“Let the word Aquarium then be the one selected to indicate these interesting collections of aquatic animals and plants, distinguishing it as a Freshwater Aquarium, if its contents be fluviatile, or a Marine Aquarium, if it be such as I have made the subject of the present volume.” With these words, Philip Henry Gosse (1810-1888) coined a term which is now familiar to all of us, in his 1854 book The aquarium: An unveiling of the wonders of the deep sea. Although the term aquarium, by Gosse’s own admission, had already been used previously by botanists, he cemented this word to describe any container holding water and life, be it plants or animals. Gosse found the word Aqua-Vivarium, which was used at the time, to be awkward and too long. With the word aquarium, he had a term that was more easily remembered, rolling off the tongue, which would therefore stick with people.

Gosse’s 1854 book caused an aquarium craze in England, with many people wanting an aquarium in their home. Note the terrarium with tropical plants. Drawing from Hibberd (1870).
The aquarium’s predecessor, the terrarium, had been invented in the 1830’s, which resulted in a craze for keeping ferns in parlor rooms in Victorian England. Gosse described the value of the aquarium as an ornamental object, but foremost as a tool to study aquatic life from the comfort of one’s home, a feature we still treasure today: “The facilities for observation thus afforded me have been augmented by means of Aquaria of various forms and sizes, which I have had made for my own private use, and of which I shall have occasion to speak in the following pages. In them I could mark with leisure and precision the manners of the creatures that were living at home, yet constantly under my eye.”
Before Gosse’s book would popularize the marine aquarium in England, however, a lot of small-scale experimentation had to be done. This started, as far as we know, in the late 18th century.
1790: The start of the marine aquarium
Even though most aquarium pioneers lived during the 19th century, there is one reference of a person keeping marine life as early as 1790. This man was Sir John Graham Dalyell (1775-1851), who kept jars with invertebrates in Edinburgh for sixty years, an impressive feat. Although a jar may not constitute an aquarium as we would define it today, it was essentially the same object, namely a container holding water and animals. Sir Dalyell sent a servant to the coast several times a week to carry a jug with seawater to his home, which allowed him to perform water changes. William Alford Lloyd (1826-1880), a prominent 19th century aquarist, describes this scene in an 1876 article: “The jar was sent to the sea to be filled twice or thrice weekly; but averaging it at five times a fortnight, and allowing four miles for each double journey from Great King Street to the sea and back, that amounted to 39,650 miles from the year 1790 to the year 1850.”
When the first salt water aquaria were kept at home, people still thought giant monsters roamed the oceans.
Although truly pioneering, Lloyd did not attempt to hide his contempt for the practice of water changes, calling it “an enormous and perfectly needless expenditure of force.” Lloyd, by then, realized that aerating and mixing seawater was important for keeping it suitable for marine life: “In the ocean, of course, various animals and plants are incessantly dying in large numbers, and their decomposing remains are prevented from permanently poisoning the water, in which other animals live and breathe, by the incessant motion to which the sea is subjected, and this motion brings the water into purifying contact with the atmospheric air which everywhere exists. It is this air, or rather the oxygen in it, which the water takes up.” It was also known by that time that plants were able to take up carbon dioxide produced by animals, and release oxygen. As Lloyd wrote: “In addition to this, vegetation grows by the action of light, and decomposes the poisonous carbonic acid gas evolved by the breathing of animals, the carbon being used to form the woody substance of the plants, and the residual oxygen being liberated for the use and benefit of the animals.”

In the early days of the marine aquarium, anemones were kept in small glass jars. Image from Gosse (1860).
Almost half a century after Dalyell started working with his jars, this symbiosis between animals and plants would first be used to establish a somewhat balanced marine aquarium.
1838-1854: Continued experiments and the development of artificial seawater
In 1838, Félix Dujardin (1802-1860) established a slightly more sophisticated marine aquarium, which was less dependent on water changes. Again, I have stretched the definition of aquarium to include jars or similar vessels. Dujardin, a French zoologist and botanist, kept various invertebrates which he collected off the coast of Brittany, and used seaweeds (Ulva lactuca) to purify the water. By placing several fronds of sea lettuce in each jar, the plants would be able to produce oxygen during the day and sequester nutrients released by the animals, if provided sufficient light. He was also one of the first to keep jellyfish. As he did not publish about his work on marine aquaria, his name is somewhat obscure in aquarium circles. He is mostly remembered for his work on protozoa, single-celled organisms.
In 1842, Dr. George Johnston (1797-1855) wrote about setting up a similar more or less balanced aquarium, in which he kept a coralline seaweed and various invertebrates, in his book History of British Sponges and Lithophytes. Johnston studied coralline and macro algae, and noticed these primitive plants were instrumental in maintaining healthy invertebrates: “Was there a need of adding any additional proof of the vegetability of the Corallines, an experiment now before me would seem to supply it. It is now eight weeks ago since I placed in a small glass jar, containing about six ounces of pure sea-water, a tuft of the living Corallina officinalis, to which were attached two or three minute Confervae (branches), and the very young frond of a green Ulva; while numerous Rissocae, several little Mussels and Annelides, and a Starfish were crawling amid the branches. The jar was placed on a table, and was seldom disturbed, though occasionally looked at; and at the end of four weeks, the water was found to be still pure, the Mollusca and other animals all alive and active, the confervae had grown perceptibly, and the coralline itself had thrown out some new shoots, and several additional articulations. Eight weeks have now elapsed since the experiment was begun,-the water has remained unchanged,-yet the coralline is growing, and apparently has lost none of its vitality; but the animals have sensibly decreased in numbers, though many of them continue to be active, and show no dislike to their situation. What can be more conclusive? I need not say that if any animal, or even a sponge, had been so confined, the water would long before this time have been deprived of its oxygen, would have become corrupt and ammoniacal, and poisonous to the life of every living thing.”
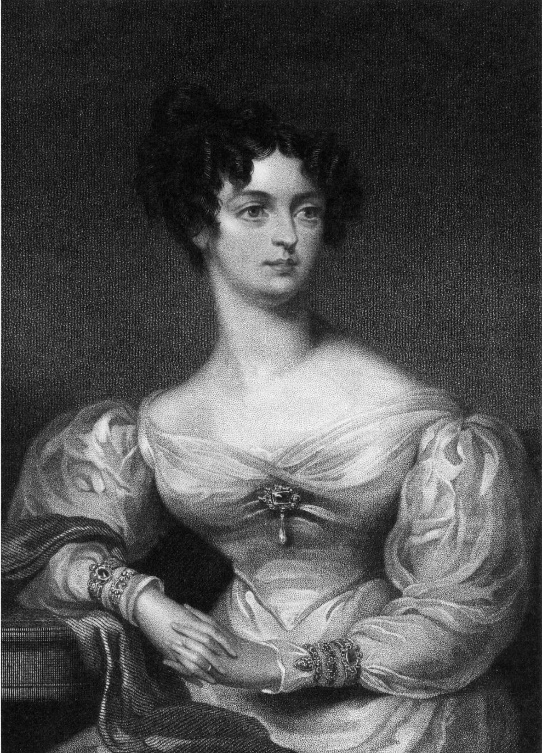
Drawing of Anna Thynne (née Beresford), whose pioneering work on marine aquaria and corals was remarkable, as at the time it was highly uncommon for women to occupy themselves with scientific study.
Although most hobbyists today are men, interestingly a woman, Anna Thynne (1806-1866), is given credit for introducing the marine hobby to London in 1846. She also established a somewhat balanced marine aquarium, in a drawing room of Ashburnham House, near Westminster Abbey. Dr. Stephen H. Ward lectured about this remarkably innovative woman: “The individual to whom is due the merit of having introduced marine vivaria into London is Mrs. Thynne. Having procured some living madrepores [stony corals] when at Torquay, in the autumn of 1846, she placed them in some seawater in a bottle covered with a bladder, and brought them safely to town. They were then transferred to two glass bowls, the seawater being kept aerated by being daily poured backwards and forwards, and being, moreover, periodically renewed by a fresh supply from the coast. In the spring of 1847, Mrs. Thynne sent for some pieces of rock, shell, etc. to which living seaweeds were attached, and subsequently depended upon the action of these for the purification of the water.” Thynne did continue to aerate her seawater, however, lest the seaweeds would not produce sufficient oxygen for the animals: “From this time I regularly placed sea-weed in my glass bowls; but, as I was afraid I might not keep the exact balance required, I still had the water refreshed by aeration.” With her method she was able to keep her seaweeds and invertebrates, including some stony corals alive for about three years, an impressive feat at the time. She fed her stony corals (Caryophyllia smithii) with boiled shrimp to keep them healthy. She also observed the release of ova and sperm from these corals: “…the Caryophylliae regularly threw out their ova at the usual season. During the day, seven of my adult Madrepores have at intervals been ejecting a whitish-blue fluid, resembling wood-smoke.” Although it was difficult for a woman to be noticed in such a conservative time dominated by men, she did manage to publish her observations in 1859, thanks to the assistance of Philip Henry Gosse.
This woodcut depicts several coldwater corals found along the British coast, which were kept by Anna Thynne and her contemporaries. On the left, contracted and expanded specimens of the Common Cup Coral (Caryophyllia smithii) are drawn. On the right, solitary polyps of the Royal Star Coral (Balanophyllia regia) are depicted. Finally, on the bottom, we see the Sandy Creeplet (Epizoanthus couchii), a colony of zoanthids. Image from Holdsworth (1860).
Around 1852, Robert Warington (1807-1867), an English chemist, and Philip Henry Gosse also started to experiment with marine aquaria. Warington used seawater from the middle of the English Channel, which he obtained at a local fish market. From the start, he aimed to use marine plants to maintain high water quality: My first object was to ascertain the kind of seaweed best fitted, under ordinary circumstances, for keeping the water clear and sweet, and in a sufficiently oxygenated state to sustain animal life.” At first he tried red and green algae, upon his friends’ recommendations, but was unsuccessful at keeping them alive. He then moved on to chlorophyta, namely the green macroalgae Ulva lattissima and Enteromorpha sp., which did stay alive and healthy. In that time, it was challenging to maintain a marine aquarium, without electricity or clean air. Even a modern city such as London was powered and heated by burning coal, which heavily polluted the atmosphere. Warington writes in 1853: “…and the water rendered clear and fitted for another experiment, it was, therefore, for greater convenience, removed into a shallow earthen pan, and covered with a large glass shade to protect the surface of the water, as much as possible, from the dust and soot of the London atmosphere, and at the same time impede the evaporation.” Despite these challenges, he was able to maintain anemones and other animals in good condition for at least a year. He added rain or distilled water to compensate for evaporation. He also kept marine fishes, such as blennies and gobies, such as a sand goby (Gobius minutus) and a pipefish (Syngnathus lumbriciformis). Unfortunately, he lost those due to poisoning of the water by paint released from the holding aquarium.

Philip Henry Gosse was the David Attenborough of his day, and popularized the study of marine life in Victorian England, with the aquarium as a valuable tool. Portrait by Maull & Polyblank, 1855, Oxford Dictionary of National Biography.
Gosse was, as he stated, less successful in his first attempts to maintain marine life due to his traveling a lot, but he continued by adding that “my ultimate object was rather the study of the habits of marine animals, to which end the Marine Aquarium was merely (or at least principally) accessory.” He published his own experiments with a marine aquarium in the fall of 1852, about a year after Warington first published about the freshwater aquarium. In the scientific world, being the first to publish a new and innovative finding is important, and this was no different back then. Gosse took this setback in stride: “Priority of publication is universally acknowledged to give a title to whatever honour attaches to a new discovery, and this I shall not dispute with Mr. Warington.” However, he did make it clear that his ideas were original, and not based on Warington’s 1851 publication: “I may be permitted to state, however, that I have for some considerable time been pursuing experiments on the same subject.” He also used a glass jar, 25 centimeters (10 inches) tall, containing about 1.7 liters (0.4 US gallons) of natural seawater. In it, he put several species of seaweeds and invertebrates such as anemones, bryozoans and polychaete worms. This worked well for about two months, until the summer heat set in. When Gosse returned home from a trip, he found his seawater jar in disarray: “The weather had set in very hot: whether this, combined with the closeness of the room, had had any effect I do not know; but on my return I found the water beginning to be offensive, a sort of scum forming on the surface, and the animals evidently dying.” It is likely that the high temperatures were more than his temperate marine life could tolerate, which may have set in a downward spiral of death, bacterial growth, oxygen depletion and more death. A similar problem would soon strike the first public aquarium in London (see below).
Two years later, in 1854, Gosse published his now famous aquarium handbook. In that same year, he also published a recipe for artificial seawater, as he found it an acceptable alternative to natural seawater. Although it was common to use natural seawater from the English Channel, Gosse described it as a tedious and expensive commodity: The inconvenience, delay, and expense attendant upon the procuring of sea-water, from the coast or from the ocean, I had long ago felt to be a great difficulty in the way of a general adoption of the Marine Aquarium.” He reasoned that “as the constituents of sea-water are known, it might be practicable to manufacture it; since all that seemed necessary was to bring together the salts in proper proportion, and add pure water till the solution was of the proper specific gravity…” He based his recipe on an analysis of seawater by Dr. E. Schweitzer, although he neglected bromine, calcium and carbonates. He latter two, Gosse argued, were easily added via shells, coral skeletons and dead calcareous algae, which also served as an attractive bottom substrate. He measured the specific gravity of his artificial seawater at about 1.026, which seems odd as I estimate its salinity at about 26 ppt. Next to its low salinity, it is doubtful that the calcareous substrate provided sufficient calcium and alkalinity to grow corals and other calcified organisms well. However, he had George Wilson analyze his seawater after several months of use, who did find traces of calcium, as well as iron, silica and iodine. Additionally, William Lloyd, who used Gosse’s artificial seawater recipe, observed growing serpulid tube worms, which apparently were able to add onto their calcium carbonate tubes.
| Ingredient | Chemical formula | Quantity | Quantity (grams or liter) | Final estimated concentration (ppt) | Natural seawater (ppt)**** |
|---|---|---|---|---|---|
| Common table salt | NaCl | 3.5 ounces | 99.22 g | 8.59 (Na) | 10 (Na) |
| Epsom salts | MgSO4·7H2O | 0.25 ounces | 7.09 g | 15.63 (Cl) | 18 (Cl) |
| Chloride of magnesium | MgCl2* | 200 grains | 12.96 g | 0.88 (Mg) | 1.4 (Mg) |
| Chloride of potassium | KCl | 40 grains | 2.59 g | 0.61 (SO42-) | 2.65 (SO42-) |
| Water | H2O** | ~4 quarts*** | ~4.55 L | 0.30 (K) | 0.38 (K) |
| Total salinity | 26.01 | 35 (list incomplete) | |||
| *It is unclear whether Gosse used anhydrous magnesium chloride or a hydrated form. For this calculation, I assumed anhydrous magnesium chloride. **Gosse wrote that he added slightly less than 4 quarts of water to his salt mixture. As we do not know the final volume of his artificial seawater blend, we can only approximate the final concentrations, although the final volume will be quite close to 4.55 liters when all salts are dissolved. ***Imperial quart. ****Based on Spotte (1992), at 35 ppt salinity, which is also found in the English Channel. | |||||
Soon after Gosse’s 1854 publication on artificial seawater, the friendly nature between him and Warington seemed to lessen. In the same year and journal, Warington published a corrigendum to Gosse’s recipe, stating that “as numerous parties have been inquiring respecting this subject, and the erroneous formula has been copied into other journals, it may prevent much annoyance as well as disappointment if this matter is set right.” When converted to the metric system, we can see that unlike Gosse, Warington came quite close to imitating natural seawater with a salinity of 35 ppt (Table 2), which is the approximate value found in the English Channel. He also wrote that it would be easier to produce seawater from natural sea salt obtained by evaporating the water, and preserving it in airtight containers. Dr. Schweitzer also used this principle to produce seawater for medicinal baths, and his cousin sold this salt in small packages. The recommended dosage of six ounces per imperial gallon of water would result in a salinity of about 36 ppt, and thus very acceptable for use in marine aquaria. After Warington’s corrigendum, in 1855, Gosse replied in kind: “If Mr. Warington supposes that I obtained from him one atom of information previously unknown to me, on the subject of making seawater from its constituent salts, he is most thoroughly mistaken. He is no less wrong in saying that I “consulted” him; since I merely mentioned what was on my mind in familiar conversation. With this, however, the public are of course not concerned, and I shall say no more on that head.” Sadly, it appears these men did not meet ever again.
| Ingredient | Chemical formula | Quantity | Quantity (grams or liter) | Final estimated concentration (ppt) | Natural seawater (ppt)** |
|---|---|---|---|---|---|
| Common table salt | NaCl | 4.325 ounces | 122.61 g | 10.61 (Na) | 10 (Na) |
| Sulphate of magnesia crystals | MgSO4·7H2O | 0.375 ounces | 10.63 g | 19.52 (Cl) | 18 (Cl) |
| Sulphate of magnesia anhydrous | MgSO4 | 0.375 ounces | 10.63 g | 1.70 (Mg) | 1.4 (Mg) |
| Chloride of magnesium | MgCl2 | 0.6 ounces | 17.01 g | 3.94 (SO42-) | 2.65 (SO42-) |
| Chloride of potassium | KCl | 0.125 ounces | 3.54 g | 0.41 (K) | 0.38 (K) |
| Bromide of magnesium | MgBr2 | 21 grains | 1.36 g | 0.26 (Br) | 0.07 (Br) |
| Sulphate of lime | CaSO4 | 0.225 ounces | 6.38 g | 0.57 (Ca) | 0.4 (Ca) |
| Sulphate of lime crystallized | CaSO4·2H2O | 0.05 ounces | 1.42 g | 0.18 (CO32-) | 0.14 (HCO3–)*** |
| Carbonate of lime | CaCO3 | 21 grains | 1.36 g | ||
| Water | H2O** | ~4 quarts* | ~4.55 L | ||
| Total salinity | 37.18 | 35 (list incomplete) | |||
| *Imperial quart. **Based on Spotte (1992), at 35 ppt salinity, which is also found in the English Channel. I compared the carbonate concentration in Warington’s recipe with the natural bicarbonate content of seawater, as most carbonate ions will convert to bicarbonate, a process which is pH dependent. | |||||
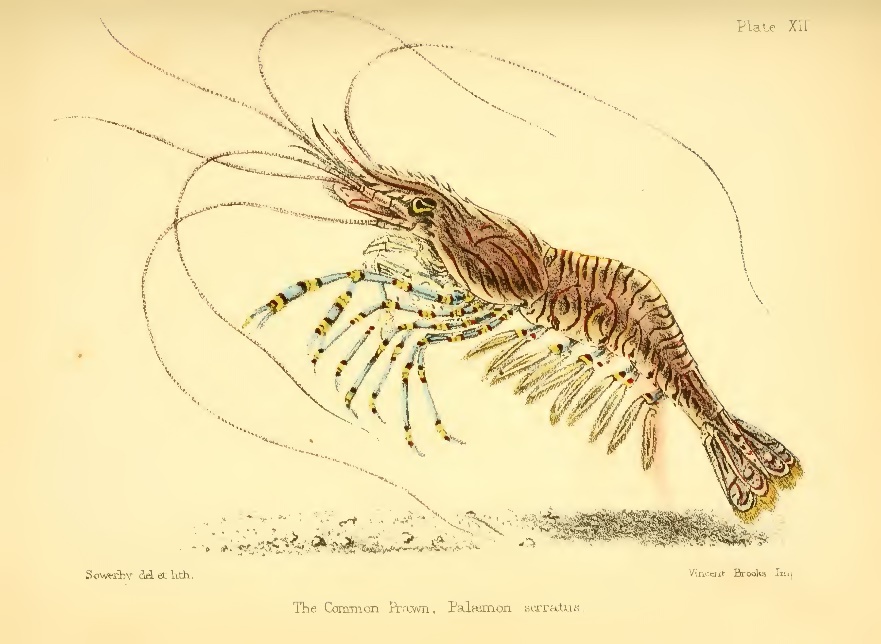
Victorian naturalists were keen observers and gifted artists. This drawing depicts the Common Prawn (Palaemon serratus). Image from Sowerby (1857).
Despite the errors in Gosse’s recipe, his 1854 experiments were more successful than in 1852. Using his artificial seawater, he kept seaweeds and several invertebrates healthy in a glass jar for over two years, including Ulva lattissima, anemones, polychaetes, barnacles, bryozoans and tunicates. After making his seawater, he filtered it over a sponge into the glass jar the next day, put in some shore pebbles, and finally some Ulva lattissima seaweed. From that point, however, Gosse acted more cautiously this time. Instead of immediately adding animals, he waited. By first introducing a marine plant, Gosse wanted to precondition the water for his animals. By that time, combining plants and animals was becoming a key strategy for a successful aquarium. Most importantly, the oxygen released by the Ulva would hopefully sustain his invertebrates, although we now know that at night, plants also deplete water of oxygen. As he put it: “This, too, is the order of nature; plants first, then animals.” Soon after introducing seaweed, he noticed green algae growing on the jar’s surface, which according to Gosse were Ulva spores: “A coating of the green spores was soon deposited on the sides of the glass, and bubbles of oxygen were copiously thrown off every day under the excitement of the sun’s light.” A week later, he introduced his animals, most of which thrived. His bryozoans, active filter feeders which require plankton, did not last long, however. Later that year, in August, he repeated this experiment with a larger quantity of artificial seawater. This time, he filled an actual aquarium as we would recognize it; a square glass tank with a volume of about 18 imperial gallons (82 liters). Despite his proclaimed successes, Gosse did add that natural seawater was to be preferred if it was easily accessible.

In the 1850’s, sea anemones were sought-after invertebrates. Notice the brown Aiptasia in the center, now considered a pest in aquaria. These color images, known as chromolithography, were state of the art at the time. Image from Gosse (1860).
Like many naturalists of his time, Gosse beautifully described the behavior of his animals. A nice example is his description of coral polyps (Caryophyllia smithii) feeding on a spider: “The feeding of the Madrepores affords much amusement; they are very greedy, and the presence of food stimulates them to more active efforts, and the display of greater intelligence, than we should give them credit for. I put a minute spider, as large as a pin’s head, into the water, pushing it down with a bit of grass to a Coral, which was lying with partially exposed tentacles. The instant the insect touched the tip of a tentacle it adhered, and was drawn in with the surrounding tentacles between the plates, near their inward margin. Watching the animal now with a lens, I saw the small mouth slowly open, and move over to that side, the lips gaping unsymmetrically; while at the same time by a movement as imperceptible as that of the hour-hand of a watch, the tiny prey was carried along between the plates towards the corner of the mouth.”
He also amusingly describes feeding a housefly, which was quickly rejected by the coral: “But the struggles of the fly’s legs perhaps tickled the Coral’s tentacles in an unwonted manner, for they shrank away, and presently released the intended victim, which rose to the surface like a cork; only however to become the breakfast of an expectant Actinia hellis [a sea anemone], which was much too wise to reject or to let slip so dainty a prey. The poor Coral evidently regretted the untoward necessity of letting it go, for his mouth,-I will not say watered, for being under water the expression might be open to criticism, but-gaped, for some time after the escape.”
Although Gosse and Warington wrote about their successes using artificial seawater, others remained proponents of the natural kind. For example, James Shirley Hibberd (1825-1890), a well-known gardening author at the time who used Gosse’s recipe, wrote in one of his books: “But artificial water is quite unsuited for animal life of any kind, until it has been brought into condition by means of growing weeds for eight or ten days, and for crustacea, star fishes, and fishes proper, it is not suitable till it has been in use for many months, and even then some species lose their health in it, and at last perish. In the case of an emergency, artificial sea-water may prove of immense value, but it is never to be used when real sea-water is obtainable.” Lloyd, however, defended Gosse against criticism, and wrote in a letter to Gosse: “In reference to what has recently been published on an improvement (or a supposed improvement) on your receipt for the manufacture of sea water, a friend of mine took me by the button and said, ‘My dear Sir, Mr. Gosse is altogether wrong; he has not salt enough; he has no…’ To which I replied, pointing to two fine Actinia dianthus in full blow in one of my vessels, ‘But if Mr. Gosse is altogether wrong, why do these Actinia flourish?’ This was unanswerable.”

A typical ornate Victorian marine aquarium, with a fountain in the middle for water aeration. Such an aquarium was decorated with rock and housed fishes, shrimp, sea stars, anemones, snails, worms, bryozoans and seaweeds, all temperate species from the British coast. Sometimes, stony corals such as Caryophyllia smithii or zoanthids (Epizoanthus spp.) were added. Image from Gosse (1856).
Perhaps the varying opinions of Gosse’s mixture were caused by the use of different animals, some of which tolerate lower salinities better than others. In addition, Gosse neither kept fishes nor mentioned whether his stony corals actually grew in their, most likely, low calcium and alkalinity environment, which obscures the limitations of his artificial seawater, even though Lloyd did observe growing serpulid tubes.
1853: The first public aquarium
Gosse’s 1854 book caused an aquarium craze in Victorian England, and drew people to the coast to look for aquarium livestock. Aquarium companies also popped up, supplying salt, aquarium and animals. The London company Sanders and Woolcott was recommended by Gosse, which produced ornate, octagonal aquaria typical for the time.
A year before, the initial spark for this trend was ignited by the first public aquarium in London. Inspired by the success of Warington and Gosse, but also Dr. J. S. Bowerbank, Dr. Cotton and Dr. E. Lankester, the Council of the Zoological Society of London agreed in 1852 that an “Aquatic Vivarium” should be constructed, a public building in which fresh and marine species were put on display. It was to be the world’s first public aquarium, an in 1853, the Fish House in Regent’s Park was opened. Gosse played an important role in the procurement of livestock for the aquarium: “Early in December, 1852, I put myself into communication with the Secretary of the Zoological Society, and the result was the transfer of a small collection of Zoophytes (corals and anemones) and Annelides, which I had brought up from Ilfracombe, and which I had kept for two months in vases in London,-to one of the tanks in the new Fish House just erected in the Society’s Gardens in the Regent’s Park. This little collection thus became the nucleus and the commencement of the Marine Aquarium afterwards exhibited there.”
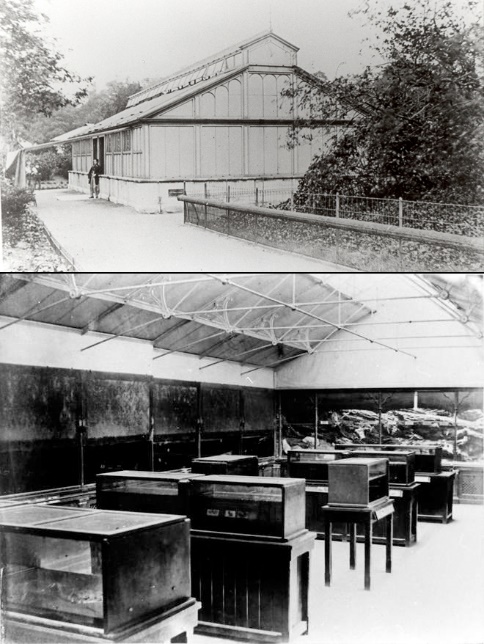
Top: Exterior south view of the Fish House in Regent’s Park, London. Bottom: Interior of the Fish House. Photographs taken circa 1875, courtesy of the ZSL.
Although the Fish House was revolutionary at the time, attracting many visitors, it did have its limitations. Water temperature, a very basic parameter today, was not kept stable in the summer as the building was constructed in a greenhouse style. This, obviously, had dire consequences for the coldwater marine life on display. William Lloyd (1876) wrote about the building’s unsuitable design: Accordingly the Regent’s Park Aquarium was made virtually as a conservatory. But it was a diametrically wrong notion, as the first summer proved; and the second summer (1854) showed this still more conclusively; and the third (1855) yet more so, the evil being an accumulating one. It was then remembered, when too late, that marine and freshwater plants and animals live in seas and rivers, where the temperature is much more restricted in range than that which obtains in the atmosphere. It was seen that success was to be obtained by representing these conditions of nature just named, and that to place such organisms in a glass house, where the rays of a summer’s sun heated a mass of imprisoned air, was to kill the animals and to stimulate the plants to unnatural growth, or rather to cause them and some of the animals to be covered with a parasitic growth of the lower green algae, which obscured them.” He went on by writing that sufficient water flow through the freshwater displays prevented them from heating up in the warm greenhouse: “The errors of this earliest aquarium were strikingly shown by its solitary merit, the latter being its fresh-water division, occupying one side of the building, where the water coursed through the tanks in a constant stream, it being clear and cool, and peopled with an adequate number of healthy animals; while on the other side of the building, and in its centre, were the marine tanks, in which the water was, and still is, turbid.”
1856-1882: The rise and decline of public aquaria in Europe

Shallow aquaria in the main aquarium corridor of the Brighton Aquarium, somewhere between 1870 and 1900. The larger display aquaria are in the background. Image courtesy of The Royal Pavilion, Libraries and Museums, Brighton and Hove, UK.
Based on the success and attraction generated by the Fish House in London, other countries would soon build their own public aquaria. The United States of America followed quickly in 1856, with an aquarium at Barnum’s American Museum in New York, and in 1859 with the Aquarial Gardens in Boston. In Paris, France, the Jardin d’Acclimatation was opened in 1860. In the same year, the Viennese Aquarium Salon opened in Vienna, Austria. Germany followed in 1864, with the Marine Aquarium Temple in the Zoological Garden in Hamburg. Next came, amongst others, the Berliner Aquarium Unter den Linden in Berlin (1869), The Crystal Palace Aquarium in Sydenham, London (1871), The Aquarium in Brighton (1873), The Manchester Aquarium (1874), Dr. Cocker’s Menagerie at Blackpool (1875), The Royal Aquarium in Westminster (1876), The Scarborough Aquarium and The Yarmouth Aquarium (1877), The Tynemouth Aquarium and Winter Garden (1878), The Aston Aquarium in Birmingham (1879) and Artis Aquarium, Amsterdam, The Netherlands (1882).
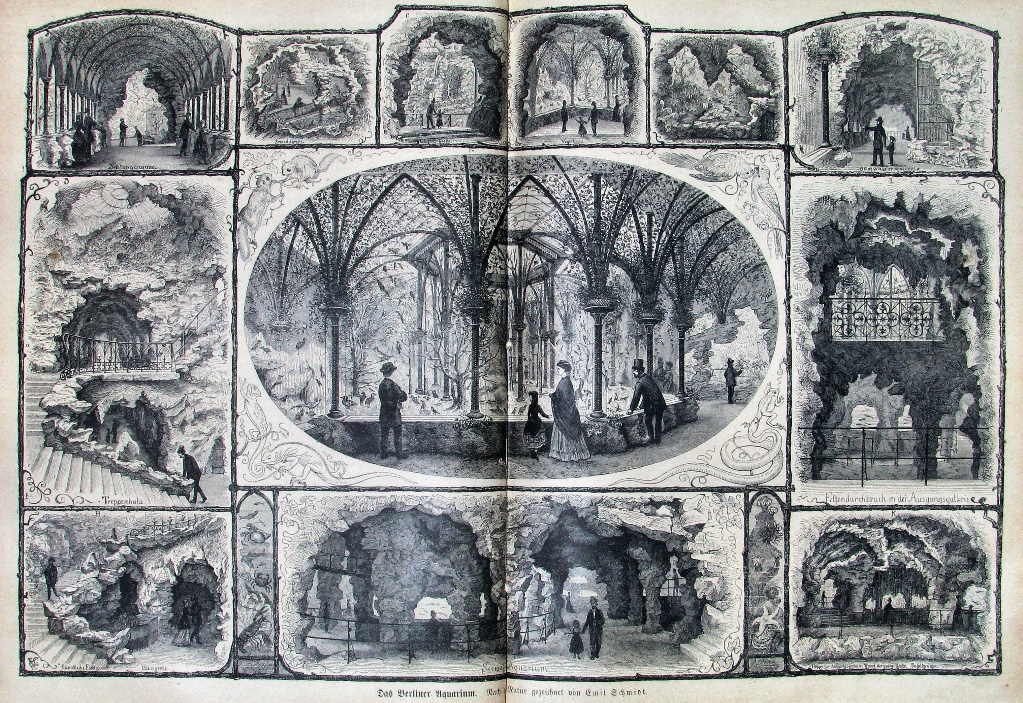
The Berliner Aquarium Unter den Linden, in the style of an artificial cave system. The marine aquarium is pictured in the lower middle. The aquarium was an initial success thanks to zoologist Alfred Brehm and chemist Otto Hermes, but declined in the early 20th century and was closed in 1910. Image: Emil Schmidt, published in Die Gartenlaube (The Garden Arbor), 1873.
One of the most spectacular nineteenth century public aquaria was the Crystal Palace Aquarium. It was established under the supervision of William Lloyd, who by then was an established name in the business. He was also responsible for the Marine Aquarium Temple in Hamburg. He even published an article about the Crystal Palace Aquarium’s opening in the esteemed scientific journal Nature: “The building undertaken by the Crystal Palace Aquarium Company was commenced in July 1870, much too late therefore to be opened when at first contemplated, April 1, 1871, though at Easter last half a dozen of the marine tanks were temporarily converted into freshwater ones, and some pike, tench, carp, eels, etc, were shown therein for three days; when the place was closed, and the progress of the works continued, and then the establishment was finally opened on August 22, 1871. It would have been well if the sea-water had been in good condition in the early part of the summer, so that advantage might have been taken of the then exceptionally cool weather to transport some of the great abundance of animals at that time on the coasts of England; but that was not possible, and then, when the water was fit, the weather became very hot, and the sending of many animals was thereby prevented. Such creatures as could be got, however, were obtained, and the opportunity is now being taken of the present increasingly colder season to add other animals constantly, so that in a short time most of the tanks will be populated.” The aquarium was immense for its time, 300 feet long and about 50 feet wide. It housed thousands of vertebrate and invertebrate animals. It had sixty-one tanks, with a capacity between 4,000 and 40 imperial gallons. In total, about 150,000 imperial gallons flowed through the building, most of which was kept in a subterranean reservoir. The aquaruim used state-of-the-art technology such as electric lighting and two steam engines, which constantly pumped water at a rate of 5,000 to 10,000 imperial gallons per hour.

Despite its shortcomings, the attraction garnered by the Fish House in London resulted in more public aquaria in Europe. In 1882, The Netherlands opened its own aquarium at Artis Royal Zoo, Amsterdam. Top: The main aquarium hall in 1920. Bottom: The same hall in the second half of the 20th century, which is still open today. Images courtesy of Artis Royal Zoo.
During his management of the successful Crystal Palace Aquarium, Lloyd assisted his friend Philip Henry Gosse with the construction of an aquarium in Gosse’s house in 1876. His son, Edmund Gosse, wrote in 1890: “He was finally moved to undertake this project from the disappointment he experienced in failing to keep alive some specimens of the scarlet and yellow Balanophyllia [a stony coral] in his earthen jars.” The ambitious home setup was described by Gosse in 1878, and was highly reminiscent of a modern reef aquarium setup. It consisted of a display tank, large sump and additional reservoir, with a total volume of 280 imperial gallons (1273 liters or 336 US gallons). The system consisted of materials such as steel, iron, red lead and cement, making me wonder about its durability and suitability for marine life. Indeed, Philip Gosse writes about the unexplainable demise of his animals in the beginning: “However it be explained, many creatures that would not live more than a few weeks, or even days, a year ago, now continue without difficulty, often coming into sight months after their introduction, in full health and beauty.” It is possible that his marine life fared better after his cement stopped leeching hydroxide, which causes abnormally high pH levels. However, Gosse was aware of this issue, as he recommended soaking cement for at least a month in his aquarium handbook. It is also conceivable that harmful copper ions leeched into his system, which in time may have been arrested by the formation of carbonate salts on copper surfaces. Water circulation between the three compartments was generated by using a hand pump, which Gosse’s servant operated several times a week. Judging from Gosse’s writing, this was no easy task: “On Tuesday, Thursday and Saturday evenings, my servant pumps till the warning-pipe streams, averaging some 675 strokes. The total of 675 strokes is performed in about half an hour.” Interestingly, he mentions the total cost for his aquarium setup, which was 60 pounds, or roughly 7,500 dollars today.

An 1880 drawing of the Royal Aquarium, Westminster, by Alfred Concanen. The aquarium was supposed to be a place of quality entertainment, but it was short-lived. The aquarium’s water supply systems did not work well, few fishes were on display, and the public soon lost interest. The aquarium and its surrounding facilities were shut down in 1903.
In the 1870’s, the aquarium craze in England subsided, which resulted in declining visitor numbers for public aquaria. Perhaps this was in part due to the inability of public aquaria to maintain high quality displays and healthy animals for a prolonged time. Fluctuations in water temperature, and possibly water quality, resulted in high mortality rates. In addition, it was difficult to ship live animals between places of the world, so lost animals could not always be quickly restocked. This often resulted in barren and turbid aquaria, which may have contributed to the public’s waning interest. At home, marine aquaria became few and far between, as they proved difficult to maintain, which is still true today. A bit frustrated perhaps, William Lloyd complained about the lack of devoted aquarium hobbyists: “I think I may write on this subject with something like authority, for I have pursued it as an exclusive occupation, zealously and unremittingly, for the last dozen years, and during that period I have collected the names and addresses of not fewer than eight thousand persons interested in aquarian matters, and for these persons I have set up, under all possible circumstances, many hundreds of Aquaria (the exact number is 3,548), and out of these not more than about the odd forty-eight are now in successful operation. All the others belonged to individuals who merely took up the thing as a transient fashion, or who, knowing and caring a little about it at first, soon abandoned it from discovering that so long as aquaria are made for houses, instead of houses for aquaria, no satisfactory result could be obtained.”
Lloyd, who by then was a leading marine aquarist, lost his job at the Crystal Palace Aquarium in 1878 as they could no longer afford his salary. A year later, he again lost his job due to financial trouble, this time at the Aston Aquarium in Birmingham. He then returned to London and worked on his Magnum Opus, a book about aquarium management and design. He would include modern technology for the time, such as steam engines and electric lighting, and honor pioneers such as Anna Thynne. Unfortunately, before finishing his book, he died suddenly in his London home in 1880. Sadly, his other main achievement, the Crystal Palace Aquarium in London, slowly declined. It was turned into a monkey house and was finally destroyed in a fire in November 1936. All that is left of it now is a ruin, which is managed by a group of dedicated volunteers. You can visit the ruins today, and wonder how amazing it must have looked in its heyday.
After the aquarium rage subsided in Victorian England, the marine hobby remained somewhat dormant until the 1950’s, when new pioneers such as Lee Chin Eng started to work with marine aquaria. From that point onwards, the hobby steadily grew into what it is now, and it seems to be growing still. Despite our knowledge on marine life and our sophisticated equipment, new scientific discoveries continue to be made and technology marches on. Public aquaria can be found everywhere, with spectacular marine displays, and it seems public interest in the oceans and marine life is higher than ever. This is opportune in a time where marine ecosystems are under great pressure from human activities.
Concluding remarks

The Crystal Palace and its aquarium were a remarkable feat of engineering in their time. Top image: The ruins of the aquarium, with two smaller aquaria partially intact on the left, and a large aquarium known as 18a in the center and right. Note the stones cemented in the aquarium’s back panel, which probably served as decoration. The holes in the floor expose the massive reservoir, which once held over 100,000 imperial gallons of seawater. Bottom image: Detailed view of the reservoir. Images by Tux-t-penguin, Deviantart.com.
It is clear that the 19th century was a pivotal time for the (public) marine aquarium, with many pioneers laying the foundations for what we know today. I sometimes wonder how they would perceive modern-day aquaria, with all their splendor and technical features. Key issues such as water temperature and water flow are handled by thermostats and streamers, water quality is maintained with filters and high quality salts, and powerful lights satisfy even the most critical inhabitants. Although the displays of Gosse, Lloyd and their contemporaries may not have been as spectacular as ours are now, it is worthy to remember that we stand on their shoulders.
Further Reading and Viewing
When this article was almost completed, I noticed a similar one on aquarium history by Albert J. Klee, of the Aquarium Hobby Historical Society of America (published November 17, 2012). The reader is referred to his interesting article, entitled Who invented the aquarium?, as well as the highly informative website Parlour Aquariums, http://parlouraquariums.org.uk. The reference list below also contains several books well worth reading.

The jars and octagonal aquaria of the mid-nineteenth century have come a long way since then, and have evolved into miniature coral reefs, with sophisticated technology. Image courtesy of Robert and Petra Worst, The Netherlands.
Two interesting videos of Victorian aquaria can be found using the following links. The first one is a video in which Jamie Craggs of the Horniman Museum and Gardens presents a mock-up of an octagonal aquarium with British marine life inspired by Philip Henry Gosse. The second video is presented by Professor Joseph Pawlik, and shows his restored 19th century tank originally made by J.W. Fiske of New York.
Acknowledgements
I wish to thank Rachel Jones and James Godwin of the Zoological Society of London, Regent’s Park, London, for their assistance with obtaining several images.
References
- Gosse PH (1852) On keeping Marine Animals and Plants alive in unchanged Sea-Water. The Annals and Magazine of Natural History, Second Series 10:263-268
- Gosse PH (1853) A Naturalist’s Rambles on the Devonshire Coast. Van Voorst, London. 451 p
- Gosse PH (1854a) On Manufactured Sea-Water for the Aquarium. The Annals and Magazine of Natural History, Second Series 14:65-67
- Gosse PH (1854b) The Aquarium: An Unveiling of the Wonders of the Deep Sea. Van Voorst, London. 278 p
- Gosse PH (1855) On Artificial Sea Water. The Annals and Magazine of Natural History, Second Series 15:17-19
- Gosse PH (1856) A Handbook to the Marine Aquarium. Van Voorst, London. 48 p
- Gosse PH (1860) Actinologia Brittanica: A History of the British Sea-anemones and Corals. Van Voorst, London. 362 p
- Gosse PH (1879) A Marine Aquarium. The Midland Naturalist 2:1-6
- Gosse E (1890) The life of Philip Henry Gosse. Kegan Paul, Trench, Trübner & Company, London. 387 p
- Hibberd S (1856) The Book of the Aquarium and Water Cabinet. Groombridge and Sons, London. 148 p
- Hibberd S (1870) Rustic Adornments for Homes of Taste. Groombridge and Sons, London. 402 p
- Holdsworth EWH (1860) Handbook to the fish-house in the gardens of the Zoological Society of London. Bradbury and Evans, London. 50 p
- Lloyd WA (1855) Memoranda on the Employment of Artificial Sea-Water in Marine Aquaria. Quarterly Journal of Microscopical Science 3:315-316
- Lloyd WA (1871) The Crystal Palace Aquarium. Nature 4:469-473
- Lloyd WA (1876) Aquaria: Their Past, Present, and Future. The American Naturalist 10(10):611-621
- MeasuringWorth website, http://www.measuringworth.com
- Parlour Aquariums website, http://www.parlouraquariums.org.uk/
- Sowerby GB (1857) Popular histroy of the aquarium. Lovell Reeve, London. 327 p
- Spotte S (1992) Captive Seawater Fishes: Science and Technology. John Wiley and Sons, Hoboken. 942 p
- Thynne A (1859) On the increase of Madrepores. The Annals and Magazine of Natural History, Third Series 3:449- 461
- Warington R (1851) Notice of observations on the adjustment of the relations between the animal and vegetable kingdoms, by which the vital functions of both are permanently maintained. Quarterly Journal of the Chemical Society of London 3:52-54. doi:10.1039/QJ8510300052
- Warington R (1853) On Preserving the Balance between the Animal and Vegetable Organisms in Sea Water. The Annals and Magazine of Natural History, Second Series 12:319-324
- Warington R (1854) On Artificial Sea Water. The Annals and Magazine of Natural History, Second Series 14:419-421
- Zoological Society of London website, www.zsl.org


0 Comments