leptoseris incrustans | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Leptoseris incrustans | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptoseris mycetoseroides | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Leptoseris mycetoseroides | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Leptoseris mycetoseroides | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptoseris papyracea | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Leptoseris porosa (now Leptoseris solida) | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Leptoseris scabra | 99 | 330 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Leptoseris scabra | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Leptoseris sp. | 153 | 510 | Hawaii | 0.07 | 1.75 | Kahng and Maragos (2006) |
| Leptoseris striata | 102 | 340 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Leptoseris yabei | 120 | 400 | Hawaii | Kahng and Maragos (2006) | ||
| Lobophyllia corymbosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Lobophyllia costata (now Lobophyllia hemprichii) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Lobophyllia hemprichi | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Madracis brueggemanni | >40 | >133 | Gulf of Mexico, Barbados | Kangh et al. (2010) and references therein | ||
| Madracis decactis | 80 | 267 | West Florida | 1 | 25 | Jarrett et al. (2005) |
| Madracis decactis | >40 | >133 | Bermuda, Florida, Bahamas, Barbados | Kangh et al. (2010) and references therein | ||
| Madracis formosa | >40 | >133 | Gulf of Mexico, Curaçao | Kangh et al. (2010) and references therein | ||
| Madracis mirabilis | >40 | >133 | Gulf of Mexico | Kangh et al. (2010) and references therein | ||
| Madracis myriaster | >40 | >133 | Gulf of Mexico | Kangh et al. (2010) and references therein | ||
| Madracis pharensis | >40 | >133 | Bahamas, Jamaica, Curaçao | Kangh et al. (2010) and references therein | ||
| Madracis senaria | >40 | >133 | Curaçao | Kangh et al. (2010) and references therein | ||
| Millepora platyphylla | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Millepora sp. | >40 | >133 | Bermuda, Gulf of Mexico | Kangh et al. (2010) and references therein | ||
| Montastraea cavernosa (now Orbicella cavernosa) | 80 | 267 | Curaçao | 0.65 | 16.25 | Van den Hoek et al. (1978) |
| Montastrea curia | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora cf. floweri | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora cf. minuta | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora cf. tuberculosa | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Montipora cf. tuberculosa | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora composita | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora erythraea (now Montipora aequituberculata) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora foliosa | 47 | 157 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Montipora meandrina | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Montipora millepora | 70 | 233 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Montipora spumosa | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora stilosa | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Montipora verrilli | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora verrilli | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Montipora verrucosa | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Montipora verrucosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Montlpora cf. tuberculosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Mycedium elephantotus | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Mycetophyllia aliciae | >40 | >133 | Jamaica | Kangh et al. (2010) and references therein | ||
| Mycetophyllia reesi | >40 | >133 | Belize, Jamaica | Kangh et al. (2010) and references therein | ||
| Napopora irregularis (now Porites irregularis) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Oculina varicosa | 40 | 133 | East Florida | 5.84 | 146 | Reed (1980) |
| Oculina varicosa | >40 | >133 | Florida | Kangh et al. (2010) and references therein | ||
| Oxypora lacera | >40 | >133 | Ryukyu Islands | Kangh et al. (2010) and references therein | ||
| Pachyseris speciosa | >70 | >233 | Okinawa | Yamazato (1972) | ||
| Pachyseris speciosa | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pachyseris speciosa | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Pachyseris speciosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pavites complanata | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pavona cactus | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pavona clavus | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pavona maldivensis | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pavona maldivensis | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Pavona maldivensis | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pavona minuta | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Pavona varians | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pavona varians | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Pavona varians | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pavona yabei | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Platygyra daedalea | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Platygyra daedalea | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Plerogyra sinuosa | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pocillopora cf. solida | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Pocillopora cf. solida | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pocillopora damicomis | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Pocillopora damicornis | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Pocillopora elegans | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Pocillopora molokensis | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Pocillopora verrucosa | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Pocillopora verrucosa | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Podabacia crustacea | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Porites astreoides | >40 | >133 | Curaçao | Kangh et al. (2010) and references therein | ||
| Porites australiensis | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Porites australiensis | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Porites cf. lutea | 70 | 233 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Porites cf. myrmidonensis | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Porites divaricata | >40 | >133 | Florida | Kangh et al. (2010) and references therein | ||
| Porites echinulata | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Porites eydouxi | >40 | >133 | Marshall Islands | Kangh et al. (2010) and references therein | ||
| Porites lichen | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Porites lobata | >40 | >133 | Hawaii | Kangh et al. (2010) and references therein | ||
| Porites lutea | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Porites lutea | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Porites lutea | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Porites solida | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Psammocora cf. contigua | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Psammocora cf. obtusangula (now Psammocora contigua) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Psammocora explanulata (now Cycloseris explanulata) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Psammocora haimeana | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Psammocora nierstraszi (now Psammocora verrilli) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Psammocora profundacella | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Psammocora profundacella | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Psammocora sp. | >40 | >133 | Chagos Archipelago, Maldives, Johnston Atoll | Kangh et al. (2010) and references therein | ||
| Psarnmocora explanulata | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Scolymia sp. | >40 | >133 | Bermuda, Florida, Bahamas, Gulf of Mexico, Jamaica | Kangh et al. (2010) and references therein | ||
| Seriatopora hystrix | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Seriatopora hystrix | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Solenastrea sp. | >40 | >133 | Belize | Kangh et al. (2010) and references therein | ||
| Stephanocoenia sp. | >40 | >133 | Gulf of Mexico, Belize | Kangh et al. (2010) and references therein | ||
| Stylocoeniella guentheri | 30 | 100 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Stylocoeniella guentheri | 45 | 150 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Stylocoeniella guentheri | 40 | 133 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Stylophora danae | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Stylophora kuehlmanni | 70 | 233 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Stylophora mamillata | 40 | 133 | Port Sudan, Red Sea | Kühlmann (1983) | ||
| Stylophora pistillata | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) | ||
| Synaraea convexa (now Porites rus) | 36 | 120 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Synaraea convexa (now Porites rus) | 70 | 233 | Takapoto Atoll, Tuamotu, Pacific | Kühlmann (1983) | ||
| Synaraea irregularis (now Porites rus) | 35 | 117 | Moorea, Society Islands, Pacific | Kühlmann (1983) | ||
| Synaraea undulata (now Porites rus) | 50 | 167 | Port Sudan, Red Sea | Kühlmann (1983) |
| Species | Maximum depth (m) | Maximum depth (ft) | Location | Reference |
|---|---|---|---|---|
| Acanthogorgia sp. | 115 | 383 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Muricella sp. | 70 | 233 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Eleutherobia sp. | 130 | 433 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Lobophytum sp. | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Carijoa sp. | 90 | 300 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Dichotella sp. | 102 | 340 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Ellisella sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Heliania sp. | 104 | 347 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Junceella sp. | 70 | 233 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Nicella sp. | 101 | 337 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Verrucella sp. | 109 | 363 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Viminella sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Pteronisis sp. | 104 | 347 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Keroeides sp. | 112 | 373 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Acabaria sp. | 47 | 157 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Dendronephthya sp. | 130 | 433 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Chironephthya sp. | 104 | 347 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Siphonogorgia sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Parisis sp. | 114 | 380 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Astrogorgia sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Echinogorgia sp. | 159 | 530 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Paracis sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Villogorgia sp. | 99 | 330 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Callogorgia sp. | 113 | 377 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Pterostenella sp. | 114 | 380 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Annella sp. | 105 | 350 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Cespitularia sp. | 55 | 183 | Great Barrier Reef, North-East Australia | Bridge et al. (2012) |
| Eunicea claigera | >40 | >133 | Jamaica | Kangh et al. (2010) and references therein |
| Pseudopterogorgia elizabethae | >40 | >133 | Jamaica, Barbados | Kangh et al. (2010) and references therein |

Tools of the trade: Dr. Pim Bongaerts uses a remotely operated vehicle (ROV) to study deep water corals on the Great Barrier Reef. Photograph by Pim Bongaerts, Ph.D.
| Species | Maximum depth (m) | Maximum depth (ft) | Location | Reference |
|---|---|---|---|---|
| Acanthopathes undulata | 259 | 863 | Oahu | Wagner et al. (2011) |
| Antipathes grandis | 127 | 423 | Maui | Wagner et al. (2011) |
| Antipathes griggi | 172 | 573 | Kauai | Wagner et al. (2011) |
| Antipathes sp. | 57 | 190 | Kau’ula | Wagner et al. (2011) |
| Aphanipathes sp. | 130 | 433 | Maui | Wagner et al. (2011) |
| Bathypathes sp. | 320 | 1067 | Oahu | Wagner et al. (2011) |
| Cirrhipathes cf. anguina | 30 | 100 | Kauai | Wagner et al. (2011) |
| Dendropathes cf. intermedia | 320 | 1067 | Oahu | Wagner et al. (2011) |
| Leiopathes sp. | 365 | 1217 | Hawai’i | Wagner et al. (2011) |
| Myriopathes ulex | 182 | 607 | Hawai’i | Wagner et al. (2011) |
| Myriopathes? sp. | 396 | 1320 | Johnston Atoll | Wagner et al. (2011) |
| Stichopathes cf. echinulata | 172 | 573 | Kauai | Wagner et al. (2011) |
| Stichopathes sp. | 396 | 1320 | Johnston Atoll | Wagner et al. (2011) |

Just another day at the office: a marine biologist photographs a rich deep water reef community. Note the barrel sponge just below the diver’s hands and the spiral-shaped black coral to his right. Photograph by Pim Bongaerts, Ph.D.
Although Leptoseris hawaiiensis is the record holder for zooxanthellate corals, other photosynthetic organisms are found in even extremer habitats. Crustose coralline algae are the deepest-occurring macroalgae, and have been found down to a staggering depth of 268 meters (893 ft.) where light is virtually absent (Table 4). It is mystifying to find what we consider to be light-dependent organisms down there. Coralline algae can even survive up to 17 months under ice with less than 0.07% of surface irradiance (Schwarz et al. 2005). Seagrasses are also found in deep waters, with their maximum depth recorded so far at 90 m (300 ft.).

Even a small patch of mesophotic reef is packed with biodiversity. Note the colorful lace corals, possibly Stylaster or Distichopora sp. Lace corals are members of the Hydrozoa class and produce a calcium carbonate skeleton, making them resemble stony corals (Anthozoa class). They reproduce by releasing miniature jellyfish into the water. Photograph by Pim Bongaerts, Ph.D.
Table 4. Deepest known benthic photosynthetic life in tropical oceans. Based on Gattuso et al. (2006) and references therein. Maximum surface irradiance is approximately 2,500 µmol m-2 s-1 (Frade et al. 2008; Lesser et al. 2010).
| Maximum depth (m) | Maximum depth (ft) | % of surface irradiance | Absolute estimated irradiance (µmol m–2 s–1) | |
|---|---|---|---|---|
| seagrasses | 90 | 300 | 1.1 | 27.5 |
| macroalgae (coralline algae and seaweeds) | 268 | 893 | 0.0005 | 0.0125 |
| zooxanthellate corals | 165 | 550 | 0.02 | 0.5 |
Symbiosis with zooxanthellae and bacteria
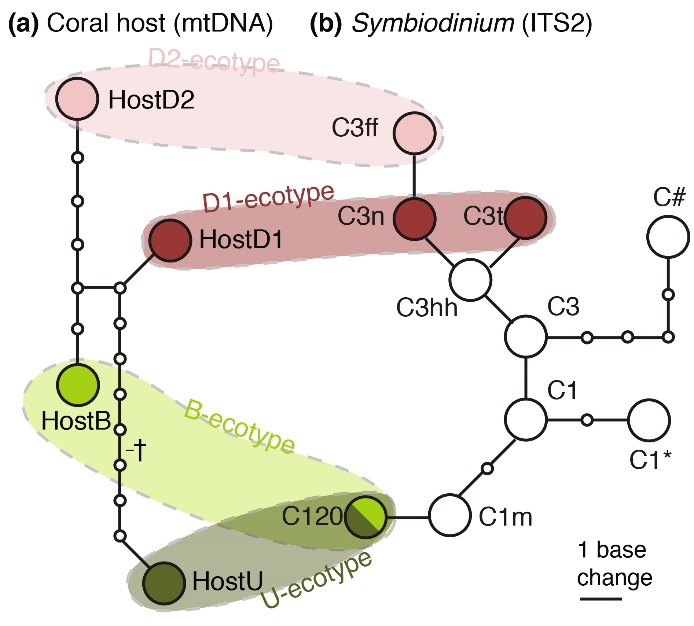
Based on DNA markers (mitochondrial DNA/mtDNA and nuclear DNA/ITS2), genetically distinct Seriatopora hystrix (a) and zooxanthellae (b) can be detected, which are referred to as genotypes. DNA analysis of deep and shallowS. hystrix colonies reveals that specific associations between coral genotypes and zooxanthellae genotypes occur. The four colored areas indicate specific combinations of coral and zooxanthellae genotypes, which together make up the different ecotypes of Seriatopora hystrix, namely the Back Reef (B), Upper Slope (U) and Deep Slope (D1/D2) ecotypes. From Bongaerts et al. (2011a).
As I described above, corals growing in deeper waters show evidence of genetic and physiological adaptation. This not only holds true for the coral host itself, but also for its symbiotic dinoflagellates, the zooxanthellae. For example, Bongaerts et al. (2011a) found that deep specimens of Seriatopora hystrix hosted genetically unique zooxanthellae, which are not found in their shallow counterparts. These zooxanthellae belong to a specific group of Symbiodinium, also known as a clade. Although all S. hystrix found in shallow and deep waters hosted clade C zooxanthellae, subtle differences within their DNA were detected that suggests various clade C subgroups exist, which may be adapted to specific depth habitats.
The figure illustrates the ecotype concept, where specific coral and zooxanthellae genotypes together make up various ecotypes. Evidence for adaptation by zooxanthellae to deeper waters is also provided by the work of Lesser et al. (2010), who found that below 61 m, the genetic composition of zooxanthellae changes. According to them, “there is strong selection for zooxanthellae that are suited for survival in the light-limited environment.”
Although zooxanthellae may provide scleractinian corals with nutrition down to a depth of 165 meters (550 feet), this is yet unclear for black corals (order Antipatharia). Although antipatharians were long considered azooxanthellate, they have recently been found to host Symbiodinium (Wagner et al. 2011). Even at depths of around 400 meters (1,333 feet), these corals can host Symbiodinium, most of which belong to clade C. As such a deep, dark habitat renders photosynthesis ineffective, many black corals could be parasitized by zooxanthellae, although this remains to be determined.

Antipatharia, colloquially known as black corals, thrive in the deep waters of Sulawesi. Here, they survive by capturing plankton and detrital matter. The role of their endosymbiotic zooxanthellae remains unclear. Photograph by Pim Bongaerts, Ph.D.
In addition to zooxanthellae, research has shown that corals are host to a diverse bacterial community. These bacteria live in mucus on the coral’s surface, within coral tissue and even within zooxanthellae (Ainsworth et al. 2015). Specific coral species associate with certain bacteria, similar to zooxanthellae (Olson and Kellogg 2010). What may be surprising to aquarists is that certain cyanobacteria are found in corals and their zooxanthellae, which fix nitrogen by converting dissolved nitrogen gas (N2) into ammonia, which is then transferred to the zooxanthellae (Lesser et al. 2007). These, in turn, use the ammonia to produce amino acids and proteins, feeding themselves and their coral host. This intricate partnership between the coral animal and its symbiotic microorganisms allows this so-called holobiont to survive in a low nutrient environment. Although certain zooxanthellae may be found on deeper coral reefs only, this is not yet clear for bacteria (Olson and Kellogg 2010). It is possible that specific species of bacteria may allow corals to persist in very low light environments, by supplying their host with specific compounds.

Analyzing mucus provides important clues about a coral’s life in the deep, such as its associations with specific bacteria. Here, Dr. Pedro Frade is sampling a stony coral collected from 90 meters (300 feet). Photograph by Pim Bongaerts, Ph.D.
Concluding remarks

Mesophotic reefs, home to fascinating marine life, deserve our protection. Note the Anthias just left off-center. Photograph by Pim Bongaerts, Ph.D.
It is clear that mesophotic coral reefs are fascinating, important and also underappreciated ecosystems. Fortunately, scientific study of mesophotic reefs is gaining traction, providing us with a better understanding of how these ecosystems function. Public aquaria are taking notice as well, with the Steinhart Aquarium of the California Academy of Sciences preparing a mesophotic reef aquarium, with corals and fishes specifically collected for this new display. Through research and education, hopefully, we will be able to protect these reefs from human disturbances, allowing them to serve as a refuge for fishes, corals and other invertebrates.
Further reading
To learn more about (mesophotic) coral reefs, please visit the XL Catlin Seaview Survey website, a unique project which aims to record the world’s reefs in 360 degree panoramic vision. Using an innovative SVII Panoramic Camera with a propeller and three Canon 5D DSLR’s equipped with wide-angle lenses, 360 degree images of coral reefs are recorded. Their website has some spectacular images which you can download. The related XL Catlin Global Reef Record also has many panoramic images and videos to enjoy.

Large shoals of blue triggerfish (Odonus niger) find refuge in the deep, between large sponges, soft corals and whip gorgonians. Notice the solitary fishes just left and right off center. Photograph by Pim Bongaerts, Ph.D.
I also would like to bring your attention to the upcoming mesophotic reef display at the Steinhart Aquarium, entitled Twilight Zone: Deep Reefs Revealed, which opens June 10th 2016. Their description:
Step inside a realm known as the twilight zone-a narrow, dimly lit band of the world’s oceans more mysterious to scientists than the surface of the Moon. In this groundbreaking new aquarium exhibit, located within the California Academy of Sciences’ newly updated Coral Reefs of the World gallery, discover the beauty and importance of the little-known reefs that exist between 200 and 500 feet below the ocean’s surface-beyond the range of traditional SCUBA gear but above the depths typically explored by submersibles. Meet spectacularly colorful fish and rarely seen invertebrates, learn about the innovative technologies that are enabling Academy scientists to study this new frontier, and discover what you can do to help protect these amazing-and vitally important-ecosystems.
Acknowledgements
I am greatly indebted to Dr. Pim Bongaerts of the Global Change Institute, University of Queensland and his colleagues of the XL Catlin Seaview Survey for providing extraordinary images. I also want to thank Charles Delbeek, M.Sc., Assistant Curator and Kelly Mendez, B.A., Senior Communications Manager of the Steinhart Aquarium for providing information about their new mesophotic display.
References
- Ainsworth TD, Krause L, Bridge T, Torda G, Raina J-P, Zakrzewski M, Gates RD, Padilla-Gamiño JL, Spalding HL, Smith C, Woolsey ES, Bourne DG, Bongaerts P, Hoegh-Guldberg O, Leggat W (2015) The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. The ISME Journal 9:2261-2274
- Bak RPM, Nieuwland G, Meesters EH (2005) Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curaçao and Bonaire. Coral Reefs 24:475-479
- Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O (2010) Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29(2):309-327
- Bongaerts P, Riginos C, Hay KB, van Oppen MJH, Hoegh-Guldberg O, Dove S (2011a) Adaptive divergence in a scleractinian coral: physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evolutionary Biology 11:303
- Bongaerts P, Sampayo EM, Bridge TCL, Ridgway T, Vermeulen F, Englebert N, Webster JM, Hoegh-Guldberg O (2011b) Symbiodinium diversity in mesophotic coral communities on the Great Barrier Reef: a first assessment. Marine Ecology Progress Series 439:117-126
- Bou-Abdallah F, Chasteen ND, Lesser MP (2006) Quenching of superoxide radicals by green fluorescent protein. Biochim Biophys Acta 1760:1690-1695
- Bridge TCL, Fabricius KE, Bongaerts P, Wallace CC, Muir PR, Done TJ, Webster JM (2012) Diversity of Scleractinia and Octocorallia in the mesophotic zone of the Great Barrier Reef, Australia. Coral Reefs 31:179-189
- Crandall JB, Teece MA, Estes BA, Manfrino C, Ciesla JH (2016) Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. Journal of Experimental Marine Biology and Ecology 474:133-141
- D’Angelo C, Denzel A, Vogt A, Matz MV, Oswald F, Salih A, et al. (2008) Blue light regulation of host pigment in reef-building corals. Marine Ecology Progress Series 364:97-106
- D’Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, Wiedenmann J (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31:1045-1056
- De Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W (2013) Surviving in a Marine Desert: The Sponge Loop Retains Resources Within Coral Reefs. Science 342:108-110
- Done T (2011) Corals: environmental controls on growth. In: Hopley D, editor. Encylopedia of modern coral reefs. Springer, Dordrecht. pp 281-293
- Enriquez S, Mendez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnology and Oceanography 50(4):1025-1032
- Eyal G, Wiedenmann J, Grinblat M, D’Angelo C, Kramarsky-Winter E, Treibitz T, et al. (2015) Spectral Diversity and Regulation of Coral Fluorescence in a Mesophotic Reef Habitat in the Red Sea. PLoS ONE 10(6): e0128697. doi:10.1371/journal.pone.0128697
- Ferrier-Pagès C, Gattuso JP, Dallot S, Jaubert J (2000) Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19:103-113
- Ferrier-Pagès C, Hoogenboom M, Houlbrèque F (2011) The role of plankton in coral trophodynamics, 215-229. In: Dubinsky Z, Stambler N (Eds), Coral reefs: an ecosystem in transition. Springer, Dordrecht, The Netherlands
- Frade PR, Bongaerts B, Winkelhagen AJS, Tonk L, Bak RPM (2008) In situ photobiology of corals over large depth ranges: a multivariant analysis on the roles of environment, host, and algal symbiont. Limnology and Oceanography 53:2711-2723
- Fricke HW, Vareschi E, Schlichter D (1987) Photoecology of the coral Leptoseris fragilis in the Red Sea twilight zone (an experimental study by submersible). Oecologia 73:371-381
- Gattuso J-P, Gentilli B, Duarte CM, Kleypas JA, Middelburg JJ, Antoine D (2006) Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeoscience 3:489-513
- Gittins JR, D’Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015) Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Molecular Ecology 24: 453-465
- Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev Camb Philos 84:1-17
- Hylkema A, Wijgerde T, Osinga R (2015) Decreased growth of Stylophora pistillata with nutrient-driven elevated zooxanthellae density is largely explained by DIC limitation. Advanced Aquarist 14(5)
- Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255-275
- Kahng SE, Maragos JE (2006) The deepest, zooxanthellate scleractinian corals in the world? Coral Reefs 25:254
- Kinzie III RA, Hunter T (1987) Effect of light quality on photosynthesis of the reef coral Montipora verrucosa. Mar Biol 94:95-109
- Kinzie III RA, Jokiel PL, York R (1984) Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar Biol 78:239-248
- Kühlmann DHH (1983) Composition and ecology of deep-water coral associations. Helgoländer Meeresuntersuchungen 36:183-204
- Lesser MP, Falcón LI, Rodríguez-Román A, Enríquez S, Hoegh-Guldberg O, Iglesias-Prieto R (2007) Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Marine Ecology Progress Series 346:143-152
- Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. Journal of Experimental Marine Biology and Ecology 375:1-8
- Lesser MP, Slattery M, Stat M, Ojimi M, Gates RD, Grottoli A (2010) Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91(4):990-1003
- Leutenegger A, D’Angelo C, Matz MV, Denzel A, Oswald F, Salih A, et al. (2007) It’s cheap to be colorful. Anthozoans show a slow turnover of GFP-like proteins. FEBS Journal 274:2496-2505
- Maragos JE, Jokiel PL (1986) Reef corals of Johnston Atoll-one of the World’s most isolated reefs. Coral Reefs 4:141-150
- Marubini F, Davies PS (1996) Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar Biol 127:319-328
- Mass T, Kline DI, Roopin M, Veal CJ, Cohen S, et al. (2010) The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. J Exp Biol 213:4084-4091
- Matz MV, Marshall NJ, Vorobyev M (2006) Are Corals Colorful? Photochemistry and Photobiology 82:345-350
- Meadows MG, Anthes N, Dangelmayer S, Alwany MA, Gerlach T, Schulte G, et al. (2014) Red fluorescence increases with depth in reef fishes, supporting a visual function, not UV protection. Proceedings of the Royal Society of London B: Biological Sciences 281: 20141211. http://dx.doi.org/10.1098/rspb.2014.1211
- Muir P, Wallace C, Bridge TCL, Bongaerts P (2015) Diverse Staghorn Coral Fauna on the Mesophotic Reefs of North-East Australia. PLoS ONE 10(2): e0117933. doi:10.1371/journal.pone.0117933
- Olson JB, Kellogg CA (2010) Microbial ecology of corals, sponges, and algae in mesophotic coral environments. FEMS Microbiology Ecology 73:17-30
- Oswald F, Schmitt F, Leutenegger A, Ivanchenko S, D’Angelo C, Salih A, et al. (2007) Contributions of host and symbiont pigments to the coloration of reef corals. FEBS Journal 274:1102-1109
- Reed JK (1985) Deepest distribution of Atlantic hermatypic corals discovered in the Bahamas. In: Proceedings of the 5th international coral reef symposium 6:249-254
- Rivero-Calle S, Armstrong RA, Soto-Santiago FJ (2008) Biological and physical characteristics of a mesophotic coral reef: Black Jack reef, Vieques, Puerto Rico. Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 7-11 July, Session number 16
- Rooney J, Donham E, Montgomery A, Spalding H, Parrish F, Boland R, Fenner D, Gove J, Vetter O (2010) Mesophotic coral ecosystems in the Hawaiian Archipelago. Coral Reefs 29:361-367
- Salih A, Hoegh-Guldberg O and Cox G (1998) Photoprotection of symbiotic dinoflagellates by fluorescent pigments in reef corals. Proc Aust Coral Reef Society 75 Anniv Conference (ed) Greenwood & Hall. pp 217-230
- Salih A, Larkum A, Cox G, Kuhl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408:850-853
- Schlichter D, Fricke HW, Weber W (1986) Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Marine Biology 91:403-407
- Schwarz A-M, Hawes I, Andrew N, Mercer S, Cummings V, Thrush SF (2005) Primary production potential of nongeniculate coralline algae at Cape Evans, Ross Sea, Antarctica. Marine Ecology Progress Series 294:131-140
- Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H (2007) Imbalanced coral growth between organic tissue and carbonate skeleton caused by nutrient enrichment. Limnol Oceanogr 52:1139-1146
- van Oppen MJH, Bongaerts P, Underwood JN, Peplow LM, Cooper TF (2011) The role of deep reefs in shallow reef recovery: an assessment of vertical connectivity in a brooding coral from west and east Australia. Molecular Ecology doi: 10.1111/j.1365-294X.2011.05050.x
- Wagner D, Pochon X, Irwin L, Toonen RJ, Gates RD (2011) Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proceedings of the Royal Society B 278:1323-1328
- Wang L-H, Liu Y-H, Ju Y-M, Hsiao Y-Y, Fang L-S, et al. (2008) Cell cycle propagation is driven by light-dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral Reefs 27:823-835
- Wijgerde T (2013) Coral Feeding: An Overview. Advanced Aquarist 12(12)
- Wijgerde T, Laterveer M (2013) Coral growth under Light Emitting Diode and Light Emitting Plasma: a cross-family comparison. Advanced Aquarist 12(2)
- Wijgerde T, Tilstra A (2014) Debunking Aquarium Myths. Advanced Aquarist 13(2)
- Wijgerde T, van Melis A, Silva CIF, Leal MC, Vogels L, et al. (2014) Red Light Represses the Photophysiology of the Scleractinian Coral Stylophora pistillata. PLoS ONE 9(3): e92781. doi:10.1371/journal.pone.0092781









Here’s a complete guide to underwater ear equalizing:
https://cleverdiving.com/does-swallowing-equalize-your-ears-while-diving/
The Valsalva technique is the standard, so pinch your nose and blow through your nose. The excess pressure on your throat pushes air through your Eustachian tubes, opening the valves and equalizing your ears.