
One of the most significant and challenging aspects of SPS coral culture is the safe maintenance of calcium (Ca2+), magnesium (Mg2+), and alkalinity because SPS coral biocalcification is the fastest on planet Earth (Cohen & McConnaughey 2003; Munn 2019). Buffering is inextricably linked to dissolved carbon dioxide (DCO2), which manifests as carbonic acid (2H+CO32-) before much assimilates as carbonate/bicarbonate (CO32-/HCO3–). Acids are reductants that suppress dissolved oxygen (DO), pH, and water’s REDOX status (oxidation-reduction potential; ORP) and we comprehend that CO2 is 282 times more soluble in seawater than oxygen (Muangkaew et al. 2002; Dickson 2010) and is thus potentially injurious.

Fig1. Clarified kalkwasser as viewed through the side of a clear plastic kalk-dosing vessel featuring a white residuum of undissolved calcium hydroxide, toxic metal hydroxides, and calcium carbonate.
Calcium emanates from abyssal hydrothermal vents where pressure prevents its spontaneous deposition and dissolves particulate kinds, while oceanic calcium remains saturated. Remarkably, depletion occurs in finite volumes such as lagoons and the Red Sea (Holmes-Farley 2002).
Recirculating systems accumulate detritus and “potion”-derived synthetic compounds including chelating agents which are absent in pristine reef environments. Therefore, Ca2+ may bind to several substrate-bound substances, many of which are probably exported by rigorous husbandry (Holmes-Farley 2002). ~450 mg l-1 (ppm) of Ca2+ and ~1350 mg l-1 of Mg2+ occur in natural reef water, where ideal aquarium degree carbonate hardness (dKH) ranges from 8 to 11 (2.857 to 3.929 meq l-1; 143 to 197 mg l-1 CaCO3) with the latter inducing rapid SPS culture.
1705 mg l-1 (ppm) of Mg2+ are lethal to 50 percent of mysis shrimp (Americamysis bahia) after 48 hours at 20 parts per thousand (o/oo; Pillard et al. 2002), albeit survivorship increases as salinity rises (Pillard et al. 2002; Holmes-Farley 2003). Regularly test system water for Mg2+ while exploiting calcium reactors and maintain concentrations just below 1500 mg l-1.
Aqueous ions participate in dynamic equilibrium by continuously combining and dissociating where pH influences proton (H+) and hydroxyl (OH–) electrostatic associations and thus acid/base speciation. Bicarbonate (HCO3–) is an acid and carbonate (CO32-) is its conjugate base. Ca2+ solubility decreases with heat in contrast to most solutes, and thus readily accretes onto warm equipment. The pKa or the pH at which CO32-/HCO3– are in equal concentration is just less than 9 at 26.5°C (Marubini et al. 2008) whereas warmer water prompts H+ dissociations and enriches CO32-. The shift combined with declining Ca2+ solubility triggers the deposition of calcite (CaCO3(solid); Holmes-Farley 2002). Nucleation is augmented at existing sites such as “kalk-stirrer” effluent, calcium carbonate crystals, or hydrophobic zones (Delbeek & Sprung 1994; Koishi 2017).

Fig 2. Small polypropylene (PP) “beans” (“poly pellets”) sold as stuffing for cuddly toys are floated on the surface of kalkwasser to extend its longevity, which are retrieved with a net during reformulation and are merely 3GBP for 500 grams.
CaCO3, MgCO3, and CaMg(CO3)2 continually accrete onto and dissolve from surfaces. The latter two rapidly veneer CaCO3 and prevent further deposition because CaCO3 will not readily precipitate onto MgCO3 (Holmes-Farley 2002). Ionic bonds between Ca2+CO32-/HCO3– and Mg2+CO32-/HCO3– sequester these divalent cations which further impedes their deposition and maintains their solubility well below saturation. 80 to 90 percent of calcium manifests as Ca2+ which complexes with the oxygens of several water ligands due to their higher electron density (δ–), whereas 10 to 15 percent electrostatically binds to sulphate (SO42-) at seawater pH which also becomes complexed with several molecules of water (Holmes-Farley 2002; Zavitsas 2005). Ca2+ also combines with borate (BO33-), fluoride (F–), and diverse assortments of inorganic and organic PO43-, albeit 3Mg2+2PO43- are more plentiful which is only partially explained by the wealth of Mg2+ (Holmes-Farley 2002). Kalkwasser may cause soluble calcium phosphate to exceed saturation and form solid and sedimentary Ca3(PO4)2 (Wiseman 1965; Holmes-Farley 2002; NCBI 2004).
Three methods of trace element replenishment are at our disposal: the Balling method; kalkwasser administration, and/or calcium reactor effluent which range from the least to the most hazardous.
All marine life must expend resources on maintaining intracellular dissolved solids below those of seawater where fish concentrations are ~0.9 percent whereas the oceans are ~3.5 percent. Osmoregulation is part of a physiological dynamic equilibrium called homeostasis, and fish swim through vertically stratified thick layers of temperature-distinct water called thermoclines which they find stressful. Whereafter, they revert to a temperature of their choosing (Donaldson et al. 2008). They are familiar with the consequences which are foreseen, and their actions are voluntary. Now imagine the same thing happening involuntarily. Its unfamiliarity is feasibly disorientating and physiologically and mentally disturbing. Cortisol-mediated anxiety impacts fish immunity long after the stressor has ceased, and teleostean stress responses are very different to our own (Odio et al. 1986; Barton 2002). Some studies have concluded that fish do not feel pain like us, yet the sensitivity of dorsal fin rays are akin to our fingertips (Rose et al. 2014; Hardy et al. 2016). The author has kept fish for over 50 years in commercial and domestic settings and the suggestion that fish experience pain and distress markedly differently to us appears erroneous. Abrupt involuntary vacillations are therefore harmful and thus fish-only commercial systems are maintained with no more than a 10 percent weekly water change, because freshly constituted and conditioned “salt mix” is biochemically distinct from that of the system. The maximum frequency for SPS reefs should be in the region of 7 percent weekly which assists replenishment of ions that rapidly deplete like manganese (Mn2+), iron (Fe3+), and the halogenic anion iodide (I–), albeit we may need to exceed this guideline in smaller aquaria where we cannot use a calcium reactor.

Fig 3. A gravity-fed kalkwasser reactor with a mini powerhead that facilitates the initial “stir” because a dripper is not a makeshift “kalk-stirrer”.
Hans-Werner Balling proposed a trace element-replenishing procedure in 1994 which was later named the Balling method. Chloride (Cl–) is a small, rapid, halogenic anion and potent oxidizing-agent that disrupts ionic bonds and is the most concentrated of balanced seawater (Balling et al. 2008). Balling fortified calcium and alkalinity with solutions of calcium chloride (CaCl2) and sodium bicarbonate (NaHCO3), whereas magnesium was sustained with a 50/50 solution of magnesium chloride (MgCl2) and magnesium sulphate (MgSO4). Whereupon ionic balance was restored using a subtractive water change with sodium chloride-free (Balling) salt. Such salts are available from resources like https://www.matuta.de/ that have an online calculator but remember to purchase anhydrous (dry) reagents or correct their masses for water of crystallization (.H2O). Above we described the Balling method as the least harmful yet we can see that the osmotic balance of the system’s inhabitants will be altered involuntarily and abruptly. Notwithstanding, the author has the utmost regard for this precise and fundamentally beneficial procedure, which is preferable to the hazards associated with calcium reactors, whereas kalkwasser is unlikely to keep pace with a dedicated SPS reef unless cooled with an evaporative chiller. See: Part II (Aslett 2024).
Kalkwasser (limewater) was first described by Peter Wilkens in The Saltwater Aquarium for Tropical Marine Invertebrates published in 1973. A heaped teaspoon of powdered calcium hydroxide is vigorously blended in a UK gallon (1.2 US gallons) of cold reverse osmosis water and left to settle for ~10 hours. The clear liquor is a caustic, saturated solution with a pH of ~13 yet it absorbs atmospheric CO2 and spoils forming insoluble calcium carbonate, so its total shelf life is ~24 hours (Fig 1.). Some of you may recall chemistry lessons where blowing bubbles through limewater with a straw caused it to cloud, albeit its viability is extended to a total of ~36 hours when covered with polypropylene “poly pellets” used for stuffing cuddly toys (Fig 2.).
Dripper:
Ca(OH)2(solid) >> Ca2+(aqueous) + 2OH–(aq.) (1.)
Aquarium:
Ca2+(aq.) + 2OH–(aq.) + 2CO2(dissolved gas) >> 2HCO3–(aq.) + Ca2+(aq.) (2.)
Ca2+(aq.) + 2OH–(aq.) + 2CO2(dis. gas) >> ® 2CO32-(aq.) + Ca2+(aq.) + 2H+(aq.) (3.)
Nothing but clarified kalkwasser was drip fed into the system in a region of vigorous flow over many hours to compensate for evaporative loss which raised pH, calcium (Ca) and carbonate/bicarbonate (CO32-/HCO3–; equations 2. & 3.; Fig 3.). IMPORTANT: add kalkwasser too quickly, and it prompts ionic insolubility known as “snowing” while causticity gruesomely corrodes the flesh of livestock. Proper kalkwasser administration buffers nocturnal pH and neutralizes acidic calcium reactor effluent, while distinct dropwise administrations of moderately alkaline solutions of sodium carbonate (NaCO3) are made fresh on each occasion. Two kalkwasser drippers are made from inexpensive clear plastic containers which are rinsed and replenished every 12 hours to ensure a continuous supply.
It is not merely the pH value that matters but the method by which it is attained. pH instrumentation has to be pre-calibrated with freshy constituted buffers before it will measure accurately on a virtual scale between pH 7 and 9. Bright, clear, and slightly sparkly reef water has a daytime pH of 8.4, a DO of 8 mg l-1 (ppm), and an ORP between 370 to 385 millivolts (mV). A robust system operating within parameters will experience no more than a nocturnal pH drift of 0.2 (8.2) which occurs naturally with adequate degas/regas, which is distinct from attaining such values with ozone because water’s dissolved solids alter at the molecular level. The readings are the same, but the waters are different. Surplus DCO2 from respiration suppresses pH and/or insufficient degas leads to gas retention that inhibits regas, where carbonic acid in calcium reactor effluent adds to the collective.
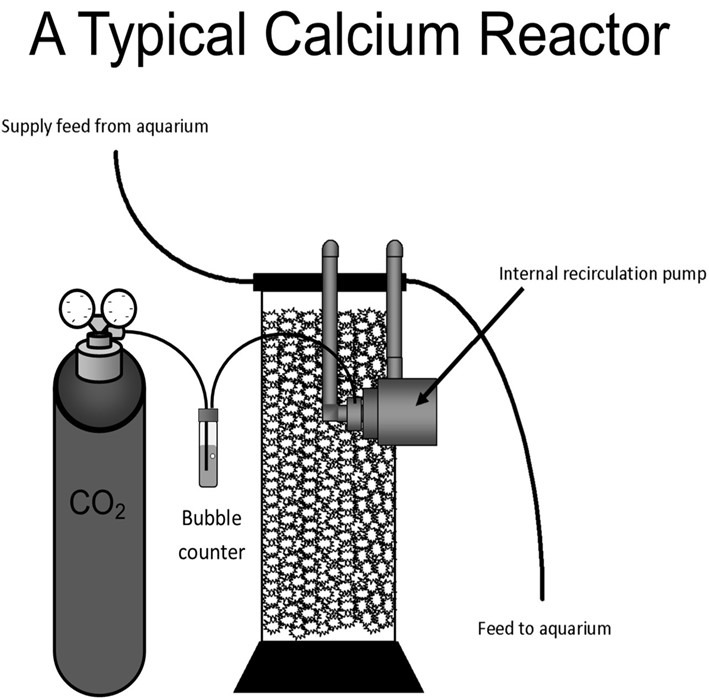
Fig 4. An illustration of a typical non-fluidized calcium reactor.
Limewater calcium hydroxide is pulverized aggregate which comprises toxic metals which precipitate and settle as insoluble hydroxides and are thus excluded when using merely the clarified liquor (Holmes-Farley 2003a; Holmes-Farley 2003b). Slurries added by “kalk-stirrers” may therefore include toxins which may be later stored in deep substrate as sulphides (Shimek 2002). Naturally occurring food grade calcium hydroxide is unlikely to undergo rigorous chemical analysis and kalkwasser’s alkalinity will likely generate false readings on aquarium test kits. Sources may comprise 0.25 percent Mg2+ and 0.024 percent strontium (Sr2+) which equate to Mg:Ca and Sr:Ca ratios of ~6.12 x 10-2 and ~2 x 10-2 respectively, which are wanting with regard to the ratios within coral skeletons and coralline algae (Holmes-Farley 2003). Notwithstanding the correct source of food grade calcium hydroxide will likely bolster these ions, yet much calcium hydroxide sold for kalkwasser may be builder’s hydrated (slaked; Ca(OH)2) lime, which may or may not prove harmless.
Kalk-stirrers introduce numerous hazards and are to be avoided, where the full horror story is available online in Aslett 2024a and thus a discussion here is unwarranted, yet their artefacts include unavoidable stagnation.
Functional calcium reactors dissolve CO2 in an isolated chamber of seawater to generate concentrated carbonic acid with a pH of 6.5 which erodes and solubilizes a solid calcium carbonate medium. Their designs should promote reactor-wide homogeneity and forestall medium zoning and fissures that may lead to stagnant pockets; however, thoroughly inspect them and their putative operation before purchase because they vary. Most designers have not accounted for stagnation most likely due to the misconception that calcium carbonate can neutralize H2S/HS– and nullify their toxicity (Fig 4.). Calcium reactors are highly efficient insofar as medium erosion and acidogenesis, but they tend to diminish DO to the point of anaerobia and flood the aquarium with DCO2 which may induce livestock decimating
hypoxia. That said, carbonic acid mediated deoxygenation is rare which renders such incidences redolent of H2S and HS–-expedited asphyxiation.
18” cubes of some 21 UK gallons (96 liters) are the smallest aquaria for which you will find a purpose-built calcium reactor, yet they must be undersized and “playing catch up” (Bertram, personal communication). Large reactors run slowly generate H2S while operating them too fast enriches systemwide DCO2, where the former is most harmful. Most small reactors are poorly designed and leak catastrophically. You cannot install them and walk away because the majority need finetuning several times per day, so hiding them in a cabinet is not for the fainthearted. The PF range of fluidized reactors from Deltec® are by far the most proficient, safe, and ergonomic which require minor adjustments merely two or three times per week. However, they are designed to adequately service 100-gallon and above systems, and they like others, must be disconnected before a vacation, drained, and recommissioned upon your return.
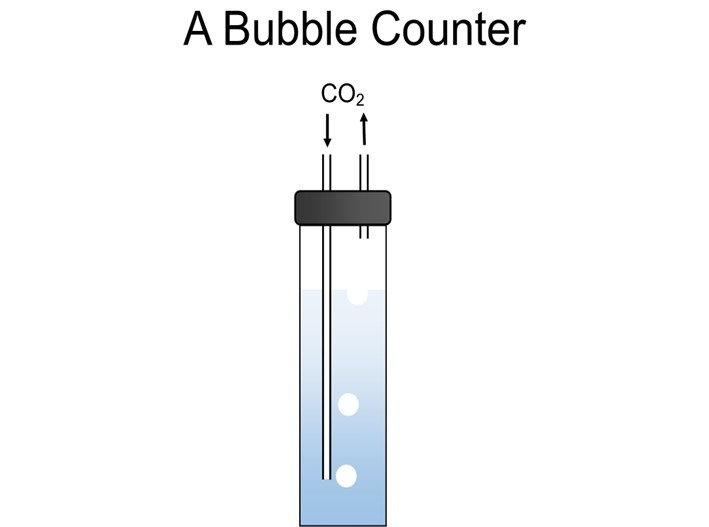
Fig 5. A traditional bubble counter where CO2 injection rates are measured by counting the bubbles rising from the long tube.
Bubble counter configurations vary greatly and several smaller reactors have quirky designs with integral bubble counters filled with reactor water. Counters must have little or no circulation and water enriched with CO2 and diminished in DO is at best bordering anoxic/microoxic. Filling a chamber with saltwater with such attributes barely moved by rising CO2 assures stagnation. Correctly designed bubble counters are remote from the reactor and filled with freshwater that cannot mix with reactor liquor throughout continual CO2 injection (Fig 5.).
Calcium reactor designers have conceded that plant life reverses metabolism to uptake O2 and liberate CO2 at night, hence aquarium CO2 regulators have gas solenoids that shut-off gas nocturnally. The solubility of CO2 in seawater ensures that counter and reactor water flood all available pipework upon CO2 cessation which appears to the unenlightened as an inconvenience rather than a deadly toxic soup. Nothing stands in its way. Non-return valves and filling the counter with glycerin prove non-inhibitory, while the author experimented with exchanging CO2 with air pressure which created difficulties too numerous to include. So when this occurs the only option is to flush the pipework to waste rather than into the reactor. Marine life has evolved in relative constancy and thus operating a reactor 24/7 provides stability and appears the only solution.
Calcium reactor-derived CO2 is neutralized by dropwise kalkwasser or the reverse lighting of a refugium, whereas no
inorganic substances appear to adequately neutralize and nullify the toxicity of H2S and HS–. Nevertheless, the harmful effects of somewhat analogous hydrogen cyanide (HCN) and H2S are mitigated by the vitamin B12 analogue, cobinamide (Jiang et al. 2016). Moreover, up drafted trickle towers or evaporative chillers can liberate surplus ozone, CO2, and some H2S.
Medium is a critical consideration where water is recirculated upwards through the reaction chamber which opposes compaction yet fissures and zoning can still occur. Hence non-fluidized medium should be amorphous with a grainsize approaching 10 millimeters. ROWAlith and ROWAlith C are silicate and phosphate reduced, excellent value, and suitable for various applications including fluidization (Fig 8.).
All media are calcite (CaCO3)-based yet some comprise magnesium carbonate (MgCO3) or they may be supplemented with a couple of tablespoons of dolomitic gravel (calcium magnesium carbonate; CaMg(CO3)2; Bingman 1997, cited in Huntington 2002).
All accreted elements become ions when coral rubble is utilized as a reactor medium which makes perfect sense and makes us feel aligned with nature. However microscopically porous scleractinian skeletons soak-up PO43- after coral demise. Mineralization thus frees an oversupply and all reactor effluents must dribble through a column of granular ferric oxide (GFO). It may be possible to “drive off” PO43- in a hot oven which recharges the eminently useful Kent Marine phosphate sponge.
The Sr:Ca ratios of the commercially available reactor media analysed by Bingman ranged from 1.3 x 10-4 to 2.5 x 10-4 (Bingman 1997), whereas typical skeletal ratios are 80 to 154 times greater at ~2 x 10-1. Coral accreted Mg2+ ranges from 0.07 to 3.5 percent and coralline algae contain over 1 percent, whereas proprietary media comprised around 135 to 408 times less (Bingman 1997; Holmes-Farley 2003; Holmes-Farley 2003a; Holmes-Farley 2003c).
Pressure and heat elicit calcite recrystallisation and marble formation on the boundary between convergent tectonic plates, and ancient skeletal and oceanic compositions were markedly different. Marble comprises merely 8 percent calcite (CaCO3) and 92 percent dolomite (CaMg(CO3)2) with a Ca:Mg ratio of ~1.5 which provides ample fortifications (www.aristonmable.eu). Pure white marble has minimal impurities and is phosphorus (P) and silicon (Si) limited; however, its long-term use may cause Mg2+ to exceed toxic thresholds. The strontium oxide (SrO) component of marble ranges from 1.0 x 10-2 to 3.0 x 10-2 percent which equates to around 9.0 x 10-3 and 2.7 x 10-2 percent Sr2+, whereas ~9.0 x 10-3 percent occurs in coral skeletons. Hence Sr2+ will be roughly correct or slightly high which may reach toxic thresholds over several months, so test regularly.
Calcium reactor throughput must be a rapid dribble which assures sufficient DO ingress. They are typically fed with a small bore sidearm of a return feed or a venturi run in reverse but preferably opt for a sufficiently small, slow, and reliable peristaltic pump operated tubing side down over system water.
Reactors are initially setup with one bubble per second whereafter effluent pH is appraised after three hours with pre-calibrated instrumentation. They have a reactor-wide pH of no less than 6.5 when operating within constraints, whereas their pH will be nearer or less than 6, and they will denitrify and liberate the intermediates ammonium and nitrite when anoxic. A visual guide to ORP is featured in figure 6. because H2S/HS–, ammonia/ammonium (NH3/NH4+), and surplus CO2 reduce water which appears dull/grey and slightly cloudy.
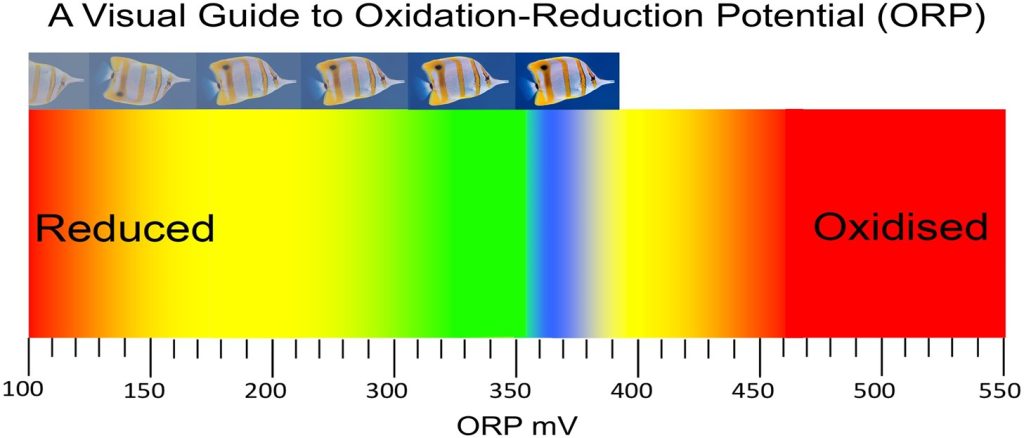
Fig 6. A visual guide to oxidation-reduction potential (ORP). Observe the brightness and clarity of the water surrounding the butterflyfish and its corresponding ORP. Water in fish-only systems should be maintained between 260 and 320 mV, whereas reef water should naturally range from 350 to 370 mV and above.
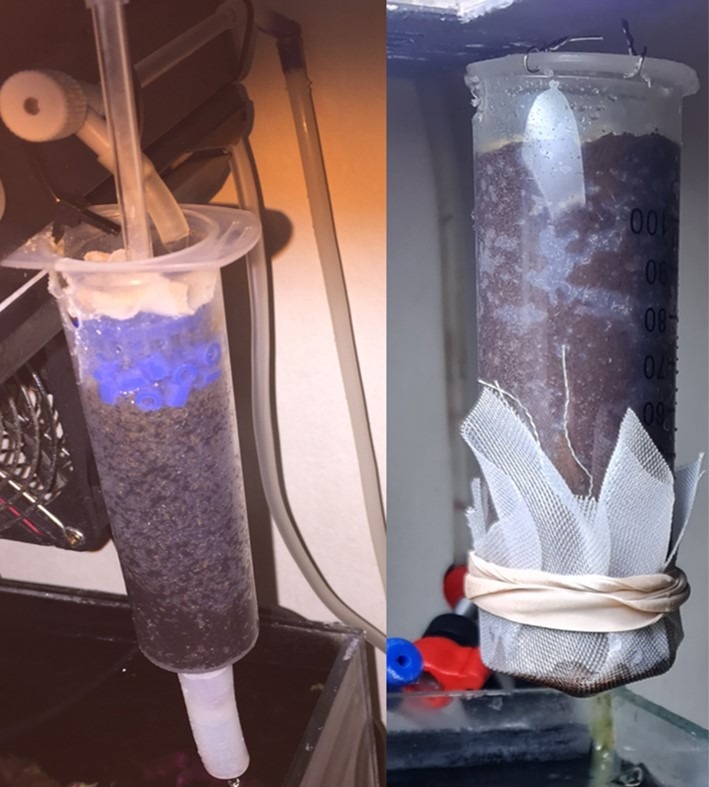
Fig 7. Two kinds of granular ferric oxide (GFO) columns removing PO43- from calcium reactor effluent. Both are barrels from large disposable syringes exceeding 50 milliliters and have “escape” holes that ensure blockages do not cause system leakage. Aslett ©.

Fig 8. Partially used ROWAlith coarse calcium reactor medium available from D-D The Aquarium Solution.
Maintaining rapid throughput and merely adjusting bubble rate is key.
Next we explore the scarcer trace elements and their maintenance with reference to DIY supplements in part VI.
Learn more about Chris Aslett and Reef Ranch HERE
References
Aslett, C., G. (2024) Teach a Person to Fish… SPS Academy Part II. https://www.reefranch.co.uk/
Aslett, C., G. (2024a) Reef Digress: Fundamental and Foundational Science: Not Simply Water. https://wwww.reefranch.co.uk/
Balling, H., W. (1994) Kalkwasser for the reef aquarium. DATZ. 08, 523-525.
Balling, H., W., Janse, M. & Sondervan, P. (2008) Trace elements, functions, sinks and replenishment in reef aquaria. Advances in coral husbandry in public aquariums. Leewis, R., J. & Janse, M. (eds.). Burgers’ Zoo, Arnhem, The Netherlands. pp 143-156.
Barton, B., A. (2002) Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integrative and Comparative Biology. 42(3), 517-525.
Bertram, S. (2004) D-D The Aquarium Solution, D-D The Aquarium Solution Ltd, 11-17 Fowler Road, Hainault Industrial Estate, Ilford, Essex IG6 3UT.
Bingman, C. (1997) Calcium Carbonate for CaCO3/CO2 Reactors: More Than Meets the Eye. http://web.archive.org/web/20010210225056/http://www.animalnetwork.com/fish2/aqfm/1997/aug/bio/default.asp
Cohen, A. & McConnaughey, T., A. (2003) Geochemical Perspectives on Coral Mineralization. Reviews in Mineralogy & Geochemistry. 54, 151-187.
Delbeek, C. & Sprung, J. (1994) The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates (Volume 1). Ricordea Publishing, Coconut Grove, Florida 33133, USA. pp 228-229.
Dickson, A., G. (2010) The carbon dioxide system in seawater: equilibrium chemistry and measurements. Scripps Institution of Oceanography, University of California, USA. https://www.researchgate.net/publication/284774361_The_carbon_dioxide_system_in_seawater_Equilibrium_chemistry_and_measurements
Donaldson, M., Cooke, S., Patterson, D. & Macdonald, J. (2008) Cold shock and fish. Journal of Fish Biolog. 73(7), 1491-1530.
Hardy, A., R., Steinworth, B., M. & Hale, M., E. (2016) Touch sensation by pectoral fins of the catfish Pimelodus pictus. Proceedings of the Royal Society B: Biological Sciences. 283(1824),.
Holmes-Farley, R. (2002) Chemistry and the Aquarium: Calcium. AdvancedAquarist.com. https://www.advancedaquarist.com/2002/3/chemistry
Holmes-Farley, R. (2003) Aquarium Chemistry: Magnesium in Reef Aquaria. AdvancedAquarist.com. Volume 11. https://www.advancedaquarist.com/2003/10/chemistry
Holmes-Farley, R. (2003a) Aquaria with low soluble metals. ReefKeeping.com. Volume 4. http://reefkeeping.com/issues/2003-04/rhf/feature/index.htm
Holmes-Farley, R. (2003b) Chemistry and The Aquarium: Aluminium in The Reef Aquarium. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/7/chemistry
Holmes-Farley, R. (2003c) Aquarium Chemistry: Strontium and the Reef Aquarium. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/11/chemistry
Huntington, S. (2002) A Guide to Using Calcium Reactors. ReefKeeping.com http://www.reefkeeping.com/issues/2002-05/sh/feature/
Jiang, J., Chan, A., Ali, S., Saha, A., Haushalter, K., J., Lam, W., L., Glasheen, M., Parker, J., Brenner, M., Mahon, S., B., Patel, H., H., Ambasudhan, R., Lipton, S., A., Pilz, R., B. & Boss, G., R. (2016) Hydrogen Sulfide–Mechanisms of Toxicity and Development of an Antidote. Scientific reports. 6, 20831.
Koishi, A. (2017) Carbonate mineral nucleation pathways. Chemical Physics [physics.chem-ph]. Université Grenoble Alpes, English. https://tel.archives-ouvertes.fr/tel-01701947/document
Marubini, F., Ferrier-Pagès, C., Furla, P. & Allemand, D. (2008) Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs. 27, 491.
Muangkaew, S., McKelvie, I., Grace, M., Rayanakorn, M., Grudpan, K., Jakmunee, J. & Nacapricha, D. (2002) A reverse-flow injection analysis method for the determination of dissolved oxygen in fresh and marine waters. Talanta. 58(6), 1285-1291.
Munn, C., B. (2019) Marine Microbiology: Ecology & Applications, Third Edition. Munn, C., B. (ed.). CRC Press, Taylor & Francis Group, London. pp 273-326.
NCBI (2004) National Centre for Biotechnology Information. PubChem Cofound Database; CID=24456. https://pubchem.ncbi.nlm.nih.gov/compound/24456
Odio, M., Goliszek, A., Brodish, A. & Ricardo, M., J. (1986) Impairment of immune function after cessation of long-term chronic stress. Immunology Letters. 13(1-2), 25-31.
Pillard, D., Dufresne, D. & Mickley, M. (2002) Development and validation of models predicting the toxicity of major seawater ions to the mysid shrimp, Americamysis bahia. Environmental Toxicology & Chemistry. 21(10), 2131-2137.
Rose, J., D., Arlinghaus, R., Cooke, S., J., Diggles, B., K., Sawynok, W., Stevens, E., D. & Wynne, C., D. (2014) Can fish really feel pain? Fish. 15, 97-133.
Wiseman, J., D., H. (1965) Calcium and magnesium carbonate in some Indian ocean sediments. Progress in Oceanography. 3, 373-383.
Zavitsas, A., A. (2005) Aqueous Solutions of Calcium Ions: Hydration Numbers and the Effect of Temperature. J. Phys. Chem. B. 109(43), 20636-20640.




0 Comments