By Joe Rowlett
The azooxanthellate (=non-photosynthetic) corals that get exported for the aquarium industry are primarily from the large family Dendrophylliidae. There are roughly 1300 species in this family, and it includes such common aquarium inhabitants as the scroll and cup corals of the genus Turbinaria, as well as Duncanopsammia, the Duncan’s or whiskers coral. [These two genera are rather unique in being zooxanthellate (=photosynthetic) members of this primarily azooxanthellate group, thus accounting for their widespread availability in the hobby.]
There are a couple of morphological characters that are important for diagnosing this family of corals. 1) The septa, which radiate towards the center of each polyp normally have a unique arrangement where the most recently formed septa (S4 or S5) fuse together to encompass an older septal cycle (this is called the “Pourtalès plan”). The presence/absence of this Pourtalès arrangement has traditionally been important for identifying members of this family to genus, though recent study has indicated this may in fact be a misleading character 2) The skeleton between the polyps is relatively porous and granular, and is termed a synapticulotheca (a feature shared with the similarly porous Porites and Acropora, amongst others) This grainy texture can be easily felt on a cleaned specimen, and it is alluded to in the –psammia (Greek: “sand”) found in many of the scientific names in this group. While neither of these characters can be seen in a live specimen, they are unique enough to easily diagnose the family as forming a single evolutionary lineage (i.e. monophyletic).

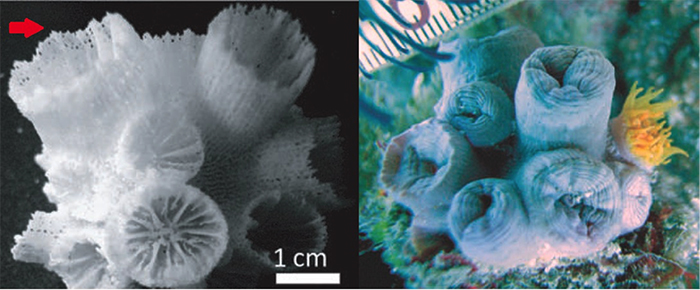
Left: T. diaphana. Right: Rhizopsammia. (Red=S1 Blue=S2) The enlarged S5 surrounds both S3 and S4 in a “Pourtalès plan” septal arrangement. Photos by Tachikawa 2005.
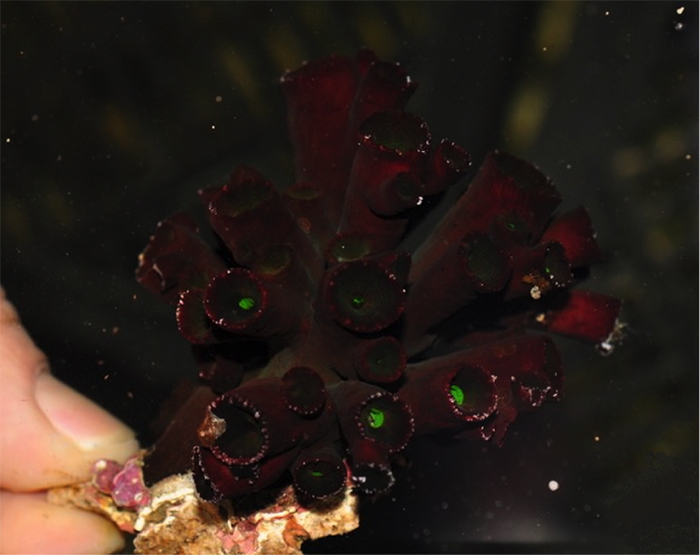
Tubastraea aurea “Orange Sun Coral”

Note how most polyps are tightly-packed on this closed colony. Photo by Marta Texiera, septal inset from Wells, 1983.
The image above is what most aquarists envision as being the “Orange Sun Coral”. As currently defined, it has the largest geographic range of any Tubastraea species, being found throughout the Indo-Pacific. It is also known from the West Atlantic, where it is an invasive species. It was first detected in Puerto Rico in 1943, and it is now the dominant stony coral in many areas, especially manmade structures like oil rigs. It can be spectacularly abundant in its preferred habitat, often forming large monospecific stands on the walls and overhangs of underwater caves and crevices subjected to strong currents.
Taxonomically there has been immense confusion regarding the correct name of this species. Many prominent aquarium references and retailers list it as T. aurea, whereas most scientific resources list that name as a junior synonym of T. coccinea. Recent molecular research has thrown a wrench into this classification, as there is now strong evidence that there are actually two separate lineages of the “orange sun coral.” How, then, do we tell them apart?
Using the molecular study of Arrigoni et al 2014 as a guide, there appear to be three possible informative differences: 1) coccinea colonies are smaller and less hemispherical 2) coccinea calices extend minimally beyond the coenosteum, giving the colony a flat appearance 3) both have 12 septa reaching the columella, but in coccinea S3 and S4 are better developed and at least half as long as the primary septa. This can develop into a full Pourtalès Plan.
But, truthfully, until a taxonomic revision is published, any species-level identification will be provisional at best.
Tubastraea coccinea? “Flat Sun Coral”
As discussed above, until this genus is formally revised, the true identity of coccinea is uncertain. The identification in this article is based on my interpretation of Arrigoni et al’s recent study of the family, with the caveat that I have not examined any skeletons to verify the accuracy of my determination. My tentative identification from photographs is based primarily on the small, amorphous colonies with little calice height. I have seen just a single series of photographs, from a cave on a Singaporean reef. This presents the possibility that this species is more associated with deep recesses than related species, which would explain its apparent rarity (absence?) in the aquarium trade. It could also be that these are just small, anomalous colonies of T. aurea. There is immense uncertainty in identifying this group as it currently stands.
Tubastraea faulkneri “Faulkner’s Sun Coral”

This specimen sits in the Smithsonian, and was identified by Dr. Stephen Cairns. Note the widely-spaced polyps. Photo by Paul Humann.
Tubastraea faulkneri was described based on specimens found growing alongside T. cf coccinea in the Galapagos Islands. Seen growing side-by-side, the two are said to be noticeably different, and presumably not just different growth forms of the same species, though this has yet to be verified with genetic analysis. Since its initial discovery it has been found occurring naturally throughout the Indo-Pacific.
The septal pattern is similar to T. cf aurea (sensu Arrigoni), but thankfully this detail isn’t vital for delimiting these two species. The critical differences are in the spacing and size of the polyps. In T. faulkneri, the coenosteum is generally equal to or greater than the corallite diameter, whereas in T. cf coccinea & T. cf aurea the coenosteum is almost non-existent due to the closeness of the polyps. The height of the polyps is reported to be 3-8mm, which is in line with T. cf coccinea, but far shorter than in T. cf aurea. The overall impression of “Faulkner’s sun coral” is a much more uniformly-flattened and sparsely-populated colony than is seen in the “orange sun corals.”
Tubastraea faulkneri has long been reported as being common in the aquarium trade, but in searching through the aquarium literature and forum posts I found relatively few specimens that could with any certainty be attributed to it. Much of what has been identified as T. faulkneri is clearly either T. coccinea/aurea or T. tagusensis. Given its wide geographic range, it is quite probable that this species does get exported regularly for sale, but it is essentially impossible to differentiate this species from T. coccinea based on a living specimen unless there are noticeably large spaces between the polyps.
Tubastraea tenuilamellosa? “Yellow Sun Coral”
In Boschma 1953, all the Indo-West Pacific “orange sun corals” were treated as a single species, whilst a distinctive Eastern Pacific form was regarded as a separate species, T. tenuilamellosa. The most recent revision of the group placed this form as a synonym of a broadly defined coccinea, but based on some easily observable differences seen within mixed-species aggregations in their natural habitat, there is a very high likelihood that there is a distinct lineage worthy of species recognition in the Eastern Pacific.
This morph is notable for being a solid pale yellow, as well as having unusually tall polyps. Boschma mentions tenuilamellosa as possessing tall calices with a deep “fossa”, the bowl-like hollow at the top of the polyp. The septa are reported to be notably thinner than in coccinea, which is where its scientific name derives from. All of these features seem to match up with images taken in situ, but until skeletons are properly studied, there is no definitive identification for this coral. In addition to populations in the Eastern Pacific, identical specimens have been regularly exported from Bali, often maricultured alongside other species to create multicolor sun coral rocks.
Tubastraea tagusensis “Tagus Cove Sun Coral”
The third commonly seen orange species, T. tagusensis, was described from the Galapagos Island of Isabela, specifically an area known as Tagus Cove. It is now known to occur throughout the Indo-Pacific, and is reported as invasive in Brazil. Its shape is similar to T. cf aurea, and it is likely to be frequently misidentified in the trade as such. Small specimens are also appearing for sale online as Dendrophyllia arbuscula, a species with a branching colony shape. In researching this article I looked far and wide for anyone with a properly identified specimen in their aquarium, but, alas, nearly every specimen identifiable as T. tagusensis has been misidentified as the easily distinguishable T. faulkneri. This species is abundant in the aquarium hobby, but it seems that no one has recognized it until now.
Tubastraea tagusensis has relatively closely-spaced polyps, though from the specimens that have been illustrated in the scientific literature there appears to be a bit more coenosteum present than in T. coccinea/aurea. This may be why it has been confused with the otherwise very different T. faulkneri. The corallites average 7-12mm in diameter, which does little to separate them from the other orange species. Where they differ is in the polyp heigh, as they potentially can reach up to 35mm, compared to 13mm for T. cf aurea. This combination of taller, closely-packed polyps gives this species a spikier look than its congeners.
Another possible difference is that some (but not all) specimens are entirely yellow, whereas T. coccinea/aurea usually will have an orange body with yellow tentacles. Whether these yellow specimens are indeed the same species or an undescribed form are impossible to know without morphological and genetic study. I have also seen shorter-polyped yellow specimens attributable to T. cf aurea, which lends some credence to these being mere color variants.

A specimen alongside a possible T. diaphana in the Red Sea. Photo by Mona Deinhart & Chris Leba.
The definitive way to separate this species is to look at the septa. In T. tagusensis, S1-3 are equal in size in mature polyps, and occasionally S4 can be similarly sized as well. This gives the corallites up to 48 septa reaching the center of the corallite, compared to just 12 for T. cf aurea.

The two colonies upper-left to the eel appear to be tagusensis. From Sri Lanka. Photo by Christian Loader.
Tubastraea floreana “Galapagos Sun Coral”
Tubastraea floreana was described in 1982 from the Galapagos island of Floreana, and appears to be restricted to the island chain. It is listed as endangered, with only one small population now known to exist. The other known populations are thought to have died off due to environmental changes brought about by a recent occurrence of an El Niño oceanographic pattern. Thus, barring its discovery in the West Pacific, this species in not likely to appear in aquariums anytime soon. Identifying T. floreana is relatively easy, as the polyps are roughly half the size of its orange congeners: 4-6mm vs. 7-13mm diameter. Unlike the other orange species discussed, there are only three septal cycles, with S3 being rudimentary. The overall impression is of a Tubastraea that has been miniaturized in size and skeleton. The colonies are reported to be a bright pink in life.
Tubastraea diaphana “Short Black Sun Coral”
The original species description of T. diaphana published in 1846 by J.D. Dana states that the colonies are “low, subramose” and black in coloration. Furthermore, the description makes special mention of the abnormally thin corallite walls present in the skeleton. These walls were thin enough that small perforations could be seen, giving the colony a diaphanous look—hence the name T. diaphana.
T. cf diaphana. Note the perforations (arrow) through the corallite walls. Photos by Arrigoni et al 2014.
Confusing the matter is a specimen identified by Arrigoni et al 2014 that appears to bear all the hallmarks of T. diaphana, save for having orange tissue instead of black. Nowhere else in the literature has there been any mention of an orange color morph of this species, nor are there other species in the genus with known color variants so extreme. This raises the question of whether these orange and black sun corals possessing diaphanous skeletons are truly the same species. Perhaps this orange specimen represents a species heretofore unknown to taxonomists. Clearly more specimens are needed before we better understand what is going on here.
The black variant of this species is regularly seen for sale, often sold alongside frags of T. cf coccinea that have been glued together on a flat base. Such pieces are exported from Bali, and are probably best avoided as rarely are all the glued pieces in good health. It’s impossible to say if the orange variant is ever exported, but it seems quite likely that it would be. Differentiating the orange variant from T. coccinea/aurea would be virtually impossible without a skeleton to examine.
Tubastraea micranthus “Branching Black Sun Coral”
Tubastraea micranthus is naturally found throughout the Indo-Pacific, and it is a recent introduction into the Caribbean, where it is feared it may become invasive. Large colonies of this branching black sun coral are perhaps the easiest species in this genus to identify, but rarely are large colonies collected for aquarists. More likely to be seen for sale are broken pieces of straight, twig-like branches that resemble a bottlebrush or young colonies with a short, bushy shape. (Note that small colonies are frequently misidentified as the non-branching T. diaphana.)
Like T. diaphana, the tissue covering the coenosteum is a brownish-black, with varying amounts of green. (Interestingly, Tachikawa 2005 reports a bright orange variation of this coral exists in the waters of Japan alongside the more commonly encountered black morph.) The colony shape is unique, being predominately arborescent and able to reach several feet in height. This led to their initial inclusion in Dendrophyllia, which has many deep-water branching species of nearly identical shape. The lack of a Pourtalès plan has traditionally been used to exclude it from the otherwise similar Dendrophyllia, but Arrigoni et al 2014 reports that this character is actually quite variable, with polyps from the same specimen often showing both character states. Genetic study did eventually corroborate its placement within a monophyletic Tubastraea.
Tubastraea micranthus is reported to be a particularly difficult species to keep. There have been reports of specimens being maintained for years, and even growing new polyps, but invariably strong water currents and heavy feedings are required. For most aquarists, this species never opens its polyps and slowly dies. In the wild, T. micranthus is unique amongst Tubastraea in preferring the type of high-flow habitats at the top of reefs favored by Acropora. This need for heavy flow and heavy feedings makes this one of the more demanding corals available to aquarists. Unfortunately, less-scrupulous vendors will usually gloss over this difficulty when selling them. Probably more than any other Tubastraea, this species often meets a grisly demise of starvation.
Tubastraea sp. 2 “Bicolored Sun Coral”
It’s not uncommon for unusual sun corals specimens to turn up for sale. More often than not, anything that isn’t easily recognizable tends to get labeled as a “dendro”, with little regard to the actual accuracy of the identification. Arrigoni et al found that some specimens that displayed a slight amount of branching were in fact molecularly related to Tubastraea.
These two undescribed species share a similar colony shape, consisting of numerous tall polyps arising from a shared base, with secondary branches budding off some of these primary polyps. The branching is generally less than is seen in a coral like Dendrophyllia arbuscula, though there are strong similarities between the two, and it is quite possible that small colonies of either could be confused. In large colonies of the unidentified Tubastraea, it seems that the shared portions of the skeleton lose tissue and become overgrown with algae, detritus and encrusting organisms. They can resemble Rhizopsammia in this regard, but the septa are generally less prominent in living specimens.
The specimens which I am identifying (tentatively) as sp. 2 can be identified by the darkly-colored oral disks, which contrast with the yellowish coenosarc. This feature has given them the common name used in this article. Colonies are composed of apparently separate clusters of polyps, with each cluster containing anywhere from 6-10 individual polyps.
Confusingly, there seem to be two different corals called sp.2 by Arrigoni et al. The first instance is used as the basis for this coral ID, but the second time it appears it is in reference to a particularly tiny (~5mm polyp diameter) and unbranched colony. The largest illustrated specimen appears to have only three polyps. Hopefully this will be explained in future publications.
Tubastraea sp. 1 “Spiky Orange Sun Coral”
This variant, which I am tentatively grouping together as the third undescribed species discussed in Arrigoni et al 2014, is identifiable from its tall polyps and orange coloration, as well as the inconspicuous septa when viewing living colonies. This may merely be a color variant of what I have identified as tenuilamellosa? in this article, as the only obvious difference is in coloration. This doesn’t seem to be a common aquarium offering, but I have seen some aquarium specimens which can be potentially groups here. Obviously, much taxonomic work remains to be done before a meaningful identification can be reached for this coral.
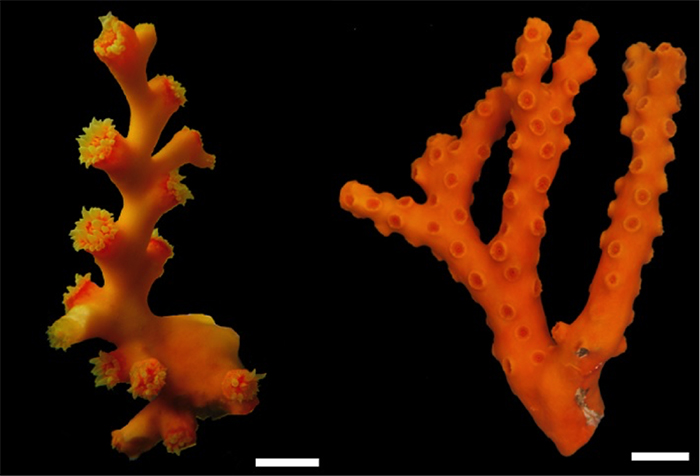
Dendrophyllia arbuscula “True Branching Dendro”
Despite the ubiquity of its name in the aquarium industry, Dendrophyllia is one of the rarest corals available to aquarists (outside of Japan at least). Most of the twenty-nine valid extant species are known only from deep, temperate waters. The genus is currently defined by its branching colony shape and Pourtalès septal arrangement, but there is now evidence to indicate these characters have convergently evolved elsewhere. There is also genetic evidence that what we currently “Dendrophyllia” may not form a single lineage.
The genus can be divided into three informal groups. The first group consists of tall, arborescent colonies made up of a few thickened branches with an axial corallite at the tip. D. cribosa and ijimai are the only shallow water members of this group. Colonies of D. cribosa form thick branches (around 6-10 branches per colony) with numerous short polyps arranged in irregular lines around the circumference. D. ijimai forms far thinner branches—the main branch being not much thicker than the side polyps—with much taller polyps extending laterally off the main branch. Both species have wide ranges and may potentially turn up for sale, likely as broken branches rather than whole colonies.
The second group consists of several species with smaller, bushier colonies. The total number of polyps is generally smaller than in Group 1, and the primary branches are typically far shorter and with far fewer polyps budding off. There is still an axial corallite, though it isn’t necessarily as obvious as it is in group 1, being little differentiated from the other polyps. D. arbuscula, which has a known depth range of 2-353m and a wide distribution throughout the Indo-West Pacific, is the only species likely to be exported. Several other species occur in the shallow waters of Japan, and these too may be sporadically collected for the Japanese market.
The scientific name arbuscula is derived from the Latin arbusculus, meaning “little tree”. This is an apt name, as the largest colonies examined by Cairns (1994) consisted of only 8 polyps. This raises the question of whether the low polyp count is an artifact of the limited number of specimens available for study, or if it is truly diagnostic for the species. This low number of polyps reported for D. arbuscula is in stark contrast to photographs online attributable to this species, with polyp counts that range from the dozens to perhaps a hundred or more.
The third and final group comprises those species possessing tall branches with polyps that grow in a zigzag (=sympodial) pattern instead of an axial corallite. D. boschmai is the only species found in relatively (40m+) shallow water, and the few aquarium specimens that I’ve seen which show this characteristic sympodial growth form are presumably of this species. This species seems to have a widespread (or possibly antitropical?) distribution, and all the specimens I’ve seen have come from Japanese aquarists.
Tubastraea sp.3 “Fathead Dendro”
The “fathead dendro” is one of the more desirable and expensive species of sun corals available to aquarists. It also happens to be one of the easiest species to keep, as the polyps tend to extend their polyps during the day for easy feeding, and colonies seem to thrive in any reasonably healthy aquarium. Colonies are comprised of large (15-20mm) polyps with a coloration that varies from a bright orange oral disk with light-colored walls to more solidly-orange specimens. There are typically not more than a dozen polyps per colony.
While the “fathead” name is certainly appropriate (few colonial sun corals have such large polyps), it turns out the “dendro” part of the name is a bit off. This really should surprise nobody, as there is never any branching pattern present in this species. It was only with the recent molecular work of Arrigoni et al 2014 that we now have a name to put to this species: Tubastraea sp. 3. Yes, another unidentified Tubastraea species!
Frustratingly for such a desirable coral, this species appears only sporadically in the aquarium trade. When they’re available, they can be had in large numbers, and then they seem to disappear from the hobby for long stretches. The reason for this—as reported by some online retailers—is that these are illegally exported without proper CITES, presumably from Indonesia. Of course, there are no CITES permits for undescribed species, so any specimens seen for sale are smuggled in under false names.
One name that is frequently misapplied to this species is D. fistula. It’s almost impossible to determine where misidentifications like this originate, but once an incorrect name gets used it tends to stick. Aquarium authors are a steady source of these misidentifications, and a recent article by Young (2013) is a fine example. He astutely recognized that D. fistula had been synonymized as Eguchipsammia fistula and published the “fathead dendro” under this new name. Thus was born a new and even more egregious misnomer. E. fistula is a deepwater species with colorless polyps and a unique twig-like colony shape that is unattached and lays half-buried in the muds of the ocean floor.
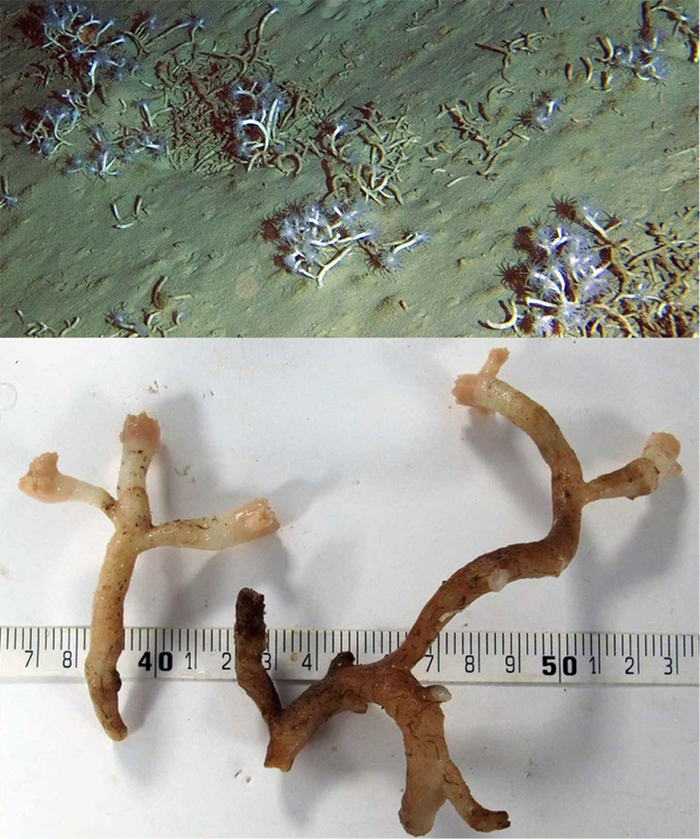
Eguchipsammia fistula, shown living in its natural deep-water habitat— buried and unattached to rock. Note the white tissue and elongate skeleton. Photos by Roder et al, 2013.
There is a strong financial incentive for aquarium retailers to disect “fathead” specimens into single-polyp frags. This can make for some confusing identification problems, as these single polyps are quite difficult to tell apart from the “solitary sun corals”, Balanophyllia. A look at the skeleton would quickly reveal the difference, as Balanophyllia has a strong Pourtalès Plan, but a live specimen would make this determination impossible. Perhaps the best tip is to look for the characteristic bicolored appearance of most “fathead” specimens.
Balanophyllia? “Solitary Sun Corals”
The solitary dendrophylliids comprise the most numerous genus in the family, with 63 species as of last count. These are quite uncommon corals amongst aquarists, and most specimens identified as Balanophyllia are just as likely to be misidentified. To wit, most aquarists who have kept specimens for any length of time have reported asexual budding along the edges, which by definition makes that specimen NOT Balanophyllia. These are likely to be single polyps of a colonial species, such as Tubastraea sp. 3.
Aquarists tend to anecdotally identify Balanophyllia based on its large polyp size, ovular shape and je ne sais quoi. Certainly large, ovular polyps that stay solitary are going to be easy to identify, but it should be noted that Balanophyllia has many small and circular species—colonies of which could easily be confused with a coral like Rhizopsammia. A name that seems to pop up with some regularity amongst aquarists and divers is B. bairdiana, though it should go without saying that such identifications are based on little more than whim and fancy. Oddly, the name is frequently used for a colonial species of unknown identity. (Do remember that discretion is the better part of valor when it comes to identifying scleractinians.)
Not surprisingly for a genus whose only real defining characteristic is its lack of defining characteristics, molecular study has shown the group to form at least two separate lineages. There have traditionally been two subgenera recognized: B. (Balanophyllia) includes the solitary species that remain attached to the reef, while B. (Eupsammia) includes those solitary species that are free-living in muddy substrates. To date, only 3 of the 63 species have been studied genetically, so it’s anyone’s guess how many further solitary lineages may be discovered.
Rhizopsammia? “Micro Dendros & False Dendros”
Rhizopsammia is one of the simplest of the colonial dendrophylliids. The polyps arise from reptoid (=stoloniferous) growth, meaning that new polyps bud from thin, encrusting strips of tissue that spread outwards from the original polyp. The end result is a colony of closely placed polyps which look solitary and unconnected.
There is some question as to the true identity of the “micro dendro” species in the aquarium trade, as Jake Adams (pers. comm.) has mentioned specimens that have been identified as Eguchipsammia by Dr. Steven Cairns. Eguchipsammia is a troublesome genus taxonomically. It originally was created to house branching specimens that lived unattached in deepwater muds. This was later expanded to include branching specimens that remained attached and that could occasionally form large colonies—such colonies can even form expansive deepwater reefs.
Further complicating matters is the recent molecular evidence indicating that Rhizopsammia and Cladopsammia likely belong to the same genus, representing variations in the amount of stoloniferous connectivity between polyps. The six species of Cladopsammia are essentially identical to Rhizopsammia save for the fact that the stoloniferous connections spread to fully cover the areas between the individual polyps, giving the appearance of a shared skeleton that each polyp emerges from. Cladopsammia can also form buds directly off of the primary polyps, which is a trait shared with its other close genetic relation, Eguchipsammia.

Rhizopsammia is quite variable in size. Note the prominent stolons in this specimen. Photo by Sabine Penisson.
The “micro dendros” are one of the least commonly collected corals, but this is not likely due to any real rarity in the wild. There are ten species of Rhizopsammia, all of which occur in shallow waters, and at least three of which have wide ranges throughout the Indo-West Pacific. These three are distinguished based on polyp size: R. verrilli are largest (up to 45mm tall and 9mm wide), R. nuda are up to 10mm tall and 5mm wide, while R. minuta are smaller still. It appears that R. verrilli has become a somewhat common export recently, with one online retailer regularly offering specimens. In the case of this seller, they have been misidentified as “Dendrophyllia arbuscula”. These can be identified by the tall polyps, which are sparsely populated on the coral. There is usually some slight amount of branching, and the septa are large and protuberant above the walls of the polyp.
Cladopsammia? “Muddy Sun Coral”
This is another poorly known genus with a confused taxonomic history. The species placed here were formerly members of Dendrophyllia, but were segregated based on their unbranched growth form. Beneath the sediment in this photo is a shared skeleton from which the polyps arise, which is the main defining feature of this clade as it now stands, but it could easily be argued that this is just an exaggeration of the stolons seen in Rhizopsammia. And, in Arrigoni et al’s molecular study, the two groups are shown to be closely related—possibly congeneric.
Dendrophyllia sp. “Pink Branching Dendro”
One species that can cause confusion is a very Tubastraea-like “dendro” which has become increasingly common in recent years. This species is being collected from relatively deep waters (30m) off Sumatra that experience unusually cool temperatures and high water velocity. Many wholesalers are selling it as an “orange sun coral”, which highlights the relative uselessness of our current trade names.
This species has been frequently misidentified since its introduction in the hobby, with one prominent aquarium publication (de Vries 2012) misidentifying it as Cladopsammia gracilis. A well-known online retailer has been selling these under the old and incorrect name D. fistula. That this is a true Dendrophyllia is based on a specimen I’ve had identified by azooxanthellate coral expert Dr. Steven Cairns at the Smithsonian.
For many aquarists this may seem like a big taxonomic “Who cares?”, but it is important to recognize the distinctiveness of this species, as the “pink brancing dendro” is much harder to maintain in captivity than any Tubastraea species. Aquarists, almost without exception, report little success getting this species to open it polyps for feeding. Given its deeper-water origins, perhaps this is a species that would benefit from cooler water temperatures. It seems that the only reliable way to get this species to extend its polyps is to blast it with impracticably heavy water currents. One aquarist who reported some success with this species mentions that a weeklong period without feeding was observably detrimental, whereas other azooxanthellate species in the same tank fared fine. As it stands, this is a species that is better left in the oceans.
Dendrophyllia is a large genus with many poorly known species. Of the three species groups in the genus, the pink branching dendro doesn’t seem to fit neatly into any of them. In fact, without having had a specimen identified by the world’s expert on the group, it would have been difficult to argue that this wasn’t just an aberrant Tubastraea. So is this an undescribed species?

A larger colony of this unidentified “red dendro”. It has been misidentified online as T. sibogae, a junior synonym of T. diaphana.
It bears mentioning that this species is attributed to Dendrophyllia based on the presence of a weak Pourtalès Plan, but this character is of questionable importance given the molecular work of Arrigoni et al 2014.When Dendrophyllia was first described it included all the species now in Tubastraea and Cladopsammia. Given the massive changes to coral classification that have occurred recently with the increase in molecular studies, it’s hard to argue that we have a firm understanding of how these different species and genera truly fit together. The “pink branching dendro” is a perfect example of that uncertainty. A species with a (weak) Pourtalès septal arrangement, a massive Tubastraea-like colony shape that extends into Dendrophyllia-esque branched lobes. This is a dendrophylliid missing link!
Citations
Adams, Jake. Eguchipsammia at Extreme Corals DE Is the First Micro Dendro We’ve Seen in a Couple Years. Reef Builders. N.p., 14 Feb. 2012. Web. 26 Dec. 2014.
Arrigoni, R., Kitano, Y.F., Stolarski, J., Hoeksema, B.W., Fukami, H., Stefani, F., Galli, P., Montano, S., Castoldi, E., Benzoni, F. 2014. A Phyogeny Reconstruction of the Dendrophylliidae (Cnidaria, Scleractinia) Based on Molecular and Micromorphological Criteria, and its Ecological Implications. Zoologica Scripta. 43:661-688
Boschma, H. 1953. On Specimens of the Coral Genus Tubastraea, with Notes on Phenomena of Fission. Studies on the Fauna of Curacao and other Caribbean Islands. 4(18): 109-119, plates 9-12.
Cairns, Stephen D. 1994. Scleractinia of the temperate North Pacific. Smithsonian Contributions to Zoology, 557: i-150. doi:10.5479/si.00810282.557.i
Cairns, S.D. 2000. A revision of the shallow-water azooxanthellate Scleractinia of the western Atlantic. Studies on the Fauna of Curacao and other Caribbean Islands, 75: 1-240.
Cairns, S.D. 2001. A generic revision and phylogenetic analysis of the Dendrophylliidae (Cnidaria: Scleractinia). Smithsonian Contributions to Zoology 615: 1–75. doi: 10.5479/si.00810282.615
Cairns, S.D., and Kitahara MV 2012. An illustrated key to the genera and subgenera of the Recent azooxanthellate Scleractinia (Cnidaria, Anthozoa), with an attached glossary. ZooKeys 227: 1–47. doi: 10.3897/zookeys.227.3612
Cairns, S.D. and Parker, S.A. 1992. Review of the Recent Scleractinia (stony corals) of South Australia, Victoria and Tasmania. Records of the South Australian Museum Monograph Series, 3: 1-82.
Cairns, S.D. and Zibrowius, H. 1997. Cnidaria Anthozoa: azooxanthellate Scleractinia from the Philippine and Indonesian regions. Memoires du Museum National d’Histoire Naturelle, 172: 27-243.
Dai, Chang-feng, and Sharon Horng. Scleractinia Fauna of Taiwan. Taipei, Taiwan: National Taiwan U, 2009.
Dana, J. D. 1846. Structure and classification of zoophytes: narrative of the United States exploring expedition during the years 1838, 1839, 1840, 1841, 1842 under the command of Charles Wilkes, U.S.N., Vol. 7. G.P. Putnam. Philadelphia. 740 p.
De Vries, Joost. “The Pink Branching Dendro Is Probably Cladopsammia Gracilis.” Reef Builders. N.p., 08 Nov. 2012. Web. 26 Dec. 2014.
Fatherree, James. “Aquarium Corals: A Look at the Sun Corals.” — Advanced Aquarist. Pomacanthus Publications, LLC, Dec. 2011. Web. 24 Jan. 2015.
Glynn, Peter W., Gerard M. Wellington, and John West Wells. Corals and Coral Reefs of the Galápagos Islands. Berkeley: U of California, 1983.
Mantelatto, M.C., J.C. Creed, G.G. Mourão, A.E. Migotto and A. Lindner. 2011. Range expansion of the invasive corals Tubastraea coccinea and Tubastraea tagusensis in the Southwest Atlantic. Coral Reefs 30(2): 397.
Roder, C, Berumen ML, Bouwmeester J, Papathanassiou E, Al-Suwailem A, Voolstra CR. 2013. First biological measurements of deep-sea corals from the Red Sea. Sci Rep 3
Sammarco, P. W., Porter, S. A. and Cairns, Stephen D. 2010. A new coral species introduced into the Atlantic Ocean – Tubastraea micranthus (Ehrenberg 1834) (Cnidaria, Anthozoa, Scleractinia): an invasive threat?. Aquatic Invasions, 5(2): 131-140. doi:10.3391/ai.2010.5.2.02
Sentoku, Asuka, and Yoichi Ezaki. “Intrinsic Constraints on Sympodial Growth Morphologies of Azooxanthellate Scleractinian Coral Dendrophyllia.” Ed. Patrick Callaerts. PLoS ONE 8.5 (2013): E63790.
Tachikawa, H. 2005. Azooxanthellate Scleractinia (Hexacorallia, Anthozoa, Cnidaria) collected from Otsuki, Kochi Prefecture, Japan. Kuroshio Biosphere. 2:1-27 +13pls.
Wells, J.W. 1983. Annotated List of the Scleractinian Corals of the Galapagos. In P. W. Glynn and G.M. Wellington, editors, Corals and Coral Reefs of the Galapagos Islands, pages 212-291, 21 plates. Berkeley: University of California Press.
Young, Ngalon 2013. Adding Non-Photosynthetic Corals To Your Reef An Introduction And Husbandry Guide. Reef Hobbyist Magazine. 4:38-44.

















































Hello!
I noticed that the first picture in this article is mine. You probably have taken it from project Noah?
Well, thank you! That makes me proud 🙂
It is Ok that you use it but I would have preferred that you contacted me first.
By the way, my name is written incorrectly (Marta Texiera). My correct name is:
Marta Rubio Texeira
If you can correct it here that would be nice.
I will be happy to share this and more pics with you but please always contact me first. Thanks!
Marta