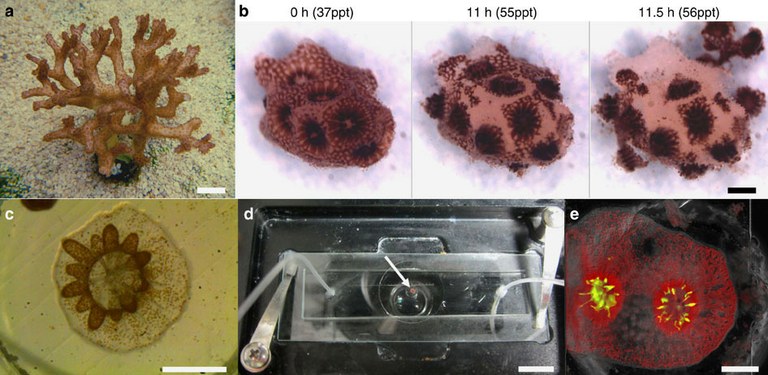
(a) A colony of P. damicornis. Polyps appear as brown dots covering the colony surface. (b) Under salinity stress, polyps from a small P. damicornis branch (0 h) are separated from each other through rupturing of the coenosarc tissue (11 h). Polyps are then ejected from the skeleton (11.5 h) resulting in the production of clonal micropropagates. (c) A settled P. damicornis micropropagate, 48 h following explantation. The polyp base is flattened against the glass surface. Extended tentacles (brown) indicate recovery from the stress experienced during the explantation process. Symbiotic algae (zooxanthellae) are visible as brown dots. (d) A coral micropropagate (white arrow) settled inside the coral-on-a-chip microfluidic device, held on a temperature-controlled microscope stage. Tubes attached to the microfluidic device allow the introduction of flow and the control of water chemistry during the experiment. (e) A P. damicornis micropropagate as seen by epi-fluorescence microscopy, based on the autofluorescence of the coral’s native GFP (green) and algal chlorophyll (red). Scale bars: (a,d) 1 cm; (b,c,e,f) 500 μm; (g,h) 20 μm.
Dr. Tim Wijgerde explains to Advanced Aquarist:
Scientists have succeeded in growing miniature corals on glass slides and keeping them alive in microscopic “aquaria“. By exposing small pieces of coral (Pocillopora damicornis) to high salinity, marine biologists triggered a response known as polyp bailout, during which the coral colony ejects its polyps into the surrounding water. These polyps are subsequently reattached to glass slides, and kept alive in a miniature flow-through aquarium with light and temperature control. This new coral-on-a-chip method, which has just been published in Nature Communications, allows scientists to study coral bleaching, growth and disease in unprecedented detail.
Here’s a snapshot of how the innovative process works:

A branch tip fragmented from a healthy coral colony is micropropagated via a polyp-bail-out response by subjecting it to a gradual increase in salinity. Micropropagates are transferred to open microwells within a microfluidic channel and incubated under controlled light, flow and temperature conditions to induce settlement. Microscopic observation is facilitated by sealing the microwell with a glass coverslip and introduction of flow via silicon tubes connected to a syringe pump. Light intensity is controlled using the microscope’s transmitted illumination system. Temperature control may be provided using a stage incubator. The coral-on-a-chip platform allows maintaining live coral micropropagates on the microscope stage for extended time periods while precisely controlling the chemical and physical environment.










0 Comments