In the mid-1980’s I bought a small aquarium laboratory ‘kit’ in order to monitor various aquaria chemical parameters. This kit, imported from Europe, included state-of-the-art testing reagents, including one for alkalinity (or carbonate hardness).
Since that time, testing for alkalinity has become commonplace among reef hobbyists and for good reason. Hobbyists now have many choices when choosing an alkalinity test kit. How accurate are these? What should we know in order to make an informed decision? To this end, four different brand ‘test kits’ were purchased and their performances evaluated.
What is Alkalinity, and Why Is It Important?
Alkalinity is the acid-neutralizing capacity of water, and is due to the presence of carbonates, bicarbonates, hydroxides as well as other bases including borates, silicates, phosphates and others. We are most interested in the bicarbonate content of reef water since it not only buffers against modulations in pH, but acts as a major inorganic carbon source for zooxanthellae photosynthesis (carbon dioxide being another source). Alkalinity is destroyed during the conversion of toxic ammonia to relatively harmless nitrate. Calcium carbonate (in the form of aragonite) constitutes a major portion of coral ‘skeletons’. In short, alkalinity is one of the most critical parameters to monitor and maintain.
(Note: It is not the intent of this article to extensively discuss alkalinity and any of the many caveats in its proper determination and sources of potential error. These have been the fodder for more than a few articles. For example, see Randy Holmes-Farley’s in-depth comments at www.advancedaquarist.com/2002/2/chemistry and www.advancedaquarist.com/june2003/chem.htm. These articles are linked to many more.)
Sampling Procedures
It is generally best to analyze the sample immediately, however, use these procedures if this is not possible. Samples used in determination of alkalinity concentrations should be gathered in clean glass or plastic containers, preferably a new container that will be dedicated to this purpose. Fill the sample bottle completely and cap tightly to avoid exposure to the air. Refrigerate (4ºC or 39ºF) the sample and try to test within 24 hours (although a holding time of 14 days is permissible). Bring the sample to room temperature before testing.
Tips for Using Test Kits
If a meter is not used to determine titration pH endpoints, then colors should be determined against a white background and under a full-spectrum fluorescent lamp (avoid high kelvin lamps when determining a colorimetric endpoint!). Also avoid highly colored and/or turbid samples when judging endpoint coloration (usually not a problem with aquarium water, but a source of potential error in the field).
In the cases of the Salifert and Seachem tests, resist the urge to return unused titrant to its container – this is a potential source of contamination!
Testing Protocol
The tests performed all report ‘Total Alkalinity’ which hereafter is referred to as simply ‘alkalinity’. The water ‘bible’ Standard Methods for the Examination of Water and Wastewater recommends one procedure for determination of alkalinity – the Titration Method. Titration (pronounced tie-tray-shun) for alkalinity is a procedure where an acid of known strength is added to a sample of known volume until a predetermined pH – the endpoint – is reached. This endpoint can be determined with a calibrated electronic pH meter and probe or by use of color indicators (where pH is determined by a color change such as bromcresol green-methyl red sodium salt changing from green to pink at a pH of ~4). Alkalinity is then calculated using the known factors. The acceptable reporting unit is ‘milliequivalents per liter’ (although aquarists commonly use other less desirable units).
I titrated 100ml of freshly sampled aquarium water with 1.6N sulfuric acid until an endpoint of ~4.2 was reached. With this volume of sample and acid ‘strength’, total alkalinity is determined simply by multiplying the titrator’s reading by 1. These were the various conversions used:
Total alkalinity (mg/l as CaCO3) divided by 50 = milliequivalents per liter.
Milliequivalents per liter X 2.8 = KH (carbonate hardness).
At least 4 tests were conducted with each test kit, and compared to multiple tests using Hach’s reagents. The results were averaged. These observations of different brands of alkalinity testing devices and procedures are not meant to be exhaustive or statistically significant. It is simply a report of comparisons of results obtained from single test kits of different makes.

Figure 2. The setup used in the testing protocol. The 100ml sample (now pink in the beaker) was titrated with 1.6 normality sulfuric acid until a pH of ~4.2 was reached.
Red Sea pH/Alkalinity Test Lab
Number of tests: 60 tests
Amount of Titrant: 15 milliliters (about 300 drops)
Test Method: Colorimetric endpoint after titration with a known amount of titrant (5 drops). Uses a printed color comparator as a reference. “Normal” range is a greenish color indicating a range of 1.7-2.8 meq/l. ‘High’ is blue, and ‘Low’ is colored yellow.
As noted, this combination test kit measures alkalinity as well as pH.
Titration Endpoint (pH): 4.06 standard units
Units: General ranges of milliequivalents per liter only, consisting of ‘Low’, ‘Normal’ and ‘High’.
Comments: This method reports only in general terms and then depends upon the observer’s color vision. Uses citric acid as the titrant. No Material Safety Data Sheet.
A small trial bottle (25 ml) of alkalinity booster is included. See test results below.
Salifert Carbonate Hardness/Alkalinity Profi Test
Number of tests: 100-200
Amount of Titrant: 50 milliliters (about 1,000 drops)
Test Method: Titration to a known colorimetric endpoint with varying amounts of reagent.
Titration Endpoint (pH): 3.93 standard units
Units: Milliequivalents per liter; KH
Comments: No Material Safety Data Sheet is included, although warnings to ‘keep out of reach of children’ (what about some ‘adults’?), ‘dyes will stain’ and so on are included. The directions apparently assume the user understands carbonate chemistry terminology (as KH and meq/l are never defined). The expiration date of the reagents is marked on the box top, but it is in German “Mindestens haltbar bis: 05-2011”, which translates literally to “Long-lasting at least to: 05-2011”. Titration endpoint is sharp and easily recognized. See test results below.

Figure 4. The Salifert alkalinity test appears to use the same reagents as Hach’s method.
Seachem MultiTest pH/Alkalinity
Number of tests: ‘over 75 tests’ – the approx. total of ph and alkalinity tests. Projected number of alkalinity tests (based on alkalinity of ~3 meq/l): At least 30.
Amount of Titrant: ~6 ml
Test Method: Titration method based on a colorimetric endpoint.
Titration Endpoint (pH): 2.9 standard units
Units: Milliequivalents per liter.
Comments: Instructions are straight forward, and results are reported in the proper unit of meq/l. No Material Safety Data Sheet is included. Reagents have no expiration date, but Seachem warranties their reagents according to their website (www.Seachem.com). A standard solution is included to check accuracy (a unique feature among these tests). Titration endpoint is easily identified and is ‘clean.’ Multiple tests can be performed with provided lab ware (see Figure 5). Lab ware is compact. See test results below.

Figure 5. Colorimetric divisions of Seachem’s alkalinity test. Top row, from left: Initial color (blue), one drop before endpoint (green-yellow), and finally, the endpoint of the titration (yellow).
Tetra
Number of tests (5 ml sample): 20, based on an alkalinity of ~3 meq/l
Number of tests (10 ml sample): 10, based on an alkalinity of ~3 meq/l
Amount of Titrant: 10 milliliters
Test Method: Titration method based on a colorimetric endpoint.
Titration Endpoint: 3.2 pH
Units: German Hardness, dH, Carbonate Hardness (KH) and (for freshwater) General Hardness (GH).
Comments: No Material Safety Data Sheet (MSDS), although reagent chemistry is listed in the instructions. The instructions could be confusing to a first-time user due to a plethora of terminology (see Units, above). The term ‘dH’ is never truly defined (it means ‘degrees of hardness’), and General Hardness and German Hardness could both be abbreviated as ‘GH’. Reagents do not have an expiration date. Titration endpoint is very apparent and ‘clean’. Lab ware (a vial) is relatively large and easy to handle. Test is quick and easy to perform. See test results below.
Tests were performed using 5ml and 10ml as per Tetra’s recommendations. In all cases, Tetra’s results were consistently higher than those obtained with the pH meter titrations.

Figure 6. Apparent colors of Tetra’s test as titration progresses. From left: Initial color, one drop before endpoint and endpoint.
Results
Red Sea
To my eye, the result appeared to be 2.2 meq/l compared to 2.7 by the ‘standard method’ (described above). This result is approximately 19% low. I did not graph the results as I did for the other tests (below) simply because judging the titrated sample’s color is so subjective.
Salifert
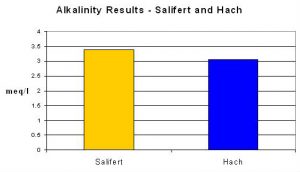
An average of Salifert’s results was 3.39 meq/l, compared to 3.05 meq/l using the procedure recommended by Standard Methods.
Seachem
This test kit reported an average alkalinity value of 2.50 meq/l compared to 2.78 using Hach’s reagents and digital titrator.
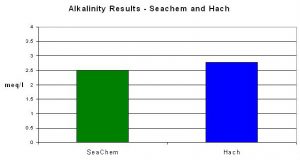
Figure 8. Seachem’s alkalinity test results were within ~10% of those obtained by the pH endpoint titration method.
Tetra
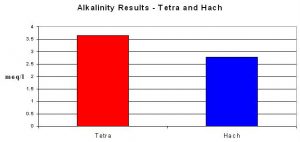
The alkalinity test by Tetra produced an average result of 3.66 meq/l. The titration/pH endpoint method averaged 2.78 meq/l.
Discussion
Salifert and Seachem test kits performed well in this ‘competition’. Both were within approximately 10-11% of the ‘true’ alkalinity value. Does it really matter to you that alkalinity is 3.3 meq/l but is reported at 3 meq/l? No two situations are exactly alike, but, generally, these easy-to-perform tests deliver results that should be acceptable to the majority of hobbyists.
The Tetra test did not perform as well. It is difficult for me to believe that the reagent was properly prepared at the factory. I tend to think the reagent was simply old and had lost potency.
And this brings up a point – Only Salifert marks their reagents with an expiration date. Although shelf life is probably at least year or more with the others, it would be comforting to know that reagents are fresh. Expiration dates are standard on Hach reagents, and it is not unheard of in the pet industry (besides Salifert, I seem to recall the German company Dupla once – and perhaps still does – marked their reagents with an expiration date). However, Seachem, on their website, states they will replace ‘old’ reagents (see that site for details).
As a general rule, reagents should be discarded 6 months after being opened. It is good lab practice to mark the bottle with the date it was opened. Project your realistic use of these kits – how often will you perform alkalinity tests in the next six months? Although large amounts of reagent might seem like a good idea, chemicals could ‘spoil’ on the shelf while waiting for use or become contaminated with use.
The Rea Sea test is, at least in this grouping, in a category of its own in that it simply reports alkalinity in rather vague ranges. This is not to say that the kit is without use – those wanting to monitor the buffering capacity of marine fish-only aquaria might find this test of value.
No Material Safety Data Sheets (MSDS) are included with any of the kits, and I did not find them on the company websites (www.redseafish.com; www.salifert.com, www.Seachem.com, and www.tetra.com). Having this information readily available would seem a good idea.
Reference
- American Public Health Association (APHA), 1998. Standard Methods for the Examination of Water and Wastewater. Edited by L. Cleceri, A. Greenberg and A. Eaton. Washington, DC.





0 Comments