
Herein we learn vital management skills that assist in the maintenance of pristine water quality (oligotrophy) while the author imparts vital background that arms the reader with sufficient insight to make informed choices, diagnose irregularities, and formulate mitigating strategies.
Unlike fish, hermatypic corals (zooxanthellate Scleractinia) help purify their environment by utilizing an extensive gamut of inorganic and organic particulate and dissolved compounds. SPS corals were not previously included in target-feeding schedules and grew phenomenally quickly and exhibited exemplary health, albeit most corals were harvested in situ on wild reefs and irradiance was administered like horsepower. Several corals merely require intense light, dissolved inorganic carbon (DIC), the energy reserve adenosine triphosphate (ATP), and calcium (Dunn et al. 2012). Wild corals acquire particulate organic carbon, nitrogen, and phosphorus (POC; PON; POP) from zoo-, nano-, and pico-plankton ranging from arthropods to bacteria, ciliates, and flagellates, while zooxanthellae proliferate in under illuminated well-nourished corals (Houlbrèque & Ferrier‐Pagès 2009).
History has taught us that SPS coral survivorship and health appears more contingent on sustained oligotrophy and photosynthetically useful radiation (PUR) than heterotrophy. However light impacts amino acid uptake, and traces of glycine, alanine, and phenylalanine stimulate scleractinian feeding responses (Houlbrèque & Ferrier‐Pagès 2009) where pork gelatin is alanine and glycine rich.
Fortuitously a lack of zooplanktonic prey will not kill SPS colonies because food inputs contribute to chronic pollution (eutrophy) which these corals cannot withstand. Akin to light (Marubini & Davies 1996; Jiang et al. 2014) they likely adapt to exploit nutrition received.
Conversely fish provide essential ambient ammonia yet stock too many, or overfeed, and reefs commence to malfunction (Aslett 2023). Functional reefs attain dynamic equilibrium where oligotrophy provides ideal conditions for all biota and as such, all potential prey items compete equally for resources. Arthropods and a smorgasbord of dietary delights are lacking in commercial fish-only systems because mandatory chemotherapeutics are lethal to invertebrates which is one reason to never mix a supplier’s water with your own. The other is fish-only systems operate between 80 to 90 mg l-1 (ppm) of nitrate and phosphate approaching 1 mg l-1. Such pollution is the direct consequence of ample feeds that maintains healthy populations of nitrifying bacteria that become waterborne to occupy a niche that might otherwise be filled with pathogens (Michaud et al. 2006) while planktonic nitrifying microorganisms remain rare in domestic aquaria (Hovanec, personal communication 2019).

Fig 1. Vitalis aquatic nutrition SPS coral food is micronized dry nourishment analogous to the fine particulate matter upon which these corals naturally dine. Each product manufactured by World Feeds Limited is formulated to satisfy species requirements by qualified and experienced scientists. Image courtesy of World Feeds Ltd ©, Doncaster, UK.
Eutrophy impacts diversity in several ways. Most sea life is nitrate-tolerant albeit echinoderms and arthropods which include sea urchins, copepods, and amphipods are particularly vulnerable (Holmes-Farley 2005). Hence a vast cross-section of potential prey find it hard to subsist which demands greater food inputs which intensifies eutrophication.
Fish forage and scavenge in oligotrophic domestic systems hence their diets require merely supplementing. Vis-à-vis captive reef fish nourishment, less is significantly more, whereas one heavy-handed feed can lead to elevated and persistent nitrate that lasts for months.
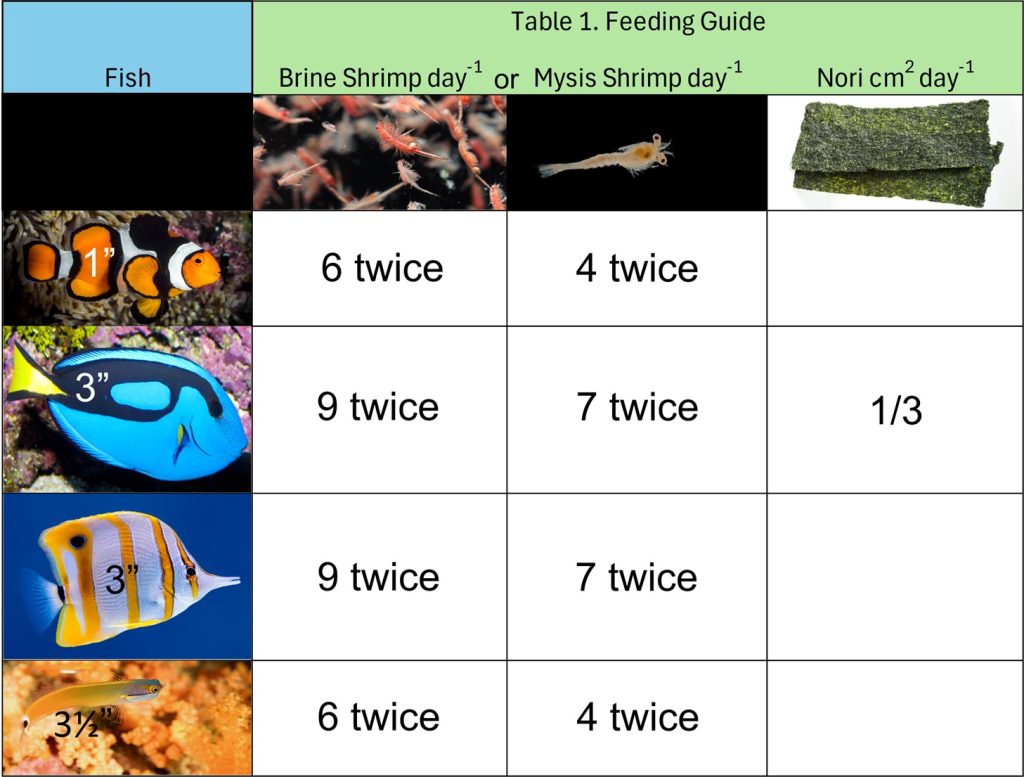
Fish must not be starved yet they will continue to eat far beyond their requirements and are thus challenging to satiate. Sufficiently nourished fish are full-bodied with abdomens continuous with their ventral flank (Fig 2.). All fish are individuals with interspecific traits yet they have personal preferences (Budaev & Brown 2011; Tosetto et al. 2017) and evaluating how an animal will behave once its natural diet is withdrawn, is at best impractical. Table 1. features well-nourished reef compatible teleosts and a nutritional guide for similar shaped and sized fish which are measured from their caudal peduncle to their snout.
Aquarists purchase janitorial fish for their SPS reefs because fish are polluters which must compensate the ecosystem for their keep. One inch of fish per 10 UK gallons was suggested previously, of which 50 percent is more appropriate.
Zooplanktivorous bony fish that browse throughout the day make befitting tankmates for SPS corals, whereas predatory piscivores like lionfish and groupers that feed on every third day excrete significant nitrogenous waste but intermittently, which will not sustain the logarithmic growth of nitrifying communities. Snappers, grunts, pufferfish, and triggerfish will devour ornamental shrimp and other welcome crustaceans and are thus non-reef safe.
Due to resource-expending osmoregulation and the meagre energy gained from the oxidation of ammonia and nitrite, nitrifying chemolithoautotroph interfission (doubling), is in the region of 30 to 40 hours (Hovanec, personal communication 2019), and as such, maturity takes two entire months to “catch up” with the biomass of recently introduced fish. Typically, nitrification is “kickstarted” with a solitary small clownfish, followed a month and a half later by a small tang or surgeonfish, and finally a longnose or copperband butterflyfish at two and half months. It is advised that SPS colonies be stocked no sooner than month three.
World Feeds Limited manufacture unparalleled specialist diets for ornamental teleosts and invertebrates. Their Vitalis aquatic nutrition is a privilege to recommend (Fig 1.).
Proprietary brine and mysis shrimp are frozen in immeasurably polluted water so an unopened frozen pouch is floated in a fresh- or salt-water reservoir for 20 minutes which should be aerated and the same temperature as the display. The contents are emptied into an ordinary fine hand net and carefully manipulated under a stream of cold mains water during which the volume will shrink. A final thorough rinse in either fresh reverse osmosis water or conditioned “salt mix” makes it suitable for fish or corals respectively, whereafter it is emptied and scraped into a polyethene bag and laid flat in a freezer. The process must be thorough but quick and efficient because defrosted feed rapidly oxidizes and turns black in air or when heated. Feeding oxidized food is very harmful insofar as it has negligeable nutritional value. Marine fish benefit greatly from ingesting freshwater because it assists osmoregulation whereas corals are stenohaline and intolerant of hypo- or hyper-salinity. Purification of this type has a meaningful impact on the trophic status of any system.

Fig 2. A severely malnourished and dehydrated yellow tang (Zebrasoma flavescens) which appears “pinched” on its abdomen and the outline of the skull and the skeleton are discernible with fluid collecting beneath the skin (oedema).

Fig 3. Rapid tissue necrosis (RTN) of an acroporid.
A cautious and diligent approach shaves the frozen block with the approximate number of prescribed zooplankton from table 1. which are applied to the surface. Alternatively, briefly wiggle the tip of the block submerged, where in time the aquarist learns to target individuals. Fish will try to nibble the block where momentary removal proves dissuasive.
Coral feeds are prepared from frozen where prewashed marine quintet offers welcome variety. Seek out oddments like rotifers and red plankton which can be cleansed in a brine shrimp net. Shave the frozen block with a conservative amount and mush between two dessert spoons, add a little tank water, and target feed using a disposable Pasteur pipette throughout wavemaker cessation. Have a set and judicious amount and stop when it is used and remember which corals were skipped and feed them first on the next occasion. A sensible frequency is twice per week which is supplemented by a biweekly nocturnal wiggle where we aim to administer 20 zooplankton for every 80 liters. Planktonic animals are only abundant in the well-lit euphotic zone at night, so hermatypes are adapted to nocturnal feeding but will capture food continuously.
The tentacles of large polyp stony (LPS) corals are most conspicuous at first light or after a feed. SPS aquarists must avoid housing azooxanthellate Scleractinia like sun corals of the genus Tubastraea, albeit their polyp extension is elicited by applying one or two zooplankton directly to their corallites. Soon after their tentacles extend and they will feed (Fig 4.). Some feed-dependent azooxanthellates are stony with small polyps.

Fig 4. Tubastraea coccinea (taxon inquirendum).
The animal and human supplement brewer’s yeast is the deceased micronized remnants of alcohol-fermenting Saccaromyces cerevisae (Ferreira et al. 2010) which comprises rare and beneficial bioactive species of chromium (Scawarz & Mertz 1959; Ferreira et al. 2010) and dietary precursors of nicotinamide adenine dinucleotide (NAD+) which is a multifunctional signal, cofactor, and substrate for various energy-requiring cellular processes including DNA repair (Elhassan et al. 2017; Orlandi et al. 2020).
Brewer’s yeast is a useful nutritional supplement for fish and corals while its insolubility and indigestibility confer its profound prebiotic and immunostimulatory qualities. Nevertheless, its soluble components likely comprise organic carbon so continual use may be analogous to SPS-killing carbon dosing (Kline et al. 2006) so use as an occasional treat.
Another major cause of eutrophication is decay like rapid tissue necrosis (RTN) which affects SPS colonies and permits the observer to watch as the flesh peels from the skeleton (Fig 3.). We must cut our losses and immediately remove and dispose of the afflicted to avoid unresolvable nutrient enrichment, albeit a thorough systemwide examination for sources of H2S and standing/stagnant water is advised to avoid a crash or prevent reoccurrence. See the preceding articles for guidance.
To learn more about the benign maintenance of calcium, magnesium, and alkalinity as SPS culture demands we utilize rapid yet inherently hazardous replenishment procedures, visit our website HERE.
References
Aslett, C., G. (2023) Holosystemics Part I: Captive Reef Function versus Malfunction. https://www.advancedaquarist.com/
Budaev, S. & Brown, C. (2011) Personality traits and behaviour. Fish cognition and behaviour. Brown, C., Laland, K. & Krause, J. (eds.). Wiley-Blackwell, Oxford, UK. pp 135-165.
Dunn, J., G., Sammarco, P., W. & LaFleur, G. (2012) Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: A controlled experimental approach. Journal of Experimental Marine Biology and Ecology. 411, 34-44.
Elhassan, Y., Philp, A. & Lavery, G. (2017) Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule. Journal of the Endocrine Society. 1(7), 816-835.
Ferreira, I., M., P., L., V., O., Pinho, O., Vieira, E. & Tavarela, J., G. (2010) Brewer’s Saccharomyces yeast biomass: characteristics and potential applications. Trends in Food Science & Technology. 21(2), 77-84.
Holmes-Farley, R. (2005) Reef Alchemy: Nitrite and the Reef Aquarium. ReefKeeping.com. https://reefkeeping.com/issues/2005-06/rhf/index.php
Houlbrèque, F. & Ferrier‐Pagès, C. (2009) Heterotrophy in Tropical Scleractinian Corals. Biological Reviews. 84(1), 1-17.
Hovanec, T. (2019) Dr. How to harness bacteria to cycle your saltwater tank quickly! | MACNA 2019. BrsTV. https://www.youtube.com/watch?v=zDI7sxqC-ss
Jiang, J., Zhang, H., Orf, G., Lu, Y., Xu, W., Harrington, L., Liu, H., Lo, C. & Blankenship, R. (2014) Evidence of functional trimeric chlorophyll a/c 2 -peridinin proteins in the dinoflagellate Symbiodinium. Biochimica et Biophysica Acta (BBA) – Bioenergetics. 1837(11), 1904-1912.
Kline, D., I., Kuntz, N., M., Breitbart, M., Knowlton, N. & Rohwer, F. (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Marine Ecology Progress Series. 314, 119-125.
Marubini, F. & Davies, P. (1996) Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Marine Biology. 127(2), 319-328.
Michaud, l., Blancheton, J., P., Bruni, V. & Piedrahita, R. (2006) Effect of particulate organic carbon on heterotrophic bacterial populations and nitrification efficiency in biological filters. Aquacultural Engineering. 34(3), 224-233.
Orlandi, I., Alberghina, L. & Vai, M. (2020) Nicotinamide, Nicotinamide Riboside and Nicotinic Acid-Emerging Roles in Replicative and Chronological Aging in Yeast. Biomolecules. 10(4), 604.
Scawarz, K. & Mertz, W. (1959) Chromium (III) and the glucose tolerance factor. Archives of Biochemistry. 85, 292-295.
Tosetto, L., Williamson, J., E., Brown, C. & Animal, B. (2017) Trophic transfer of microplastics does not affect fish personality. Animl Behviour. 123, 159-167




0 Comments