
Advances in technology and utilization of LEDs in particular continue to bring new analytical devices to market and many of these instruments are smaller and much less expensive than those of only a few years ago. Hanna Instruments has introduced two colorimeters (called ‘Checkers’) specifically designed to test for calcium and iron. We’ll pit the inexpensive Hanna Iron Checker and reagents against a $1,150 Hach DR890 colorimeter, Hach reagents, and a Hach Certified Iron Standard. The Hanna Calcium Checker will be challenged by Hanna’s own Certified Calcium Standard. How do the results compare, and are these instruments worthy of your consideration? What are the strengths and weaknesses of these two instruments?
What is a Colorimeter?
A colorimeter is a device that can measure light transmitted through (or absorbed by) a liquid. Since addition of a specific chemical reagent to a solution containing the target substance may cause the sample to become colored, a colorimeter measures the light (often that produced by a monochromatic light source as a light-emitting diode, or LED) absorbed/transmitted by the colored solution after the zero reference of a non-colored (blank) solution has been made. Hence the concentration can be calculated by the instrument’s programmed logic.
Importance of Calcium and Iron in the Aquarium
Calcium is, of course, a critical chemical parameter in reef aquaria. It is extracted from the water column by calcifying plants and animals. Calcium concentrations vary according to different references but 400 to 420 mg/L are the generally accepted levels. On the other hand, iron is found in seawater at very low concentrations (0.002 to 0.02 mg/L depending upon reference). Iron is a critical element in many biochemical processes and is an important factor in photosynthesis. At very low concentrations, iron can be a limiting element for photosynthesis.
Hanna Instruments
Hanna Instruments (Woonsocket, Rhode Island, USA) has been in business since 1978, and today offers over 3,000 products to its customers worldwide. Many of their products are of interest to aquarist and Hanna has in fact targeted the aquarium market. For more details, see ‘Contact Information’ near the close of this article. Hanna imports the Checkers and their reagents from Europe (Romania to be exact).
Instrument Programming
The iron and calcium Checkers have a number of pre-programmed features. In case of the iron unit, perhaps the most important is a timer (some instruments costing 15x as much lack this). Other programming includes high light, low light, inverted cuvette, under range, over range, low battery and dead battery. The Calcium Checker’s minimum reading is 200 mg/L while its maximum is 600 (and this is how under- and over-range samples are reported). No timer is required for calcium testing and low battery and dead battery is signaled. Quite a lot programmed into instruments the size of a pack of playing cards.
Manufacturer’s Specifications: Iron
High Range Iron Colorimeter (Model HI-721): Manufacturer’s Specifications Range: 0 – 5 mg/L Resolution: 0.01 mg/L Readout: Liquid Crystal Display (LCD; ~1/2″) Accuracy @ 25C (77F): ±0.04 mg/L; ±2% of reading LED Maximum Wavelength: 525nm Temperature: 0-50C (32-F) Humidity: 95% non-condensing Battery: 1 AAA Auto Shut-off after 3 minutes of non-use, and 10 seconds after measurement Weight: ~ 2 ounces
Hanna’s reagent for iron is similar to Hach’s and converts all soluble iron and most insoluble forms of iron in the sample to soluble ferrous iron. The ferrous iron reacts with the 1-10 phenanthroline indicator in the reagent to form an orange color in proportion to the total iron concentration.
Manufacturer’s Specifications: Calcium
Marine Calcium Colorimeter (Model HI-758):
- Manufacturer’s Specifications Range: 200-600 mg/L
- Resolution: 1 mg/L
- Readout: Liquid Crystal Display (LCD; ~1/2″)
- Accuracy: ±6%
- LED Maximum Wavelength: 610nm
- Temperature: 0-50C (32-122F)
- Humidity: 95% non-condensing
- Battery: 1 AAA
- Auto Shut-off after 10 minutes of non-use
- Weight: ~2 ounces
Hanna’s calcium test used a modified zincon (1-carboxy-2′-hydroxy-5′-sulformazylbenzene) procedure to test indirectly for calcium. In the test procedure, calcium displaces zinc from an EGTA (ethylene glycol tetraacetic acid) complex, which is then analyzed for. A blue color develops and is proportional to the amount of zinc displaced by calcium. Zinc is then measured colorimetrically. Reagent ‘A’ contains mostly sodium tetraborate decahydrate, a pH buffering agent followed by small amounts of zinc and sodium hydroxide.
Possible Interferences with the Zincon Calcium Analysis Method
An interference is a positive or negative effect on result of the target substance by an extraneous factor during analysis. For instance, the EDTA titration method is a popular method for calcium analysis but is one subject to a positive interference by strontium as well as a few other substances. Hanna’s method is not the EDTA method (it is instead the zincon method). I wondered of potential impact on the calcium result by another metal (strontium). I performed a quick analysis of deionized water spiked with a few crystals of strontium chloride and found the sample developed a blue color indicative of calcium. I cannot say for sure if strontium is a positive interference or if the strontium chloride (A.C.S. grade) used to spike the blank contained a small amount of calcium. From my limited data, I suggest this test may be subject to interferences.
Testing Protocol
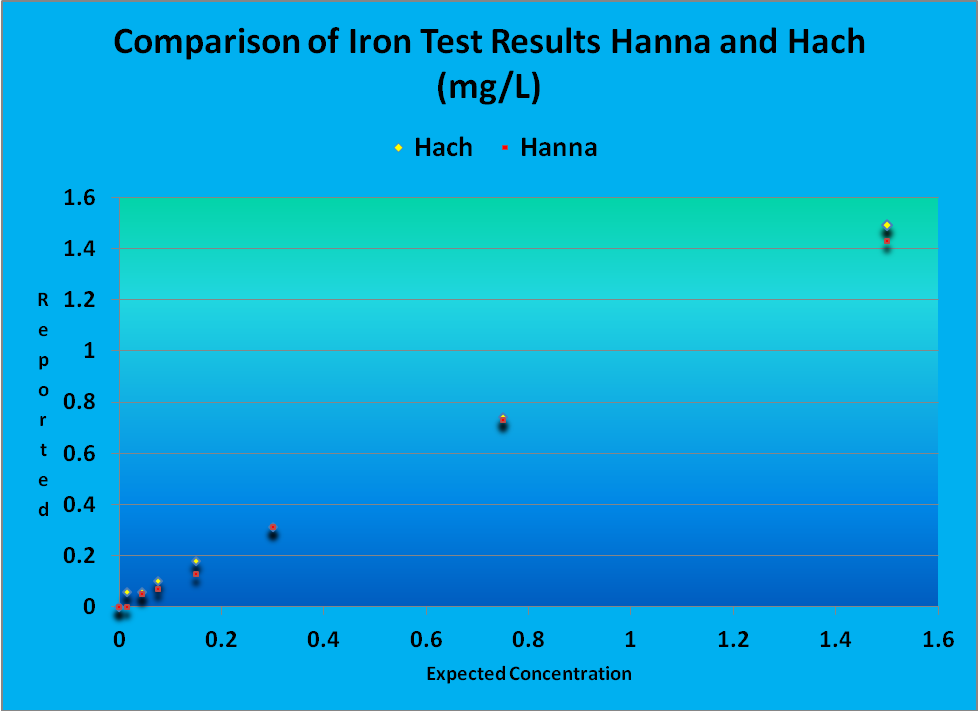
A solution containing 3 mg/L iron (a drinking water standard from Hach Company) was initially tested, and then diluted and tested by a Hach DR890 colorimeter and a Hanna Checker. Deionized water served as the control for both instruments. Results are shown in Figure 1.
A Certified Laboratory Standard (400 mg/L as Calcium, ±20 mg/L) checked instrument calibration. It consists of two 10-mL cuvettes – one contains a blank (unreacted sample) and the other a reacted sample containing a known amount of calcium. The Certified Standard I received contained 400 mg/L (±20mg/L) and results of 4 checks I performed were 399, 401, 399, and 400 – close enough for me! The Standard is marked with an expiration date (expect a shelf life of about 6 months). One standard check should be performed for about every 10 calcium analyses for quality control purposes. The Standard is relatively inexpensive at ~$15 US plus taxes and shipping if applicable. I highly recommend this option.
A standard was prepared from deionized water and commercially available calcium chloride. Once a baseline figure was obtained, this sample was diluted by 10% several times. These results are in Figure 2 below.
A single sample grabbed from the waters of a natural reef was analyzed several times in order to judge my laboratory technique. Results are shown in Figure 3.
Results
A comparison of results of iron testing by Hanna’s Checker and a much more expensive Hach colorimeter are shown in Figure 1.
Figure 2 shows the results of a standard calcium solution diluted several times to span the range of results reef hobbyists might expect to see.
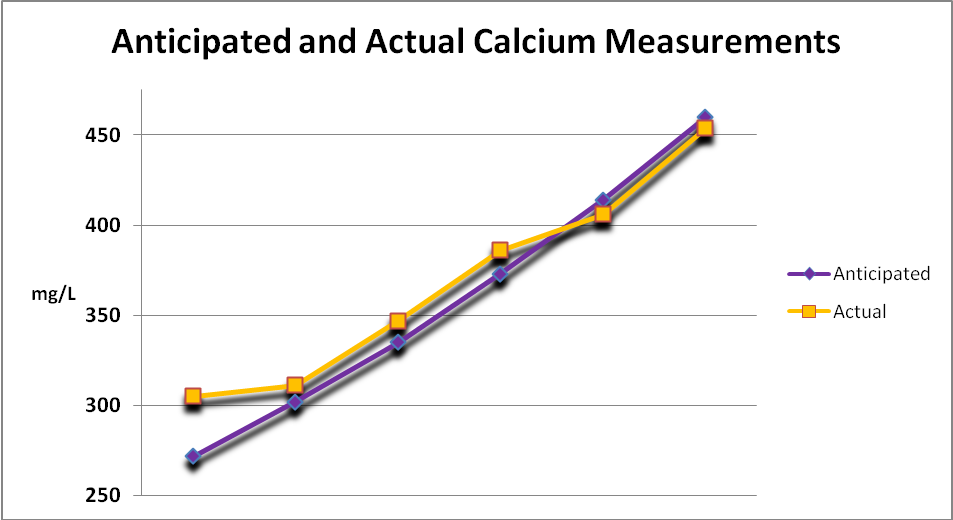
Figure 2. The blue markers and line shows the anticipated results of a calcium standard and multiple dilutions while the yellow line and markers are the actual test results.
Analyses of Calcium and Iron in a Water Sample from a Natural Reef
As a matter of curiosity, a water sample was gathered from the littoral zone at the 4-Mile Marker Reef in Kailua-Kona, Big Island of Hawaii and tested for Calcium and Iron. Salinity of the sample was 34 ppt. When we consider variations of calcium naturally found in seawater (400-420 mg/L) and the margin of error Hanna reports for their instrument (±6%) we could expect acceptable measurements of natural seawater to be in the range of 376 – 445 mg/L. Figure 3 shows the results.
Iron tests all generated readings of ‘zero’ (or below the detection of the instrument – chart not shown).
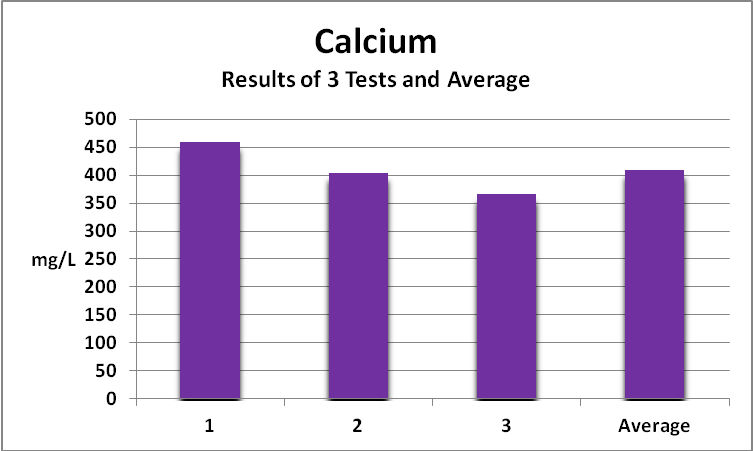
Figure 3. Calcium content of natural seawater as measured by a Hanna Marine Calcium Checker. Test #2’s result was slightly above the 6% margin of error while that of Test #3 fell slightly below.
Time per Test: Hach v. Hanna: The more difficult the task, the less likely it is to be performed hence ease of use and time invested are important considerations. Hanna’s iron test can be performed in about 6-7 minutes (including reagent dissolution and 3-minute chemical reaction times. Hach’s recommended procedure includes a 3-minute reaction period). The Calcium test requires about 3-4 minutes.
Changing the Battery: Some instruments require a certain degree of talent in order to change the battery. Not so with the Checker brand – simply remove a small Philips Head screw and slip a new AAA battery in.
Conclusions
It appears that the lower detection limit of the HI721 High Range Iron Checker is somewhere around 0.05 mg/L Total Iron (suspended and dissolved ferrous and ferric iron). The 721 is probably most useful for hobbyists specializing in freshwater or marine planted aquaria where iron is routinely dosed due to high demand. Due to detection limitations of the Checker, it suitable for analysis of natural and artificial seawater only if a high concentration of iron is known or suspected to exist (the reagent chemistry is sound and no normal interferences are expected).
For marine hobbyists measuring calcium of about 400 mg/L, expect readings of about 375 to 425 or so when practicing good laboratory technique. Hanna uses a highly diluted sample (~100x) and a small cuvette path length (~17mm) to extend the range of this test hence care should be exercised when performing the test procedure. The high dilution factor might account for the advertised accuracy of ±6%. Your dedication to properly performing this test is perhaps the most important factor. Be aware some components of natural and artificial seawater might introduce positive interferences.
Likes
- Instrument Price
- Iron Reagents are suitable for use in fresh, brackish, and seawater (be aware of instrument minimum detection limits. Calcium reagents are suitable for concentrations of 200-400 mg/L, likely limiting it to brackish and saltwater analyses)
- Good Accuracy for the price, especially for iron
- Reagent Price: ~36¢ per iron test; ~80¢ per calcium test
- Calcium reagents are marked with an expiration date
- Built-in timer (iron only)
- Instrument Error Messages
- Material Safety Data Sheet (MSDS) available on-line
- Hanna offers optional calcium and iron standards for quality assurance
Dislikes
- Reagent foil packages are large and not easily bent to allow pouring of reagent into the test tube
- Cuvettes are tall and easily tipped over
- Calcium test requires use of deionized water that is not supplied
Bottom line
Both instruments are recommended if the minimum and maximum detection limits meet your requirements and good laboratory practices are maintained. Good job Hanna!
Suggested Retail Price
The going price for the HI-721 High Range Iron colorimeter and the Calcium tester (HI-758) seems to be $50.00 – $55.00 US each. Additional iron reagents were found online $8.99 for 25 tests. Calcium reagents were priced at $19.50 for 25 tests. The Hanna Calcium Standard (highly recommended) is about $15 US. Shipping and taxes might be additional.
Optional Accessories
The Iron and Calcium Checkers come standard with two 10-milliliter cuvettes (test tubes) and caps. Should the need for additional cuvettes arise, order Hanna part number HI731231 (4 cuvettes); the part number for 4 additional caps is HI-731225. These part numbers are identical for all Checkers, regardless of test. Hanna offers a Calcium Standard (HI758-11) for about $15 US; a similar standard for Iron is offered by Hanna (HI721-11; 1 mg/L) is also available for ~$10 US. Cuvette Cleaning Solution (HI93703-50; 230 mL) will clean dirty vials but really isn’t needed if good lab practices are maintained. Other options include Cleaning Tissues (I use Kim Wipes™ or camera lens paper). Batteries (AAA) in my opinion should be purchased locally.
Other Instruments from Hanna
Many of Hanna’s other instruments may be of interest to aquarist, including:
- HI-727 Color of Water (0-500 Platinum-Cobalt Units; absorbance @470nm)
- HI-736 Phosphate Ultra-low Range (0-200ppb; Ascorbic Acid Method)
- HI-718 Iodine (0-12.5 mg/l; DPD Method). Note that chlorine and bromine are positive interferences to the DPD method of analysis.
- HI-706 Phosphate High Range (0-15mg/l; Ascorbic Acid Method)
- HI-717 Phosphate High Range (0-30mg/l; Ascorbic Acid Method)
- HI-764 Nitrite Ultra-low Range (0-200 ppb)
- A Magnesium Checker is under development
- Although Hanna markets the HI721 as able to analyze ‘High Range’ iron, there is no ‘Low Range’ instrument offered
Contact Information
Hanna Instruments has targeted the aquarium market and has devoted a webpage to hobbyists. See: [email protected]
Or write to:
Hanna Instruments, Inc.
584 Park East Drive
Woonsocket, RI 02895
Testing Procedures and Suggestions
Since Hanna’s Marine Calcium Checker involves a series of steps and a large dilution factor, perhaps we should review things a hobbyist can do to ensure acceptable results. The iron test is easier to perform but basic requirements still apply. First of all, find a comfortable, uncluttered, and well-lighted area for your miniature laboratory.
Recall that the normal amount of calcium found in freshly mixed seawater (~400 mg/L) depends on a specific gravity of about 1.025 or salinity of about 35 parts per thousand (ppt).
Good laboratory practices should be followed to ensure consistent repeatable results. The manufacturer’s directions are a good starting point – follow them! Treat the glass cuvettes with respect. Keep them clean, avoid using them if they become nicked, scratched or otherwise damaged. Don’t allow the testing sample to sit for very long after the test is completed – this might result in inaccurate measurements and can stain the cuvette’s glass. Don’t add the powder reagent to the cuvette when it is in the Checker device. This invites fouling of the cuvette chamber, subsequent cleaning and the possibility of instrument damage.
Hanna’s liquid reagents have a screw top with a snap ring to prevent spillage during shipping and for security reasons. Once the cap is removed, discard that annular ring remaining around the top. It is an accident waiting to happen – you’ll eventually grasp that loose ring and spill the bottle’s contents (I speak from experience!).
The Meniscus
Meniscus (from the Greek word menikos or “crescent”) is a crescent shape formed at the surface of a liquid and its container. A concave meniscus is formed when the liquid adheres to the container. Figure 4 shows how to correctly read the surface of a concave meniscus.
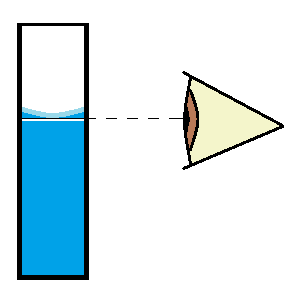
Figure 4. For best accuracy, properly filling the reactor chamber (test tube or cuvette) is a must. The bottom of the curved water surface (meniscus) should be even with the top of the filling line. Observe this by holding the cuvette at eye level.
An Inexpensive Cuvette Holder
While on the subject of the cuvette – I highly recommend use of some sort of cuvette/test tube rack to minimize the chance of spillage during the testing procedure. You likely have one already if you have access to a basic laboratory. If not, and you’ve got a few bucks to spend, check with a local lab supplier or online vendor. A perfectly serviceable cuvette holder can be made by drilling an 18mm (11/16″) hole in a short piece of dimensional lumber (such as a 2″ x 4″). Cheap but effective.
Tips on Sampling and Testing
Test results will be only as good as the care taken during the sampling and testing procedures. Samples should be analyzed immediately (usually ‘immediately’ means with 15 minutes but this time can be stretched to perhaps an hour). If this is not possible the samples must be preserved.
Sample Preservation Methods for Calcium and Iron
Proper sample preservation requires use of reagents normally found only in a laboratory setting. For the vast majority of hobbyists, these procedures should be avoided by analyzing the sample immediately. Use of acidic and caustic reagents should not be attempted unless you have access to proper personal protection devices and are trained to use them while handling laboratory chemicals.
This procedure is required if the sample cannot be analyzed within an hour or so. For calcium, collect the sample in a pre-cleaned plastic or glass bottle (using a detergent followed by rinsing with 1:1 nitric acid and deionized water). Adjust pH to <2 with nitric acid and refrigerate for up to six months at 4C (39F). Before analysis, warm the sample to room temperature and adjust the pH to ~7 with potassium hydroxide.
Iron samples should be collected in clean plastic or glass bottles. Adjust sample pH to <2 with nitric acid and store at room temperature. Before analysis, adjust pH to 3-5 with sodium hydroxide.
Waste Disposal
None of the reagents used in the iron or calcium tests are particularly hazardous, but avoid contact or inhalation with any reagents. Wear all applicable personal safety devices. Reacted samples can be flushed down the drain. Note that the zincon calcium reagent has a tendency to stain.
Thoughts of Product Quality Control in the Reef Aquarium Industry
Like many, I was disturbed when I recently learned of heavy metals contamination of activated carbon marketed by a well-known international supplier of aquarium goods. For two months this company shipped potentially contaminated carbon. To their credit they recalled their product but real damage had been done to this company’s reputation, not to mention the possible harm to more than a few aquarium inhabitants.
By chance, just a few days prior to this announcement, I attempted to mix a standard solution of calcium chloride (commercially available from this same marketer with a stated minimum guaranteed analysis of 33% calcium and a maximum of 37%). I calculated the amount required to mix a standard of just under 600 mg/L calcium. I have access to a relatively well-stocked wet lab and practiced conscientious laboratory techniques. Analyses were then performed by the Hanna Calcium Checker and checked against a Hanna laboratory-certified calcium standard. Test results were consistently below the expected concentration suggesting that ‘guaranteed analyses’ of the calcium chloride were incorrect. This revelation, followed by the incident described above, made me recall a few other events where marketers’ hyperbole did not match results generated in my laboratory. In fact, I began purchasing laboratory equipment in the early 1990’s due to ‘too-good-to-be-true’ claims made by a self-promoting and self-proclaimed aquarium ‘guru’. Two phrases come to mind: ‘Caveat emptor’ and ‘Trust but verify’.
In Closing
All items tested were obtained through normal retail channels.
Questions? Comments? Leave them in the comments section below. Private correspondence should be address to [email protected].




0 Comments