I began rearing the common clownfish, Amphripion ocellaris in relatively large numbers in late 1972. This was the first actual commercial scale culture of a tropical marine aquarium fish. Soon after the success with clownfish I wanted to broaden the numbers of species under culture and the neon goby was an excellent candidate. It was a small species, very easy to maintain, spawned very easily in a small tank, and was in some demand in the then rather small, marine aquarium hobby. It was important to inform hobbyists of these developments and so over the years I usually wrote a short article on each new species that we brought under culture. At the time, the most prestigious marine aquarium magazine (out of the two then in print) was The Marine Aquarist published by Dr. John Miklosz (Terry Siegel, was the co-editor). I wrote a number of articles for this magazine, including the following one on propagating the neon goby. This article, recently updated, contains some information originally reprinted from the February 1975 issue of The Marine Aquarist.
The neon goby, Gobiosoma oceanops, is one of the largest species of the genus and probably the most common goby in marine aquariums. It is a cleaner species and even though of small size (2 to 3 inches as adults) the neon goby does well in a community tank. It will engage in cleaning behavior with Pacific as well as Atlantic marine fishes. We often keep them with large clownfish and frequently observe symbiotic cleaning behavior. The clownfish assumes a head up position and slowly flutters its fins while the neon goby swims with rapid, jerky movements over the fins and sides of the clownfish looking for parasites. A quick shake and resumption of normal swimming posture by the clownfish breaks the cleaning pattern and sends the goby on its way. The neon and its close relative, the shark-nosed or gold lined goby, Gobiosoma evelynae, make an interesting and colorful addition to any marine tank and even benefit the occupants through their parasite cleaning behavior.
As the neon gobies mature, they begin to pair for mating and this can cause great problems in the close confines of an aquarium. Once a pair is established they forcibly reject any others of their species and even a 100- gallon tank is not big enough for three neon gobies. However, if six to eight or more gobies are present in a tank, pairing and aggressive behavior is muted, and aside from a few minor squabbles, the fish can co-exist.
Once a pair is identified, they can be easily induced to spawn by providing a suitable spawning habitat. The neons, like other gobies we have worked with, are secretive spawners. They select an overturned shell, small pipe or inside of an aquarium ornament as a spawning site. Spawning has always occurred on the underside of a surface and the attached eggs extend downward into a restricted cavity. Spawning usually takes place in the early morning hours, although I have observed it to occur in afternoon and early evening hours as well. It is difficult to observe the actual deposition of eggs because of the secretive spawning site, but there seems to be several periods of egg deposition by the female followed by fertilization by the male. Both sexes twitch and wiggle side by side under the shell during the spawning process. The female leaves the site after spawning and only infrequently visits the eggs during the incubation period.

A close up shot of neon goby eggs after 7 days of incubation. The well- developed eyes indicate that hatching is close. The eggs are about 2 mm long and hatched soon after the photo was taken.
The male spends much time caring for the eggs during incubation by moving his body and fins over the egg patch at frequent intervals. He readily leaves the eggs for short periods to feed and explore, but soon returns and rests upside down underneath the egg mass. The female often rests above the shell at the entrance to the nest cavity or cruises about the general vicinity. The shell containing the spawn can be removed for examination and replaced without any harm to the eggs or disruption of the male’s incubation behavior. The male cares for the eggs throughout the incubation period, which, depending upon the temperature may be between 6 to 8 days.
A large female at the height of reproductive activity may lay 500 to 600 eggs, but the usual spawn size is about 250 eggs. The egg capsule is about 2 mm long and 1 mm in diameter and is completely transparent. The entire development of the embryo, from the first division of the blastodisc through to hatching, can be observed through the transparent chorion, or egg “shell” casing. The attachment of the egg consists of a mass of fiberous threads extending from the base of the egg capsule to a sticky pad that adheres to the substrate. The eggs are placed very close to each other and the newly laid spawn has the appearance of, an undulating patch of clear globules. After the eyes become pigmented, the patch takes on a silvery appearance. The embryo begins development with the head pointed towards the base or attached end of the egg capsule. The embryo turns around in the egg capsule on about the third day of development and completes incubation with the head developing within the stelate, distal end of the capsule Feddern (1967) and Valenti (1972) both describe embryonic development of neon goby larvae and these papers should be consulted for technical details on larval development. Valenti states that larvae that do not reverse in the egg capsule at 50 hours, but complete development with the head at the base of the capsule, do not hatch. Our experience has shown that hatching takes place regardless of the position of the larvae. At hatching, the capsule ruptures by the head of the larvae wherever it happens to be positioned, and the larvae forces itself out the opening. I have never observed a larvae fail to hatch because of a reversed position.

This newly hatched neon goby is only 4 mm long and retains a remnant of the yolk sac that will provide energy for early development.
(Note: At first, the larvae were recovered similar to recovery of clownfish larvae in a large tank. The light from a flashlight was aimed at a corner of the tank shortly after hatching occurred at night. The larvae were attracted to the light and concentrated in that corner where they could be siphoned out into a bucket and then transferred to the rearing tank. This was too laborious a method for commercial culture however, and we soon discovered that the shell or pipe section that contained the egg mass could be removed on the day that the hatch was expected and hatching could be stimulated manually. This was done by exposing the nest to a relatively bright light and then gently stroking the eggs with a feather from a sea gull. Within a few minutes of stimulation with the feather if the eggs were ready, they would begin to hatch en mass and the larvae would pop off the nest like popcorn. We could determine when the eggs were due to hatch by counting 7 days from the spawn and also by the size of the eye of the embryonic larval fish compared to the size of the residual yolk sac in the embryo. The embryo was ready to hatch when the size of the yolk sac was about the same size as the eye of the embryo. This seems to hold true for clownfish as well.)

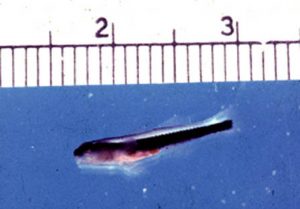
A neon goby larva at 16 days old, not far from metamorphosis into a bottom dwelling juvenile. The late larva is still transparent and lives above the bottom in the open water.
In one instance that I was fortunate enough to observe through the microscope, a malformed goby larva was completely encased in a normal egg capsule. The larvae appeared to be missing a portion of the notochord and the body wall about the gut was completely absent leaving the gut and yolk sac exposed within the egg capsule. This condition was observed shortly after hatching of the spawn and this larva had eroded the egg capsule in the vicinity of the gut instead of at the head. These observations indicate that hatching occurs as a result of the release of some substance, probably a proteolytic enzyme, originating in the vicinity of the gut and released at the mouth, that breaks down the egg capsule in the area of the head.
(Note: In nature, and sometimes in marine aquaria, neon gobies nest in small, deep holes with only a tiny opening just large enough to allow passage of an adult goby. I have no idea how the tiny larvae get from the hatched egg, entwined deep in the nest with other eggs, to the open waters of the tank, but they do. Perhaps the male helps as I have seen a male neon goby appear at the entrance of his nest, open his mouth, and then release a tiny, new-hatched larvae that then swam up into the water column. Not once, but twice did I see this!)
An early juvenile transformed overnight into a benthic living goby with dark blue and black coloration.
The larvae are quite small (4 mm long) upon hatching and usually carry a residual yolk. Feeding usually begins about 12 hours after hatching, depending upon the state of development at the time of hatching. Small living organisms are required as a first food. The larval stage of the neon goby is rather prolonged. First metamorphosis into the adult coloration and behavior pattern occurs at about 18 to 20 days, although it may extend to 40 days under adverse conditions. The larvae are reared under a carefully simulated pelagic environment.
(Note: At the time that this article was written, late 1974, great secrecy surrounded the culture of marine tropical fish. There were only two commercial, marine tropical fish hatcheries at that time, Aqualife Research, and Neptune’s Nurseries. Aqualife Research was my own small, struggling company and Neptune’s Nurseries was a sister company of the rich and powerful Aquarium Systems that had recently entered the field with my old friend Frank Hoff at the helm. Although the technology for rearing marine tropical fish was relatively simple, especially at that time, it was a new application of a fledgling technology and it held the promise and potential for a new and lucrative industry. There was a tendency to make it appear that we were in possession of special secrets worthy of protection from industrial spies and that our problems were less significant than those of the other participants in this new endeavor. Therefore I took pains in writing my articles to document our success in rearing various species without describing the “carefully simulated pelagic environment.” In those days, a bare 75-gallon tank surrounded with black plastic sheeting, with a two bulb fluorescent fixture resting on the top of the tank and two air stones spaced centrally in the tank. Rotifers, brine shrimp, and water changes completed the picture. Actually not a whole lot has changed in 30 years.)

Tank reared neon gobies can be held in large numbers in grow-out tanks. The two month old neon gobies in this 75 gallon tank were reared from two spawns.
The early juveniles take up a benthic mode of life shortly after the first color appears on the transparent larvae. A faint blackening of the sides quickly becomes a bright sliver of electric blue and the cupped pelvic fins attach the early juvenile to the tank substrate. Growth is rapid after this point in development is attained and sub-adult size is reached within 3 months. The young gobies can be paired at this time although first spawning is still 2 or 3 months in the future.
We have spawned about ten pairs of tank-reared gobies to date and have noted no obvious difference between wild and tank-reared fish, either in morphology or reproductive success. Growth continues after spawning commences and when the fish are ten months to a year old, they are full adult size and are at the height of reproductive activity. Spawning takes place every 10 to 12 days depending on temperature, and we have had pairs spawning in every month of the year. The spawning period in nature is February to April (Feddern, 1967); however, we have been able to spawn neon gobies every month of the year in the laboratory.
References
- Feddern, H. A. 1967. Larval Development of the Neon Goby, Elacatinus oceanops, in Florida. Bull. Mar. Sci. Vol. 17, No. 2, pp 367-375.
- Valenti, R. J. 1972. The Embryology of the Neon Goby, Gobiosoma oceanops. Copea, 1972, No. 3. pp 477-482.




0 Comments