- Location
- Connecticut
I just ran tests for ammonia on ammonia chloride solutions I prepared with tap water. Both kits are advertised as being compatible with salt and fresh water.
The Elos (Expry 11-16) test was completely inert, giving no color change whatsoever for NH4Cl concencentrations from 1 to 1250 ppm.
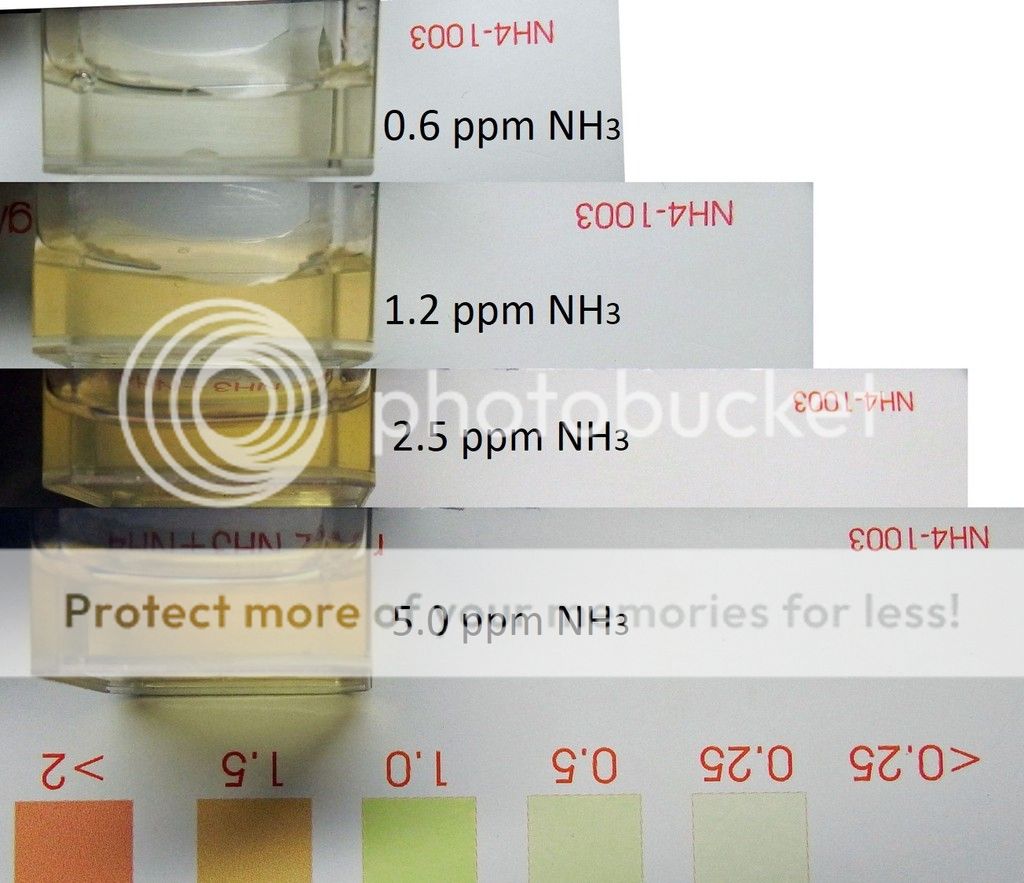
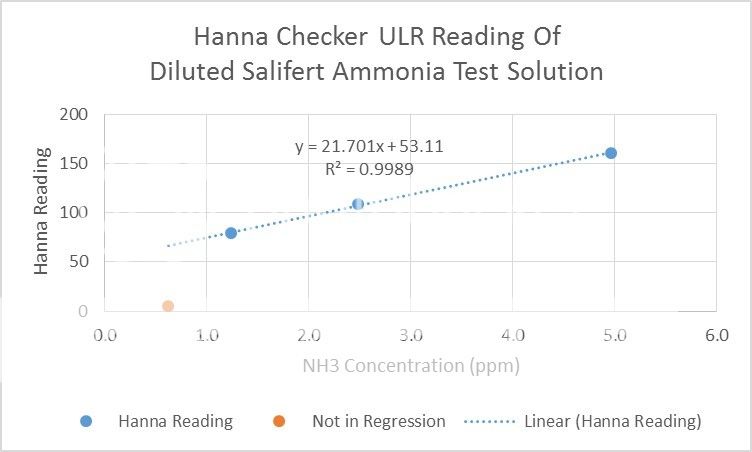
Salifert (Expry 9-15) was only a little better. It indicated 1.5 ppm for a 16 ppm solution. At 5 ppm, there was a hint of color.
Am I missing something or could these tests really be this bad?
Dan
The Elos (Expry 11-16) test was completely inert, giving no color change whatsoever for NH4Cl concencentrations from 1 to 1250 ppm.
Salifert (Expry 9-15) was only a little better. It indicated 1.5 ppm for a 16 ppm solution. At 5 ppm, there was a hint of color.
Am I missing something or could these tests really be this bad?
Dan