The bulb tentacle anemone, Entacmaea quadricolor is one of the more popular anemone species in the aquarium trade and luckily, one of the easiest to keep. Their colors are highly variable from brown to green to red (the most desirable of which are the rose-colored varieties) with various combinations and patterns. In reading through the literature, both print and on the Internet, one can find a lot of speculation on its ecology and behaviour.
One of the most perplexing phenomena involves the formation of bulbs on the tips of the tentacles. In aquaria, the tentacles of bulb tipped specimens commonly become gradually “stringy” for lack of a better term, loosing the characteristic bulb tips in the process. Some have speculated that feeding is important, others that anemonefish need to be present to stimulate the anemone while some have felt that light intensity may play a role. However, in the specimens that I have seen in the wild, bulb tips are commonly observed even in areas or depths that may not immediately be presumed to have high light levels. It is also not unusual to find specimens with a mixture of bulb tips and smooth tips in the same animal.

Male Premnas biaculeatus in E. quadricolor, Solomon Islands. Note that not all the tentacles have bulb-tips; some are even intermediate in form. J.C. Delbeek
At the Waikiki Aquarium, there was a 350-gallon anemone and clownfish exhibit that had been in operation for about 8 years. This was an open system with a slow trickle of water from a saltwater well. There was a bottom filter plate covered with coral gravel and live rock, with an airlift in each corner. A Little Giant 3MD pump was attached to one of the airlift pipes and water was returned via a subsurface return to create a gentle current. The exhibit contained a collection of anemones including a Merten’s sea anemone Stichodactyla mertensii, several Heteractis crispa, and a large number of E. quadricolor (these had multiplied via fission several times over the years producing over twenty offspring). Each anemone was fed pieces of previously frozen shrimp once a week. The tank also contained fifteen tank- raised Amphiprion ocellaris, a pair of A. leucocranos clownfish, a mimic filefish ( Paraluteres prionurus ), a Valentin’s toby ( Canthigaster valentini ), two coral beauties ( Centropyge bispinosus ), a six-bar angelfish ( Pomacanthus sexstriatus ) that was replaced in 1996 with a gold flake angelfish ( Apolemichthys xanthopunctatus ) as the six-bar had gotten too large and was moved to another exhibit. The tank was lit by two 250 W, 5500 K metal halide pendants when first set up in 1994, as well as receiving direct natural sunlight through overhead acrylic roofing for about two hours in the middle of the day. In 1996 one of the fixtures failed and was never replaced. In 2000 the remaining fixture was replaced due to failure with a four foot fluorescent fixture containing two 48 W actinic/white URI lamps. During this entire period the Entacmaea rarely exhibited bulb tips. In some cases, a few tentacles on an anemone would develop bulbs, and in some cases an entire anemone would exhibit bulb tips for a few weeks but not in any predictable pattern and not all the Entacmaea in the tank would exhibit bulb tips. Since the some of the anemones without bulb tipped tentacles had clownfish and they were all feed on a regular basis, this left light as the remaining suspected factor to be investigated.

The Waikiki Aquarium’s Reef Partners exhibit, highlighting clownfish and anemones, 1997. Photo taken with natural light. Note a few tentacles with bulb tips here and there. J.C. Delbeek.

The Reef Partner’s exhibit in 1999, note the total lack of any bulb tips on any of the anemones. J.C. Delbeek

The skylight system over the old gallery space. The Reef Partners exhibit was situated near the bottom of the picture where the PVC pipes supplying seawater can still be seen. J.C. Delbeek

The new Reef Partners exhibit in the newly renovated south Pacific gallery, July, 2002. J.C. Delbeek

The Reef Partners exhibit installed in its new location on the outside deck (the tank skirt, workbench and graphics have not yet been installed). Note the heavy shade cloth over the exhibit that was in place for the first few months. The umbrella was removed when the deck was opened to the public. J.C. Delbeek
In October 2001 this exhibit was taken down and moved to an outside location due to renovations that were to be done to the South Pacific Gallery the exhibit was located in. The outside location was on a wooden deck in the public area and was placed in full sun next to a tree. Feeding, water source, water motion and filtration remained the same. The fish population was slightly changed with a copperband butterflyfish and a pair of bicolor angels ( Centropyge bicolor ) added to replace the coral beauties. Due to concerns of possible light shock as well as increased ultraviolet light exposure, an acrylic panel was placed over the tank along with a double layer of shade cloth. The shade cloth was removed in December but the acrylic cover remained until the animals were moved. Once the shade cloth was removed, the Entacmaea began to lighten slightly in color and just about all of them developed a bi-color pattern with white to cream colored tips, some even with green hi-lights, and a bulb present on each tentacle. In late May of 2002 the animals were moved into their new exhibit inside the building and the tank was removed.
In 1999 I had taken light measurements in the anemone exhibit. Upon observing the presence of bulb tips in the anemones in the outdoor exhibit in 2002 I decided to measure light levels in this exhibit when the chance arose and compared it to the data I had gathered in 1999.
Methods
In August 1999, a LiCor LI-1000 data logger was used in combination with a calibrated LiCor LI-193SA spherical quantum sensor to measure PAR (photosynthetically active radiation) levels over a three-day period, at a depth of 24″. The LiCor unit took continuous PAR measurements from 07:15 until 18:30. Every fifteen minutes the maximum and mean PAR values from this time period were calculated and stored in memory. Measurements were cut short on the third day due to exhibit maintenance. The data was then transferred via an ASCII dump and serial cable from the data logger unit into a computer and graphed using MS Excel.
On August 1st, 2nd and 3rd of 2002 it was decided to take PAR measurements again. The original tank had been removed but another, identical tank was still outside so this was moved into the exact same position as the anemone tank. The tank was filled with water and gravel placed inside, and the LiCor sensor was placed 24” below the surface. The same acrylic cover as originally used was then placed over the tank and PAR measurements were taken. Shade cloth was then added and PAR measurements were again taken on August 16th, 17th and 18th (the delay was due to several weeks of high cloud and overcast conditions). No values were collected prior to 08:30 or after 18:00 since the threshold PAR level of 50 uM/m2/s was not reached and the data logger was not triggered to record. The mean and maximum values for both shaded and normal conditions were then graphed and compared to those taken in 1999 (figures 1 to 8).
Results
Figures 1 and 2 (mean and maximum PAR) show that light levels in the indoor tank began to gradually increase at 07:00 as the sun rose into the sky. The greatest increase began at 10:30 corresponding to the sun rising above the concrete wall of the building and shining directly onto the tank. There was then a slight drop in PAR around 11:15 and again at 12:45. These dips in light intensity between 11:15 and 12:45 each day were due to the shadow of one of the skylight support beams falling over the sensor (see photo 5). However, since the shadow was narrow, other areas of the tank would still have received full sunlight. The greatest PAR levels occurred between 11:00 and 13:15 reaching a maximum 1448 uM/m2/s at 12:00 when the sun was shining directly into the tank and no shadows from the skylight hardware were over the sensor. The sharp drop after 13:15 was due to the sun passing behind the concrete wall on the ocean side of the building and thus, direct sunlight no longer fell on the water surface of the tank, though indirect light continued to impact the exhibit until sunset. Drops in PAR seen on one day but not others were most likely due to passing clouds. From 07:15 to 10:45, and from 13:15 to 18:15 PAR levels did not exceed 300 uM/m2/s. Therefore, for 8.5 hours PAR levels were less than 300 uM/m2/s and maximum levels were attained for only 2.5 hours.
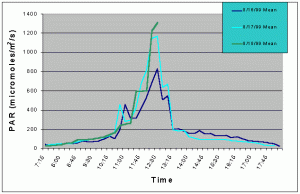
Figure 1. Mean PAR levels recorded over three successive days in the indoor anemone exhibit in August 1999.
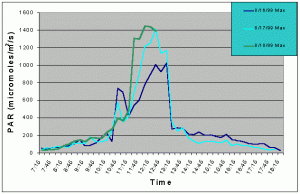
Figure 2. Maximum PAR levels recorded over three successive days in the indoor anemone exhibit in August 1999.
Figures 3 and 4 show the mean and maximum PAR values in August of 2002 without (8/1-8/3) and with (8/16-8/18) shade cloth installed on the outdoor exhibit. At the outdoor location, buildings and nearby trees impacted the light field at certain portions of the day. The most noticeable ones can be seen in figures 3 and 4 at 12:15 and 14:45 when there was a dramatic drop in mean and maximum PAR values due to the shadow of a palm tree passing over the sensor. When no shade cloth was used maximum PAR values exceeded 500 uM/m2/s between 09:00 and 17:15 and between 09:45 and 15:45 maximum PAR values exceeded 1500 uM/m2/s. The maximum PAR value reached was 2634 uM/m2/s at 13:45. When shade cloth was added to the cover, PAR values (both mean and maximum) were dramatically lower and did not rise above 500 uM/m2/s over the duration of any of the three days.
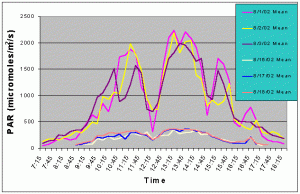
Figure 3. Mean PAR levels with and without shade cloth recorded over three successive days in August 2002.
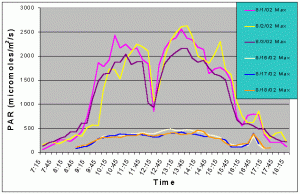
Figure 4. Maximum PAR levels with and without shade cloth recorded over three successive days in August 2002.
Figures 5 and 6 compare the mean and maximum PAR values of the indoor exhibit versus the values of the outdoor tank without shade cloth. It is clear that the outdoor tank not only received higher PAR levels but also did so for a much longer duration.
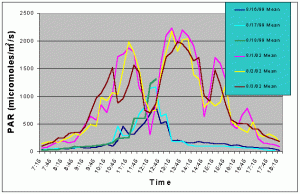
Figure 5. Comparison of mean PAR levels without shade cloth recorded over three successive days in the outdoor tank in August 2002 and in the indoor exhibit in August 2002.
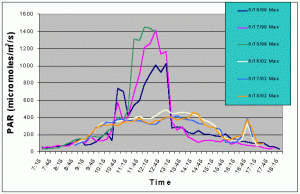
Figure 6. Comparison of mean PAR levels without shade cloth recorded over three successive days in the outdoor tank in August 2002 and in the indoor exhibit in August 2002.
Figures 7 and 8 compare the mean and maximum PAR values of the indoor exhibit versus the values of the outdoor tank with shade cloth. In this case, the outdoor tank tended to exhibit two to three times higher PAR values than the indoor tank between 07:15 and 10:15, and between 13:15 and 17:15. However, from 10:15 to 13:15 the indoor tank exhibited much higher levels than the outdoor tank.
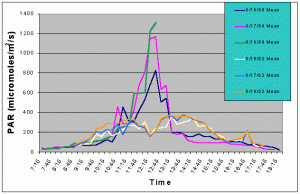
Figure 7. Comparison of mean PAR levels with shade cloth recorded over three successive days in the outdoor tank in August 2002 and in the indoor exhibit in August 2002.
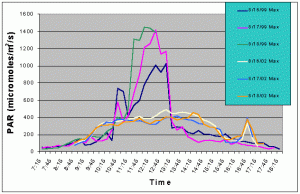
Figure 8. Comparison of maximum PAR levels with shade cloth recorded over three successive days in the outdoor tank in August 2002 and in the indoor exhibit in August 2002.
Discussion
The factors that are involved in bulb tentacle development in E. quadricolor have been speculated upon for several years. Most of these have been based upon anecdotal cause and effect observations, speculation and rumors perpetuated via print and electronic media. To be sure, there are many instances where bulb tips disappear and reappear for no apparent reason. Combine this with the fact that there are at least two recognized forms of E. quadricolor (shallow and deepwater), and it becomes apparent that predicting the behaviour of these animals becomes problematic. This study, by no means the definitive word, offers documented evidence for the role of light in the development of bulb tips.
There were several sources of potential error. Although this investigation compared data over two distinct time periods (1999 and 2002), they were taken in the same month (August) and the differences in light levels between the indoor and outdoor locations are significant and not likely due to differences in the years. Another source of error may be that the light measurements taken in 2002 occurred several months after the animals were removed from the exhibit. This means that the light levels from October 2001 till May 2002 may have been slightly lower and the photoperiod would have been shorter than in August 2002. However, even under these conditions light levels would still have been greater than those recorded indoors in 1999 and bulb tips did appear in the outdoors exhibit during this time frame. Photoperiod would have been shorter than in August of 1999, but since these difference would have been most apparent at the beginning and end of the day, when the indoor exhibit would not have received direct light anyway, it is unlikely it would have had any great effect on the final results.
From this investigation it was readily apparent that both light intensity and duration were extended in the outdoor location compared to the indoor location. This makes it tempting to assume that both factors may be of importance, but they may also be mutually exclusive; unfortunately this cannot be determined from the data in this study.
Since being moved indoors in May 2002, many of the E. quadricolor in the new exhibit (basically in the same position as the old indoor exhibit) have retained their bulb tips but some show signs of loosing them. Several E. quadricolor were also placed in a 5500 gallon reef exhibit that receives more hours of direct sunlight than the new anemone exhibit. Several of the anemones in this exhibit have moved to various locations in the tank (shallow vs. deep, low current vs. high current) so it will be interesting to note if they retain their bulb tips and for how long.
Observations on Sexual Reproduction in Entacmaea and Stichodactyla
Reproduction in E. quadricolor takes two forms, sexual and asexual. Asexual reproduction has been well-documented by hobbyists and was a common occurrence in this exhibit as well resulting in a large population of these anemones such that the live rock was nearly invisible when all the anemones were expanded. Sexual reproduction also occurs in this tank (see Sprung and Delbeek, 1997) and to the best of my knowledge, is the only such population in captivity that regularly does so each year. Spawning usually occurs between April and May between 0700 and 0900 a few days after a full moon has occurred. In April 2002 we were fortunate to once again witness not only the sperm release but also the release of planulae. Sperm release can occur in the morning but we have also observed it occurring in the late afternoon and this was the case this year. The exhibit was found to be rather cloudy in the late afternoon of April 25th and the incoming water flow was increased to ensure the sperm did not foul the water by being allowed to accumulate for too long and lowering oxygen levels. Four days later, on the morning of April 29th numerous green sphere-like objects were observed floating in the water. Upon closer examination it was found that the tentacle tips of several colonies of E. quadricolor contained these same spheres. Occasionally a few were observed being released from the tips of the tentacles. From previous experiences we knew these to be anemone embryos (see Sprung and Delbeek, 1997 for photos of the embryos and settled juveniles). A few days later there were still some visible in the tentacles and these were observed to be actually rotating and moving within the tentacles, and were more oblong in shape. It appeared as if the embryos had developed into planulae in the anemone and would shortly be released as such into the water. From my observations it would appear that E. quadricolor is dioecious, with males releasing sperm into the water column to be taken in by the females where their eggs are fertilized internally. A few days later embryos/planulae are released into the water column via the tentacle tips. Sprung and Delbeek (1997) also documented the presence of zooxanthellae in juvenile E. quadricolor only a few days after settlement. This time a few embryos were placed under a microscope and were found to contain zooxanthellae as well. Therefore, not only does the female brood the embryos for a short period but she also supplies them with their initial supply of zooxanthellae. As a result it is probable that these planulae can either settle very quickly and metamorphose into fully functional anemones, or they can spend a great deal of time in the plankton till they find a suitable substratum to settle on, without the need for feeding.
As an aside, Stichodactyla gigantea is also dioecious in nature with the females ingesting the sperm and fertilizing their embryos internally. However, unlike E. quadricolor, the embryos are internally brooded, developing into miniature copies of the adult. These juveniles are then egested from the mouth where they then drift in the current till they settle onto the substratum (L. Sharon, pers. comm., 1999). We were lucky to receive four of these juveniles in October 1999 from a coral farm in Belau (Palau); two green ones and two brown ones with an average oral disc diameter of approximately 4 cm (1.6 inches). These were placed in floating baskets in outdoor holding tanks in direct sunlight. Within the following three years one of the brown ones developed blue tentacles and all four had grown such that they had to be separated into individual baskets. At the present time (August 2002) the oral disk diameters of all four average approximately 30 cm (12 inches) when fully expanded. That means that there has been an almost eight fold increase in diameter in three years. I am confident that if the anemones had been directly feed on a regular basis they would have grown even larger (and perhaps faster) in those three years.

The tank raised S. gigantea in the new Reef Partners exhibit, a part of the new south Pacific gallery, August 2002. J.C. Delbeek
This brings up the question of age. Fautin and Allen (1992) proposed that large anemones could be more than a century old. While I do not dispute that some anemones may indeed be very long lived, to infer that something must be old simply because it is large is not entirely valid in my opinion. In the case of our S. gigantea, I think it would be safe to say that they should attain a diameter of close to a meter in less than ten years. We also have a S. mertensii that we have had for 12 years that is almost a meter in diameter when fully expanded. However, I should point that, as with giant clams (e.g. Tridacna gigas ), the growth rates of partner sea anemone species may be fairly rapid in the first decade, only to slow with advancing age. So yes, old anemones may be large but the opposite may not be necessarily true.
Bibliography
- Fautin, D.G. and G.R. Allen 1992. Field Guide to Anemonefishes and Their Host Anemones. Western Australia Museum, Perth, Western Australia.Sprung, J. and J.C. Delbeek. 1997. The Reef Aquarium. Volume 2. Ricordea Publishing, Coconut Grove, FL, USA.






0 Comments