
Wastes collected from a protein skimmer were analyzed for ‘types’ of phosphorus. Results help explain some puzzling observations made by hobbyists.
Phosphorus would likely be of little concern to hobbyists if it was not for the fact that it might promote ‘nuisance’ algal growths, yet its presence is a double-edged sword since it is essential for maintenance of a healthy life. The amount of phosphorus required by humans ranges from 800 to over 1,200 milligrams per daily. Animals (including corals and fishes) require phosphorus in their diets in order to maintain proper metabolism and is an essential element in bones, teeth, enzymes, cell membranes and so on. In an aquarium, phosphorus is added to the water column in a number of ways. Introduction of food and elimination of wastes and natural decay of once living tissues will add phosphorus to an aquarium. Even dust particles (consisting of shed human skin cells among other things) will add phosphorus. It can also be added by water added for replacement of that which has evaporated.
While phosphorus export is possible through a number of mechanisms (chemical sequestration, algal uptake, water changes, etc.) this article will focus on that removal by protein skimming and, by extension, kalkwasser additions.
Phosphorus Additions to the Reef Aquarium
Phosphorus is an essential element for life. Indeed, the major phosphorus addition to an aquarium might well be intentional feeding of captive fishes and invertebrates. In aquaculture facilities, it has been estimated that as much as 20% of feed remains uneaten. Other studies suggest the percentage is as high as 30% (Kibria et al., 1997). In addition, of that food actually eaten only a fraction is retained by the fish with most excreted as dissolved and particulate forms.
Since phosphorus is essential for life, it effects in case of death and decomposition must be considered as well. Decay of once living tissues, whether plant or animal, will add phosphorus to the water in an aquarium. Also consider that bio-erosion of substrates (whether naturally or through chemical degradation such as dissolution of some calcareous substrates by carbon dioxide in media reactors) will also add phosphorus.
Phosphorus Used in Water Treatment
Larger water supply utilities are required by law to meet federal standards on amounts of lead and copper in drinking water. These two elements are generally not present in water as it leaves the treatment plant, but instead may be dissolved from a homes water pipes (copper and pipes using lead solder) by aggressive water. To protect you from potentially harmful effects of these metals, water utilities will add phosphates to the water supply (sometimes in the form of acid-hydrolyzable P such as sodium hexametaphosphate). This chemical coats the pipes’ insides and can minimize the effects of corrosion. While generally good for you and your health, your drinking water may contain elevated levels of phosphorus. Left untreated, top-off water added to compensate for water evaporated from an aquarium will add phosphorus. If no effort is made to remove this phosphorus, it can eventually rise to high levels through cycles of concentration.
The Phosphorus Cycle
Nature had been recycling materials long before we humans grasped the concept (we merely need to observe that the landscape is not covered with dinosaur poop to prove the point). The Phosphorus Cycle is but one of many natural processes. Phosphorus is not found naturally in a pure form; instead it is combined with elements, usually in a mineral form. Slow weathering of rocks releases phosphorus to the environment although much of it is never becomes dissolved in water. However, some phosphorus becomes ‘bio-available’ (in the form of orthophosphate) and is used by primary producers such as plants and algae. In turn, plant material is used by animals in their metabolism. Dead and decay of plants and animals releases some of this phosphorus back to the environment. Under anaerobic conditions, some of the phosphorus becomes soluble in water and the process begins anew.
Glossary
- Filtrate
- Water filtered through some sort of strainer in order to remove suspended particles. Since all forms of P can be found in suspended particles, removal of these via filtration allows determination of the dissolved form directly and the suspended portion mathematically.
- Skimmate
- Material removed by the foam fractionation process and collected (usually as brown liquid/solids) in the skimmer’s collection cup.
- Parts per billion
- ppb, equivalent to one microgram per liter.
- Parts per million
- ppm, equivalent to one milligram per liter.
- Phosphorus
- A non-metal element that is essential for life. Its chemical symbol is P and has an atomic weight of 30.97.
Phosphorus can be found in water in various forms or species, including:
- Reactive Phosphate or Orthophosphate: Reactive P (also called ortho-phosphate) is ‘bio-available’ for promotion of algae growth. Reactive P can be dissolved or suspended. Colorimetric tests for phosphates commonly available to hobbyists measure only orthophosphates, although small amounts of easily hydrolyzable inorganic and organic P might be included in the result. The most abundant forms of orthophosphates occurring between pH 5 to 9 in aquatic environments are HPO4-2, H2PO4–, PO4-3, HPO4-2, and H2PO4 (Stumm and Morgan, 1981).
- Acid-hydrolyzable (A-H) Phosphate or Condensed Phosphate: Acid-hydrolyzable P can be found as dissolved or suspended forms. It may be in pyro-, meta-, tripoly-, and other forms of polyphosphates (such as hexametaphosphate). The term ‘acid-hydrolyzable’ is preferred over ‘condensed’. Phosphorus in the sample as measured by the sulfuric acid hydrolysis procedure, then pre-determined orthophosphates are subtracted (EPA, 1979). The acid-hydrolysis method reports dissolved and particulate condensed phosphate that is converted to dissolved orthophosphate through acidification of the sample (APHA, 1998). It is referred to as Dissolved Hydrolyzable P or Total Hydrolyzable P, when filtered or unfiltered samples are tested respectively.
- Total Phosphorus: The sum of organic and inorganic forms of phosphorus. As with reactive and condensed P, Total P can either dissolved or suspended. It is determined colorimetrically only after a severe oxidative digestion process where Total P is converted to orthophosphate. Samples had to be diluted since the P content exceed the maximum detection limit (3.5 mg/l as PO4 3-).
- Total Dissolved Phosphorus: Phosphorus form present in water that had been filtered through a 0.45 micron membrane then subjected to an oxidative digestion process. This process removes most suspended particles, including many bacteria. Total P includes both dissolved organic and inorganic P.
- Organic Phosphate: Formed primarily by biological processes and includes animal wastes, uneaten foods that are in various stages of degradation. Organic phosphorus is converted to orthophosphate only by oxidative destruction of the organic matter (APHA, 1989). Total organic P maybe quantified by subtracting dissolved hydrolyzable phosphorus and orthophosphate values from total phosphorus (EPA, 1998). Dissolved organic P maybe quantified by subtracting dissolved hydrolyzable P and dissolved ortho-phosphate from the Total Dissolved Phosphorus (TDP) result.
- Inorganic Phosphate: That phosphorus generally considered to be combined with calcium in alkaline, calcareous water (or aluminum and/or iron in an acidic medium) is a sum of orthophosphate and acid-hydrolyzable phosphate (although it may include some organic P as a result of the acid digestion process).
Calculations Used to Determine Phosphorus Species
Phosphorus ‘types’ (species) consists of reactive (ortho-) phosphate, phosphate that is converted to ortho-phosphate via acid hydrolysis (Acid-hydrolyzable) and Total Phosphorus (where violent digestion by strong acid and a persulfate oxidizer are used to convert the phosphorus to an easily measured form). In addition, organic, inorganic, soluble and particulate forms can be determined when additional testing protocols are used. See Figure 1.
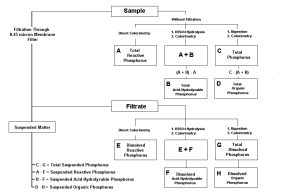
Figure 1. Flow chart for determination of various phosphorus species in aqueous matrices. From ‘Standard Methods for the Examination of Water and Wastewater’, 20th Edition.
Procedure
The aquarium used in these observations was of approximately 45-gallons (170 liters) and connected to a 100-gallon sump (379 liters), for a true water volume of about 110-gallons (416 liters). The aquarium is lightly loaded, containing a few invertebrates and two fishes.
Filtration consists of mechanical means (filter pads) and an ETSS model 800 downdraft skimmer powered by a Danner centrifugal pump. The sump contains a small amount of marine alga illuminated by a 21-watt LED spotlight. Circulation between the sump and tank is provided by a Maxi Jet 1200 powerhead.
Samples of skimmate and aquarium water were collected in glass bottles previously rinsed with 1:1 hydrochloric acid followed by another rinse with de-ionized water.
APHA Color as reported by a Hach spectrometer averaged 86 apparent color units (on a scale of 0-500 platinum-cobalt units) and appeared to be the color of weak coffee. Semi-solid material that had collected on the skimmer’s chimney and collection cup was removed with a plastic spatula and added to the skimmate bottle and homogenized as much as possible through vigorous shaking.
Samples analyzed for dissolved phosphorus were filtered through 47mm Millipore 0.45m filters that had been soaked in de-ionized water for 24 hours previous to use.
Reactive Phosphorus was determined using a Hach spectrometer (DR 2800) and the Ascorbic Acid Method (PhosVer3 reagent in a TnT vial). Since the sample was colored and turbid, pretreatment with a blanking reagent preceded testing.
Samples for Acid-hydrolyzable and Total Phosphorus used reagents within TnT vials (using the Acid Hydrolysis and Persulfate Digestion methods, respectively). A Hach DRB block heater digested the samples at recommended temperatures and times. The Ascorbic Acid method was used to determine A-H and Total P after digestion and cooling.
A 50 mg/l phosphorus quality control standard was analyzed, with a result of 49 mg/l (recovery of 98%).
The methods shown in Figure 1 were used to determine various P types.
Testing for calcium and magnesium employed the Hach titration sequential harness method. A clean endpoint for magnesium was not obtained and therefore is not reported. Calcium seemed to be elevated in the sample, but confirmation is needed.
Salinity and specific gravity were determined via use of a calibrated refractometer.
Skimmate Analyses
Preliminary analyses of skimmate held some surprises, such as very low salinity. Additionally, much of the color is removed by filtration, suggesting the matter is not dissolved but exists in fine particulate form.
Even though the salinity is slightly reduced, the calcium content was elevated. I intended to analyze the sample further, but ran out of some lab supplies. These are on order (along with other new equipment). In any case, here are preliminary results:
- pH = [email protected]ºC
- Salinity/Specific Gravity = 26 parts per thousand/1.020 (probably due to evaporation), since skimmate salinity has be observed to be as low as 13.8 ppt.
- Calcium = 576 mg/l as CaCO3
- Conductivity = 45 milliSiemenscm
- Suspended Solids = 264 mg/l
- Color, Apparent at 455nm and 8.41 pH (exceeded limit of 500 Platinum-Cobalt (PtCo) units). Sample diluted and Apparent Color calculated to be 1,816 units. To determine True Color, sample was filtered through a 0.45 filter. Result was 180 PtCo units.
- Turbidity (unfiltered sample) =179 NTU; filtered sample = 4 NTU.
Conclusions and Discussions
This testing makes it clear this protein skimmer did remove phosphorus, although the process may not be particularly effective as a sole method of P control, and phosphate could accumulate in the system if other removal mechanisms are not used.
The most interesting information revealed concerns the form of phosphorus in this aquarium’s water. I would guess that very few hobbyists (or professional aquarists, for that matter) test their systems’ water for anything other than ortho-phosphate using the easily performed one-step standard procedure. Let’s take a look at the results of tests on this tank’s water and the unfiltered skimmer waste (the skimmate). See Figure 2.
As we can see, the test result reported the aquarium water to contain ‘zero’ reactive phosphate (which, of course, is not correct. There is phosphate in the water but its level is below the detection limit of the instrument). However, the unfiltered skimmate contains a considerable amount of reactive phosphate. These results, in and of themselves, would suggest the skimmer does a fine job of removing phosphorus. Further testing suggests otherwise. See Figure 3.
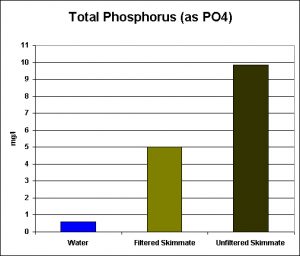
Figure 3. Total Phosphorus in aquarium water, and filtered & un-filtered skimmate. (Total Phosphorus = Reactive Phosphate + (Acid hydrolysable Phosphate – Reactive Phosphate)). Note: This is the same sample analyzed and reported in Figure 2. As expected, total phosphorus concentrations are higher than ortho-phosphate.
The amount of total phosphorus in the aquarium water is quite high, relatively speaking (0.6 mg/l) while that in the filtered/unfiltered skimmate samples is even higher.
A second round of testing was performed on another skimmate sample. These results are shown in Table 1.
| Reactive P | mg/l as PO4 |
|---|---|
| Total Reactive P | 1.93 |
| Dissolved Reactive P | 0.04 |
| Suspended Reactive P | 1.89 |
| Acid Hydrolyzable P | mg/l as PO4 |
| Total A-H P | 5.5 |
| Dissolved A-H P | 0.4 |
| Suspended A-H P | 5.1 |
| Suspended | mg/l as PO4 |
| Total P | 7.43 |
| Total Dissolved P | 0.44 |
| Total Suspended P | 6.99 |
| Organic P | mg/l as PO4 |
| Total Organic P | 0* |
| Dissolved Organic P | 0* |
| Suspended Organic P | 0* |
An aliquot of the skimmate was filtered (procedure discussed below), hence both filtered and un-filtered samples were analyzed for ortho-phosphate, acid-hydrolyzable phosphate and total phosphate. Since slight variations in the results did not add up to 100% (although they were close), I elected to use the mathematical method shown in Figure 1 to calculate Total Phosphorus for illustrative purposes in this article.
This second round produced different results than in the first tests – although the amount of unfiltered skimmate total phosphorus in these two sets is reasonably close, there was much less dissolved phosphorus. The reason for this is unknown.
Even though there are mysteries preventing explanations of why the forms of phosphorus were so different, there are two clear carry-home messages:
- Although the easily performed ascorbic acid method for ortho-phosphate determinations is widely used in the reef aquarium hobby, it is a poor indicator of true phosphorus content. Total and acid-hydrolyzable phosphorus forms are present even when a commonly available ‘test kit’ suggests the ortho-phosphate content is at or near zero. The slow conversion of the two former forms (along with normal inputs) will provide phosphorus for algae growth.
- Protein skimming will remove phosphorus, with the bulk of it being in the acid-hydrolyzable form. Since this form could be combined with calcium in alkaline solutions (such as reef aquarium water), it is tempting to think that calcium additions (especially high-pH kalkwasser solutions, with localized high pH at the drip-feed point) may play an important part in phosphorus export through protein skimming. Indeed the calcium content of the skimmate was found to be unusually high. More testing is required, and this will be reported later.
Ken Feldman has conducted considerable research in this area, and his report can be found here:
http://www.advancedaquarist.com/2010/2/aafeature/
My feeble attempts and results reported here would be a mere footnote in his work.
Postscript
As a matter of interest, I decided to conduct phosphorus analyses on the fish food I use, as well as another of the export mechanisms (P uptake via uptake by the marine alga Chaetomorpha). See Figure 4.
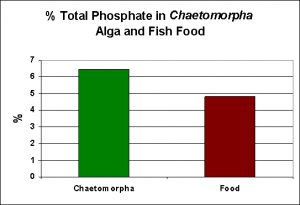
Figure 4. Phosphorus content of a food marketed for marine fish, as well that of an alga commonly used for nutrient export.
Some fish food manufacturers (Hagen for example) claim that any phosphorus content exceeding 0.9% is too much. One of the foods I use, Kent Marine (Platinum Chroma Xtreme) does not list its phosphorus content, so a Total Phosphorus test was performed. As Figure 4 shows, this food contains almost 5% Total P. Experts have calculated that fish convert just a fraction of consumed phosphorus to biomass, with the majority being released a dissolved and particulate forms of phosphorus.
Prevention of phosphorus accumulating in a system is less expensive than many forms of removal, hence the phosphorus content of a food should be considered. Look at the food’s label for a guaranteed analysis.
Questions? Comments? Please leave them in the comment section below. If you prefer a private discussion, I am best reached at [email protected].
References
- American Public Health Association, 1998. Standard Methods for the Examination of Water and Wastewater, 20th Edition, L. Clesceri, A. Greenburg and A. Eaton, Eds. United Book Press, Baltimore.
- Feldman, K., 2010. Elemental analysis of skimmate: What does a protein skimmer actually remove from aquarium water? http://www.advancedaquarist.com/2010/2/aafeature/
- Kibria, G., D. Nugegoda, R. Fairclough, and P. Lam, 1997. The nutrient content and the release of nutrients from fish food and faeces. Phytobiologica, 357:165-171.



Great sharing!
google