
When I first started work in a laboratory, determination of various aqueous constituents was a laborious task, beginning with mixing standards, manually graphing their absorbances, and then making comparisons with samples’ absorbance tests results. We manually recorded this information and charted it on graph paper. It was state-of-the-art for the 1970’s. We would be (and are) amazed with today’s analytical instruments. Compact, battery-operated colorimeters (a type of spectrometer) use conveniently packaged chemicals to quickly determine concentrations of various aqueous substances.
Of particular interest to me in this new world of laboratory instruments are devices used to analyze for alkalinity and phosphorus. Hanna Instruments offers relatively inexpensive colorimeters called ‘Checkers’ priced at about $50-$60 each (including a small supply of reagents, 2 test tubes, and carrying case). These instruments are small and highly portable. But how accurate are they, and are they worthy of your consideration? This article will attempt to answer these questions.
Hanna Instruments
Hanna Instruments (Woonsocket, Rhode Island, USA) has been in business since 1978, and today offers over 3,000 products to its customers worldwide. Many of their products are of interest to aquarist and Hanna has in fact targeted the aquarium market. For more details, see ‘Contact Information’ near the close of this article. Hanna imports the Checkers and their reagents from Europe (Romania).
Why Test?
Since you’re reading an article about testing for phosphorus and alkalinity, I’ll make an assumption you understand the role they play in aquaria. If you’d like to learn more about these, see:
- Phosphates: http://reefkeeping.com/issues/2006-09/rhf/index.php
- Alkalinity: http://www.advancedaquarist.com/2002/11/chemistry
Glossary
The following terms are used in this article:
- Cuvette
- A vessel for holding water, especially a transparent laboratory vessel (such as a test tube)
- Colorimeter
- A device for determining the concentration of a substance dissolved in liquid by comparing the intensity of its color with that of standard solution(s) of known concentration(s)
- mg/L
- milligrams per liter, essentially the same thing as ppm
- ppm
- parts per million
- Reagent
- A substance for use in a chemical reaction, especially for analysis
- Spectrometer
- For our purposes, an optical instrument capable of measuring intensity of transmitted light at a specific (but varying) wavelength, especially for determining the concentration of a dissolved substance
- Titrant
- The liquid reagent used in titrations
- Titration
- A method for determining the concentration of an aqueous substance by adding a liquid reagent of known concentration and measuring the volume necessary to convert the substance from one form to another
Methods and Materials
First of all, the results shown here are simply comparisons of those gathered by different analytical means. No standards were tested, and I am operating on the assumption that results of Hach’s EPA-approved methods and a ‘laboratory-grade’ spectrometer are most accurate. In addition, only one Hanna instrument each was used in these comparisons.
Water, gathered from a functioning marine fish-only aquarium, was used for the testing.
Samples tested for phosphate were gathered in acid-washed glassware and analyzed within a few hours’ time. Initial analyses indicated the phosphate content was at the upper detection limits of both instruments used (a Hach DR2800 spectrometer and the Hanna HI-713 colorimeter). Simple dilution with deionized water brought the phosphate to concentrations spanning the full range of both instruments and to levels realistically found in many reef aquaria. The Hanna and Hach devices both use the ascorbic acid chemistry method for analyses.
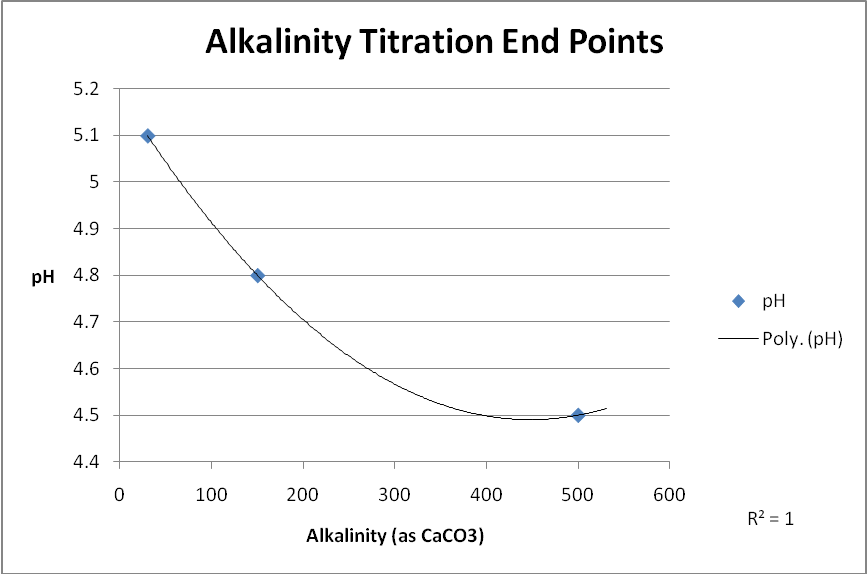
Comparing alkalinity results is not as straight-forward. The Hanna device estimates alkalinity through colorimetric analysis. Apparently Hanna has made correlation of chromatic shifts (indicating pH) and the impact of an acidic titrant on alkalinity. While making for a quick and convenient test, there are caveats. First, the amount of alkalinity determines the titration endpoint. In addition, the presence or absence of phosphate and/or silica also affects testing protocol (See Table 1 for titration endpoints).
| Test Condition | End-Point pH Values | |
|---|---|---|
| Alkalinity Concentration (as ppm CaCO3): | 30 | 4.9 |
| 150 | 4.6 | |
| 500 | 4.3 | |
| Silicates, Phosphates present or suspected | 4.5 | |
For comparative purposes, alkalinity values were determining using the Hanna’s colorimetric method and an end-point pH titration method. Hach reagents (sulfuric acid, either 0.160 or 1.600N) were used to titrate a magnetically-stirred sample. A calibrated meter monitored pH.
End-points depended upon alkalinity values shown in Table 1 while Figure 1 shows various endpoints based on alkalinity concentrations. Figure 2 is a photo of equipment used in measuring alkalinity according to EPA guidelines.
With background information out of the way, we can now turn our attention to Hanna’s HI-755 and HI-713 devices.
Alkalinity: Hanna Instrument HI-755
Hanna advertises these specifications for their Alkalinity Checker:
| Item | Specification |
|---|---|
| Range: | 0 to 300 ppm (mg/L) (some retailer ads incorrectly say 250 ppm) |
| Resolution: | 1 ppm (or mg/L, if you prefer) |
| Accuracy : | ±5 ppm (mg/L) ±5% of reading @ 25°C (77°F) |
| Battery Type: | One 1.5V AAA (included) |
| Light Source: | LED @ 610 nm |
| Light Detector: | Silicon photocell |
| Environment: | 0 to 50°C (32 to 122°F); 95% Relative Humidity maximum, non-condensing |
| Auto-off: | Yes, after ten minutes of non-use |
| Dimensions: | 81.5 x 61 x 37.5 mm (3.2 x 2.4 x 1.5″) |
| Weight: | 64 grams (2.25 oz.) – actually a little heavier (~75 g – yes, I checked!) |
| Colorimetric method, using a liquid pH indicator (bromcresol green) |
As discussed in detail in the Methods and Materials section, the results from the Hanna Checker were compared to analyses performed by the acid titration method using endpoints dictated by alkalinity concentrations and the presence of phosphates and/or silica. The results are shown in Figure 3.
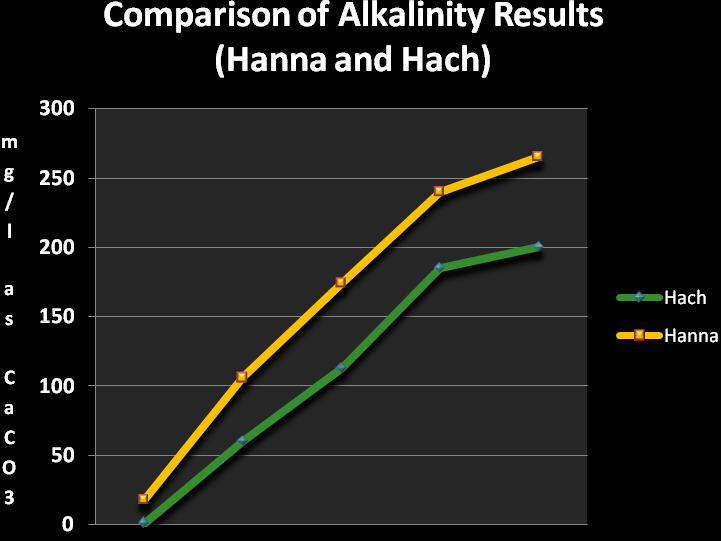
Figure 3. The Hanna Alkalinity Checker consistently reported values higher than those measured by the pH-endpoint titration method, but see Discussion (below).
Next, we’ll look at the Hanna Phosphorus Checker. Hanna advertises these specifications:
Phosphorus (Low Range), Hanna Instrument HI-713
Suitable for freshwater, brackish and seawater. Model Tested: HI-713 Phosphorus, Low Range
| Item | Specification |
|---|---|
| Range: | 0.00 – 2.50 ppm (mg/l) as Phosphate (PO43-) |
| Resolution: | 0.01 ppm (mg/l) |
| Accuracy : | ±4 % of reading @ 25°C (77°F) |
| Battery Type: | One 1.5V AAA (included) |
| Light Source: | LED @ 610 nm |
| Light Detector: | Silicon photocell |
| Environment: | 0 to 50°C (32 to 122°F); Relative humidity: 95% maximum, non-condensing |
| Auto-off: | After two minutes of non-use, and ten seconds after reading |
| Dimensions: | 81.5 x 61 x 37.5 mm (3.2 x 2.4 x 1.5″) |
| Weight: | 64 grams (2.25 oz.) |
| Analytical Chemistry: | Ascorbic Acid Method: Ammonium molybdate and potassium antimonyl tartrate react in an acid medium with ortho-phosphate to form phosphomolybdic acid that is reduced to molybdenum blue by ascorbic acid. |
| Interferences: | Silicates in excess of 10 mg/l; highly colored water (such as visibly yellow water) |
As discussed in detail in the Methods and Materials section, the results from the Hanna Checker were compared to analyses reported by a Hach 2800 spectrometer and ascorbic acid reagent. The results are shown in Figure 4.
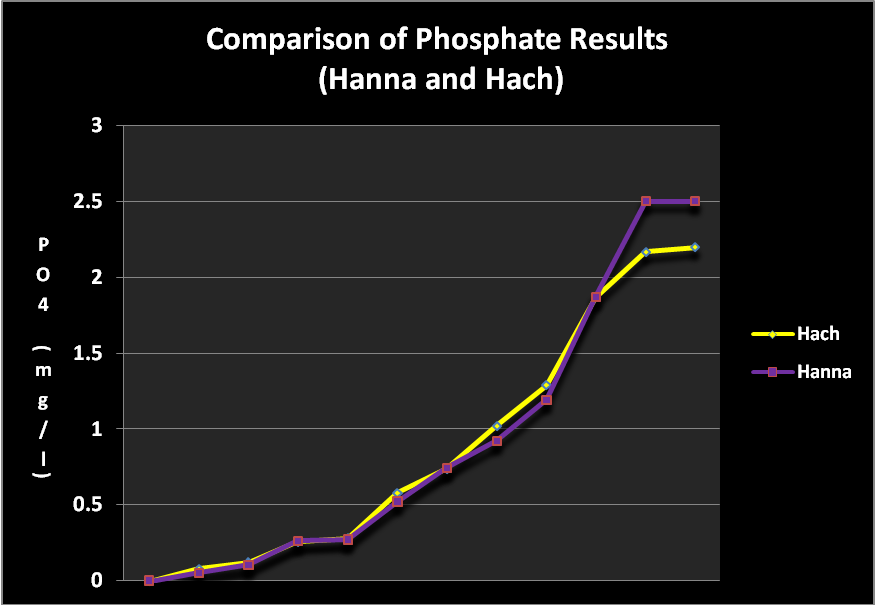
Figure 4. The results generated by the inexpensive Hanna Checker are remarkably close to those reported by a $3,500 spectrometer.
Comments and Recommendations
In many cases, results from colorimeters (and other photometric devices such as spectrometers) are superior to visually judging (comparing) colored samples. In fact, Standard method states: Visual comparisons of treated samples in Nessler tubes is not better than 5% (more often 10%) whereas photometric analyses can be reliable to within 3%, depending upon many factors. Naturally, the photometric device can eliminate personal biases of the user as well as accurately and precisely differentiate between small increments of sample absorbance/transmission.
If photometric analyses are potentially superior, the question of device engineering and quality becomes the issue (that is, will an inexpensive device deliver results comparable to an expensive one?). Under the conditions stated in the Methods and Materials section (above), these observations and recommendations are made:
Hanna Alkalinity Checker
These observations apply to the Hanna Checker HI-755 (Note the instrument is marked ‘Checker Marine’ suggesting it is intended for seawater analyses only):
Likes:
- Easy to use
- Results are generated in seconds
- The procedure is simple – no drip titration is required (just add reagent, shake, and read)
- Does not require any manual conversions (results are expressed as parts per million calcium carbonate)
- Replacement reagent is inexpensive
- Auto-shutoff
- Reagents marked with expiration date
- Small sample size (10 milliliters)
- Spare cuvette included
- Directions and MSDS available online
Dislikes:
- Alkalinity measurements were consistently higher than those generated by the titration/pH end-point method
- Multiple measurements are not possible without zeroing the instrument each time
- No Material Safety Data Sheet included (see below for link)
- Reagent will stain skin and clothing (all procedures using organic dyes carry the same risk)
- Battery replacement requires a small screwdriver and removal of a tiny screw
Recommendation (Alkalinity):
Though my quick comparisons showed the Hanna Alkalinity Checker delivered results consistently higher than those obtained with an EPA-approved method, I am impressed with the speed and ease of measurement. If I were to establish a correction (and comfort) factor to apply to the Hanna Checker’s results, this would be my method of choice for establishing alkalinity concentrations.
I recently received a Hach catalog advertising their version of a colorimetric alkalinity test. Hach’s chemistry, just as Hanna’s, apparently uses bromocresol green sodium salt in the analysis method. It seems this method is becoming widely accepted, if only for estimations (this is not an EPA-accepted procedure).
Recommendation: Yes, with some reservations
Footnote:
- 1 ppm Alkalinity as CaCO3 = 0.02 milliequivalent per liter (meq/L)
- 1 ppm Alkalinity as CaCO3 = 0.056 degrees Carbonate Hardness (dKH, or German Hardness)
Hanna Phosphate Checker
These observations apply to the Hanna Checker HI-713 (Low Range Phosphate):
Likes:
- Results compare very favorably with those generated by a much more expensive device
- Much less expensive than a full-blown spectrometer or photometer
- Easy to use and portable
- Results are generated in several minutes (including a 3-minute reaction time)
- The Checker incorporates a timer
- The procedure is simple – Add reagent, shake and bake for several minutes, and read results in parts per million as phosphate
- Replacement reagent is competitively priced
- Auto-shutoff
- Reagents work in fresh, brackish, and saltwater
- Reagents marked with expiration date
- Low Battery, Dead Battery, Under Range, Over Range, Inverted Cuvettes, High Light and Low Light errors are displayed when appropriate
- Small sample size (10 milliliters)
- Spare cuvette included
- Directions (but not MSDS) available online
Dislikes:
- Test results for phosphate are displayed for only 10 seconds after they are reported
- Multiple measurements are not possible without zeroing the instrument each time
- No Material Safety Data Sheet available
- Battery replacement requires a small screwdriver and removal of a tiny screw
Recommendation (Phosphate): Yes!
Footnote: To convert PO43 to P, divide by 3.066
Reagent Cost Comparisons
The following approximate prices are for replacement reagents only, and do not include instruments (unless noted), taxes, insurance, or shipping costs.
Phosphate:
- Hanna Phosphate Reagents: $27.99 U.S. per 100 tests
- Hach PhosVer3: $28.19 U.S. per 100 tests (10 milliliter sample)
- Footnote: The Hanna HI-714 phosphate colorimeter can use Hach reagents (ascorbic acid powder pillows).
Alkalinity:
- Hanna Alkalinity: $9.99 U.S. for 25 tests (~40 cents per test)
- Hach: Alkalinity for digital Titrator, $59.17 – includes sulfuric titration cartridges, phenolphthalein and bromcresol green/methyl red reagents. $219 includes reagents and Digital Titrator. To comply with EPA guidelines, a pH meter is also required, and a magnetic stirrer is useful.
- Hach’s new colorimetric Total Alkalinity test (25-4,000 mg/l as CaCO3): 25 tests for $31.45 ($1.26 U.S. per test) using only their spectrometer models DR2800, 3900 and 5000 (Note: These spectrometers begin at about $3,500 U.S.)
Material Safety Data Sheets (MSDS)
The two kits I obtained did not include Material Safety Data Sheets. Here is a link to the Alkalinity reagent: http://www.hannainst.com/sds/SDS_HI%20755S_2010-12-14.pdf I could not find a link to the Low Range Phosphate reagents on Hanna’s website at the time of this writing.
Sampling Procedures
“Garbage in; Garbage out” is a saying applicable to results generated when improper sampling procedures are in place. Use reasonable care when gathering samples for analyses. It is recommended that the vials (cuvettes) supplied by Hanna are used when sampling to avoid contamination.
Alkalinity: Analyze immediately or fix sample by refrigerating at 4°C (39.2°F) for up to 24 hours. Bring to room temperature before analysis. If the sample is gathered in a container other than Hanna’s cuvette, either clean plastic or glass is OK, and it should be completely filled to avoid prolonged exposure to any air trapped in the bottle. Filter if the sample contains excessive suspended particles.
Phosphate: Analyze immediately. Use the Hanna 10 milliliter cuvette to draw the sample if possible. If not possible, use clean plastic or glass containers – they should be scrupulously clean – preferable acid-washed with 1:1 hydrochloric acid and rinsed with de-ionized water. To fix the sample, exclude particulate matter via filtration and refrigerate at 4°C (or 39.2°F). Maximum holding time is 48 hours.
Treat the cuvettes with respect, and keep them clean and free of scratches. Don’t allow the treated samples to remain in the cuvettes any longer than necessary as the chemicals might stain the glass. If the vials do become stained, gently clean them with paper used for sensitive applications (such as that used to clean camera lenses). In extreme cases, a dilute bleach solution may be required to remove the stains. Some hobbyists keep the vials filled with deionized water between uses to prevent spotting within them.
Other Instruments from Hanna
Many of Hanna’s other instruments may be of interest to aquarist, including:
- HI-727 Color of Water (0-500 Platinum-Cobalt Units; absorbance @470nm)
- HI-736 Phosphate Ultra-low Range (0-200ppb; Ascorbic Acid Method)
- HI-718 Iodine (0-12.5 mg/l; DPD Method)
- HI-706 Phosphate High Range (0-15mg/l; Ascorbic Acid Method)
- HI-717 Phosphate High Range (0-30mg/l; Ascorbic Acid Method)
- HI-764 Nitrite Ultra-low Range (0-200 ppb)
Contact Information
Hanna Instruments has targeted the aquarium market and has devoted a webpage to hobbyists. See: [email protected]
Or write to:
Hanna Instruments, Inc.
584 Park East Drive
Woonsocket, RI 02895
Additional Comments
These products were obtained through normal retail channels, and descriptions, specifications and other information were current at the time of writing.
Questions? Comments? I am best reached at: [email protected] or sound off in the comments below.
References
- Holmes-Farley, R., 2006. Phosphate and the reef aquarium. http://reefkeeping.com/issues/2006-09/rhf/index.php
- Holmes-Farley, R., 2002. Chemistry and the aquarium: Solving calcium and alkalinity problems. http://www.advancedaquarist.com/2002/11/chemistry





0 Comments