Imagine an instrument that has
the potential of instantly reporting your corals’ well-being
– specifically an estimate of zooxanthellae chlorophyll content.
One could think of it as a ‘coral bleaching early warning
device.’ This isn’t science fiction, but it is a lot of
science.
Based on technology developed by NASA, Spectrum Technologies
markets a ‘chlorophyll meter’ – the FieldScout CM1000.
This instrument is a simple point-and-shoot device (so simple a
caveman can do it, as the TV commercial says). The CM1000
immediately analyzes, averages and stores data for future
reference.
If this sounds too good to be true, perhaps it is as there are
some downsides – price, and an inability to measure chlorophyll
through more than an inch or two of water – but we’ll examine
these ‘handicaps’ later in this article.
I learned of the CM1000 when I happened across the Spectrum
Technologies site while doing a web search on a peripheral
subject. Intrigued, I called Spectrum and explained that I wanted
to estimate chlorophyll content of zooxanthellae within corals.
This question stumped the available technical staff. The
CM1000’s target markets are golf courses, commercial
greenhouses, orchards, etc., so mine was probably the
‘oddball question of the day.’ They were able to tell me
that the instrument would not accurately measure chlorophyll
content through a water column. However, if the corals were to be
removed from the water, this instrument would likely measure
zooxanthellae chlorophyll a. They would check with the
inventor. Again, the same answer – theoretically yes, but not
absolutely certain. Spectrum agreed to send a unit for testing
with two conditions – I report the results, and, if satisfied
that the unit would generate meaningful data, I would purchase
it. If this device worked with corals, it could allow easily
generated insights on long-term photoadaptation or
photoacclimation processes and pigmentation shifts without
destructive sampling.
Theory of Operation
The FieldScout CM1000 Chlorophyll Meter determines Relative
Chlorophyll Content Index through measurement of two
‘light’ wavelengths – 700 nm (red) and 840 nm
(near-infrared). The device senses these wavelengths from the
light source and those reflected from the targeted surface. Since
chlorophyll absorbs red light (700nm) and reflects near-infrared
(840nm), the instrument compares the results of these
measurements and calculates an estimate of chlorophyll
content.
This is made possible by internal workings including, among
other things, beam-splitters, cutoff filters, two toggle-switch
actuated diode lasers, a reflectance standard, a dark reference,
a photodiode and a microprocessor tied into a liquid crystal
display. Quite a lot packed into a small, battery-operated
handheld unit.
The FieldScout takes one measurement of ambient and reflected
light per second (without the optional GPS – one measurement is
taken every 3-4 seconds with the GPS).
The microprocessor calculates a relative Chlorophyll Index and
displays this number (0-999) in the LCD. An average is calculated
among multiple readings. These, along with the number of
measurements made, are displayed along with the instantaneous
relative Chlorophyll Index (See Figure 1).

Figure 1. The control panel of the
FieldScout. The LCD indicates an instantaneous Chlorophyll
Index of 264, with a running average of 242 among 19
measurements. A built-in light meter indicates light intensity
(Brightness, or BRT) of 1.
Testing procedures must be fairly standardized for consistent
results. The Field Scout does not provide the lighting source for
chlorophyll measurements so ambient lighting must be used. Light
intensity should be intense, and illuminate both the
instrument’s light meter and the target. The CM1000 does,
however, include two red lasers for sighting purposes. Spectrum
recommends that the target object should be no closer to the
sensor than 28.4 cm (11.2 inches); at this distance the field of
view is 1.10cm in diameter (0.434 inches). There is also a
recommended maximum distance of 183cm (72 inches – The field of
view at this distance is 18.8cm, or 7.4″ in diameter).
Product Evaluation
This purchase would represent a substantial portion of my
annual budget so I had to quickly but carefully determine if the
FieldScout would be of value. Many questions had to be answered.
The product was evaluated on several parameters, and my comments
are as follows:
First, I had to make a determination of coral reflectance. To
do so, I used an Ocean Optics USB2000 spectrometer with a fiber
optic cable and reference standard (Spectrolon diffuse standard,
>99%). A dark reference was taken, followed by that of the
Spectrolon standard, and finally the reflected light from a
coral’s surface. Reflectance is figured mathematically by the
Ocean Optics software. The result suggested that the FieldScout
would work with corals. See Figure 2.

Figure 2. The reflectance of a brown stony
coral, Pavona species. This scan suggested the CM1000
had a good chance of measuring chlorophyll content within
zooxanthellae.
Measurement Repeatability
Spectrum advertises measurement repeatability is ±5%. I
compared indices (of a green plant) taken over a range of light
intensities. The individual data sets were indeed in good
agreement. However, comparison of indices in all data sets
revealed differences of up to 13%. I need to investigate this
further, but it appears that lower light intensity causes
slightly higher measurements and, conversely, high light
intensity measurements are relatively lower (these measurements
were made with natural sunlight throughout the day in clear, part
cloudy and overcast conditions, with a range of 1 to 6 on the
meter’s ‘Brightness’ scale). Spectrum suggests that
higher light intensity increases resolution and perhaps that is
the explanation.
Since we’re interested in trends, this is probably not
that much of an issue. However, I will work to standardize
conditions as much as possible when taking measurements (see my
thoughts in ‘Discussion’ below). And, as a footnote:
Can Artificial Light Sources Be Used? Spectrum
Technologies recommends natural sunlight as the source. However,
it is possible to use artificial light as the source if two
conditions are met. First, the light must be intense (at least
250 – 300 micromol·m²·sec, or about 15,000 lux. A built-in light
meter estimates light intensity and reports it on a scale of 0-9
on the LCD display. A brightness of ‘1’ is the minimum
amount of light required for proper measurements). Second, use
either a lamp using direct current (DC) or alternating current
(AC) at 60 hertz. Spectrum specifically recommends tungsten or
halogen lamps (probably due to the amount of red and near-IR
energy produced). I can’t think of a reason why many (if not
most) metal halide lamps could also be used. The meter must be
modified for use with light sources operating at 50 hertz.
Incidentally, an error message of ‘Excessive Light’ is
prompted when the sensors are saturated with light. Maximum
sunlight has not generated this message, but it is a possibility
under some of the higher wattage metal halide lamps.
Laser Sighting
The CM1000 includes two diode lasers for sighting. These are
3mW maximum output in the red spectral range of 635-670nm. These
‘laser pointers’ are quite bright and can be seen in even
the most intense Hawaiian sunlight. Each of these lasers are
slightly angled resulting in their beams intersecting about 30cm
(12″) from the instrument’s lens – this is a very
convenient tool for instant verification that the unit is the
proper distance from the target as well as for sighting.
Environmental Conditions
The Chlorophyll Meter seems to be a rugged unit. Its housing
(made of heavy plastic) is said to be dust-proof. Since I’ve
managed to occasionally splash the meter with seawater, it seems
to be splash-proof as well – but not water-resistant and
certainly not water-proof). Spectrum recommends operating
temperatures of between 0 and 40°C (32-104° F).
As with any electronic unit, this meter should be handled with
care and environmental extremes avoided. Spectrum supplies a
sturdy plastic carrying case with foam insert at no additional
charge.
Power Supply and Battery Life
The CM-1000 requires two AAA alkaline batteries. Although
other batteries can be used (such as NiCads), Spectrum recommends
alkaline batteries in order for the battery charge indicator to
work properly. Battery life is rated as ‘good’. Without
the optional GPS, Spectrum says 3,000 measurements are possible
on one battery set. This seems a bit of an overestimation,
however, I have had to replace batteries only once in 4 months of
usage.
The CM-1000 automatically shutdowns after 20 minutes of
inactivity in order to conserve batteries. The LCD will also
display a ‘Low Battery’ warning when the batteries reach
20% of full charge.
Data Storage
In its basic configuration (i.e., without optional data
logging) the FieldScout can store up to 64 Chlorophyll Indices
and these are available through the ‘Recall Data’
function. Oddly, the chlorophyll meter can count up to 250 data
points in a data series.
Now that we understand the theory of operation and instrument
function, we can get down to business and test the CM1000. The
first test involved green algae and measured the instrument’s
ability to discriminate among small increments of ‘known’
chlorophyll concentrations. The second test involved corals over
a timescale of months.
Test One – Green Algae
A simple test was devised to determine if the Chlorophyll
Meter could recognize small differences in chlorophyll content of
green algae.
Procedure
A 2-liter sample of ‘greenwater’ was analyzed for
suspended solids. The sample was divided into aliquots of
increasing volume. The procedure outlined in Standard
Methods was used, and equipment included an analytical
balance (Sartorius), a drying oven at 103°C, and glass microfiber
filters (Whatman, 934-AH, 47mm with pore size of 1.5 microns).
This procedure allows determination of the weight of particulate
matter suspended within the sample and when divided by area,
arrives at weight per area (in this case, milligrams per square
centimeter). It was assumed that the entire suspended solids’
weight was due to chlorophyll a content (which, of
course, it isn’t. However this method errs on the side of
caution, and we see that the meter can distinguish between very
small incremental increases of chlorophyll content). Multiple
readings (in sunlight) of each algae sample/filter were made and
the average index was charted. See Figure 2.
Results
The results suggest that the instrument is capable of
detecting small differences of chlorophyll, and that the trend
appears to be linear at these concentrations. The final portion
of this test was conducted outside in conditions of varying
sunlight intensity, and persistent trade winds made holding the
filters steady difficult. My curiosity was satisfied, and I did
not wish to repeat the 4 hour test. See Figure 3.
Notice that the meter reports a Chlorophyll Index in the high
60’s for a clean, white glass microfibre filter. This is due
to the reflective properties of the filter. See comments below
about the reflective properties of a coral skeleton.
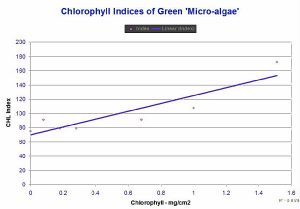
Figure 3. Relative Chlorophyll Indices
(‘Chl Index’ on the Y-axis) and approximate chlorophyll
content in milligrams per square centimeter.
An Inadvertent Test – Corals
Many questions had to be answered before I would be
comfortable with results. The first question – How far could the
two reference beams penetrate a water column? Since red
wavelengths are quickly absorbed by water (and near-IR even more
quickly), it was of little hope that ‘in-aquaria’
measurements could be made. Although the meter could detect
chlorophyll content to a depth of about 10cm, the results were
also low, and sometimes erratic. It appears that the corals have
to be removed from the water in order to test their zooxanthellae
chlorophyll content (but see remarks for a potential way of
getting around this. See comments in ‘Discussion’).
The second problem is with the coral itself, more
specifically, the reflective properties of the white coral
skeleton beneath the thin layer of tissue. Spectrum warns that
the reflectance of a light-colored or white surface may give a
false reading. This seems to be true (as indicated by the results
with the glass microfibre filter). It also seems true for coral
skeletons, as the Chlorophyll Index of a reflectance standard
made of a polished (and chemically bleached) Porites
skeleton indicates a index of about 55 (mean of 25 readings made
over a range of light intensities). In other words, the base
index for a coral is ~55, and a measurement near this number
would indicate total bleaching. For what it’s worth, a
measurement of 55 is about 5% of the meter’s maximum
measuring capability.
In any case, I began monitoring the chlorophyll index of
captive corals in one of the Natural Energy Laboratory’s
(NELHA) outdoor tanks.
Results of “Test” Two
Figures 4 and 5 shows the Porites evermanni specimen
before and after a bleaching episode. If the chlorophyll indices
are any indication, the CM1000 noted a drop in zooxanthellae (or
zooxanthellae chlorophyll a) well before any visual sign
of bleaching was apparent.

Figure 4. A nicely colored ‘Lobe’
coral (Porites evermanni) and average relative
Chlorophyll Indices (with dates and number of measurements).
The coral is healthy and robust.

Figure 5. June 28, 2005 – The same
Porites colony shown in Figure 5 – it is obviously in
distress. Chlorophyll indices as low 58 were noted. This
suggests almost total bleaching has occurred, an observation
verified by sight alone.
This colony is maintained with other propagated Porites
evermanni in an ‘open system’ outdoor tank utilizing
natural sunlight as the actinic source. All P. evermanni
specimens suffered bleaching, while other Porites
colonies (P. lobata), Pocillopora meandrina and
Pavona varians did not. These colonies showed no drop in
their Chlorophyll Indices.
It is not known why only the Porites evermanni
colonies bleached. Was it over-illumination, resulting in chronic
photoinhibition and ultimately death or expulsion of
zooxanthellae? If so, what does this suggest about theories of
colorful coral pigments and their suggested links to
photoprotection? Could ultraviolet radiation have played a part?
Why would the captive corals lose resistance to UV? Could it be
due to diet, or possibly lack of nutrition (due to insufficient
water motion resulting in poor particle delivery)? The parade of
questions is almost endless. Most important, though, is the
concept that bleaching (in some cases) could be
predicted and preventive measures could be taken to
limit the impact.
Discussion
The CM1000 is not inexpensive – it retails for about $2,200,
plus shipping. Is it worth the price? That really depends upon
your situation. Almost certainly, this instrument would not
appeal to the average hobbyist. However, professional aquarists,
coral farmers and researchers may find this instrument of use.
The potential for predicting bleaching events would be of great
value to those with large capital outlays invested in their
livestock, brood stock and systems. Although still under
investigation, this unit could also be of benefit to scientists
wishing to monitor zooxanthellae content/health with a
non-invasive means. Anyone who has ever extracted chlorophyll
a with appropriate organic solvents and quantified
chlorophyll content via spectrophotometric means (for instance,
using the equations of Jeffrey and Humphrey, 1975) will really
appreciate what this instrument has the potential to do.
It may come as a surprise that the purchase price really is a
breakthrough – previous setups had costs exceeding $60,000. A PAM
(pulse amplitude modulation) fluorometer senses chlorophyll
through fluorescence, and these units start at about $5,000. Even
with cost aside, a PAM meter requires careful setup, and
evaluation of resulting data is time consuming.
Even more important than price, we potentially have a simple
to use, point-and-shoot meter capable of examining zooxanthellae
chlorophyll content of the same coral sample (no
destructive sampling) over time and under differing environmental
conditions. The experimental possibilities are almost unlimited.
While a PAM meter tells us fluorescence and suggests relative
chlorophyll content (less chlorophyll generally equals less
min/max fluorescence) and is excellent for monitoring short-term
and dynamic photosynthetic processes, it is not particularly good
in allowing glimpses into long-term responses. The CM1000, on the
other hand, seems to allow long-term monitoring of zooxanthellae
-its ease of use and relatively large sampling area are genuine
pluses.
At this point – based on very early observations – any falling
Chlorophyll Index over the course of just a few days should
encourage increased monitoring. Of course, this instrument will
be of little use in predicting catastrophic bleaching events due
to extremely high temperature (a ‘stuck’ heater), rapid
and severe salinity modulations, etc. However, those ‘long
term’ stressors resulting in bleaching (associated with high
UV dosage, poor water motion potentially resulting in nutrient
deficiencies, toxicity issues, etc.) might be corrected before
serious bleaching and coral fatalities result. On the other hand,
an increasing relative Chlorophyll Index could indicate a
response to increasing nutrient (such as nitrogen) content in the
water. It is an interesting thought that perhaps (and this is
only a hypothesis) loss of coloration could be predicted with a
rising chlorophyll index.
It is theoretically possible to estimate the accessory pigment
content (peridinin, chlorophyll c) of zooxanthellae
based on chlorophyll a content. There is a lot of work
to do in this area, and I think I have enough years left to at
least scratch the surface.
The scant evidence at present suggests that long-term
bleaching events (as opposed to cataclysmic ‘immediate’
bleaching) of corals within aquaria may be predictable. Granted,
the initial observation of reduced chlorophyll content could
possibly be due to other factors – photoacclimation (Titlyanov et
al., 1980), seasonal variance of zooxanthellae photopigments
(Stimson, 1997), etc. I personally believe (just a hunch) the
bleaching event was due to excessive ultraviolet radiation
resulting in chronic photoinhibition.
I think it is possible to obtain underwater measurements if an
underwater housing were to be used. It is possible that the
instrument could be fitted with an air-filled tube and light
sources to allow in-situ measurements within aquaria (See Figure
6). The air-filled chamber will be about 12″ in length and
will also act as a range guide in order to quickly and
conveniently gage sensor-to-coral distance. This distance should
provide relative Chlorophyll Indices of approximately 1cm². Light
sources will probably be 12v halogen lamps (one in a waterproof
housing). I expect some problems with laser reflection, and will
have to perform some spectrometer work to standard lighting
sources. At present, I don’t see these obstacles as
insurmountable. One has to wonder of the possibilities of a
modified Field Scout in an underwater housing for measurements in
situ.
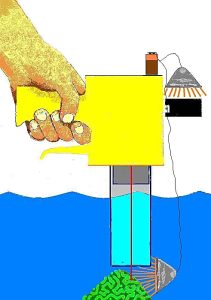
Figure 6. My concept of modifications
required in order to take chlorophyll measurements within an
aquarium. An air-filled tube prevents major attenuation of red
light and infrared energy generated by a submersed halogen
lamp. Another battery-operated halogen lamp provides reference
wavelengths to the CM1000’s light meter. The vertical red
line is the instrument’s laser sight.
For more info, visit
www.specmeters.com. I
will personally answer email directed either to the AAOM Forums,
or
[email protected].
References
- Jeffrey, S.W. and Humphrey, G. F. 1975. New
spectrophotometric equations for determining chlorophylls
a, b, c¹ and c² in higher
plants, algae, and natural phytoplankton. Biochem. Physiol.
Pflanz. 167: 191-194. - Stimson, J., 1997. The annual cycle of density of
zooxanthellae in the tissues of field and laboratory-held
Pocillopora damicornis (Linnaeus). J. Exp.
Mar. Biol. Ecol., 214(1-2): 35-48. - Titlyanov, E.A., M.G. Shaposhnikova and V.I. Zvalinskii,
1980. Photosynthesis and adaptation of corals to irradiance. I.
Contents and native state of photosynthetic pigments in
symbiotic microalga. Photosynthetica 14(3): 413-421.




0 Comments