Well, I wrote a book about tridacnid clams (Fatherree 2006a) and have been traveling around talking about them at club meetings and conferences for a couple of years now. I generally talk about providing them with sufficient lighting and whether or not they should be feed in aquariums, but I consistently get hit with the same story and questions wherever I go. With great regularity, someone tells me about losing a tridacnid, often one they’ve had for a while, for no apparent reason. And, of course, they want to know what happened to it. According to many of the stories, such losses often occur when everything else in a tank is fine (even other tridacnids), ruling out any general water quality problems. So, I call these cases “mysterious clam deaths.”
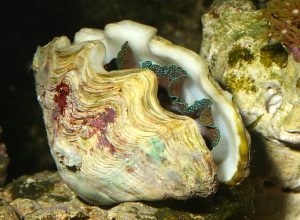
This is what advanced gaping looks like. The shell is wide open, but the mantle is flaccid and is not extended.
With this in mind, I’d like to give you an overview of some of the things that can kill tridacnids, most of which aren’t so mysterious. Many tridacnids fall due to stresses incurred from collection and shipping, but this would typically occur shortly after making it home, if they make it to a home. So, I won’t spend any time on that. Instead, I want to go over things that, again, could cause a tridacnid to die after it has been in an aquarium for a longer period of time. For now I’ll tell you that I get the impression that the majority of mysterious clam deaths are due to microbial or lighting issues, but we’ll get to that momentarily.
First, I want to point out that, much to the dismay of clam keepers, in many cases there are no problem-specific symptoms of particular troubles. There are no treatments, or at least no easy treatments for many things, either. Instead, in so many instances, you may not know that anything at all is wrong with a tridacnid until it starts to gape open, and then it’s usually too late to do anything to help it – even if there is some way to help it. Gaping simply means that a tridacnid’s shell sits open, typically more than normal, but the mantle tissue is rather flaccid and not extended as far as normal (or at all), with the clam typically reacting sluggishly (or not at all) to any sort of stimuli.

The shell margin of this gaping T. maxima is also discolored, again indicating that the clam has been in poor health for some time.
This is indeed the number one sign of trouble, but it’s also oftentimes the only obvious sign of trouble. Gaping can be brought on by just about anything that has negatively affected a tridacnid’s health to the point that it is near death, which could be environmental trouble, such as poor water quality, or disease, such as a bacterial infection of some sort, or any number of other maladies. So, gaping is a sure sign of an impending death, but seeing it probably won’t be of much help when it comes to deciding what, if anything, to do. Besides, after being in the hobby for the better part of 20 years, I don’t recall a single incidence of a tridacnid recovering, despite any form of treatment, once it has reached a state of health so poor that it begins to gape.
And with that said, here’s the list:
Bleaching
Tridacnids carry a complement of zooxanthellae, just as symbiotic corals do, and when conditions are unsuitable they can bleach out, just as symbiotic corals do. Basically, bleaching results from the loss of zooxanthellae and pigments in a tridacnid’s mantle tissue causing it to lighten up in color. There are several things that can cause this to occur at some scale or another, but unacceptable increases in light intensity (“light shock”) and/or temperature are the main culprits.

This specimen of T. derasa is in the process of bleaching out. The signs are obvious, as a significant portion of the mantle has lost its color.
Tridacnids have several means to protect themselves from over-illumination and can adapt to increased lighting over time. However, if the amount of light, especially UV light, a tridacnid receives increases faster than it can adapt, the result is literally an overload of the photosynthetic process (Osmond 1981 and Asada & Takahashi 1987). Unacceptably high temperatures (and sometimes low) are also problematic and can have the same effect on photosynthetic systems.
However, despite its sometimes lethal effects, bleaching is not a cause of mysterious clam death, as it has obvious effects on the coloration of a clam, which anyone should notice. Patchiness can develop when it starts, and if the condition progresses a tridacnid will turn almost completely white. This often takes days, or even weeks, too. So, you’d definitely see it and it would be obvious that something’s wrong. No mysterious deaths.
Predatory and Parasitic Snails
There are several species of snails in the genera Cymatium and Chicoreus that prey on tridacnids, some of which can grow as large as 10cm in length (Govan 1995). Most are smaller than this though, and can enter a tridacnid through the byssal opening, or can puncture the mantle by attacking from the shell margin or elsewhere when the shell is open, and then begin to eat a tridacnid’s soft tissues. So, if you happen to spot a suspicious snail on the outside, or especially on the inside, of a tridacnid, you’ll need to get rid of it immediately. Algae eating turbo snails, astraeas, conchs, and other well-known non-carnivores are fine, but I’d be wary of anything else. Fortunately, as far as I know these predators are not offered in the hobby, and I don’t personally know of anyone that has lost a tridacnid to a predatory snail.

These are pyram snails, hiding under the bottom of a T. derasa. They’re small and light colored, so you really have to look closely for these.
Conversely, a number of parasitic snails belonging to the genera Pyrgiscus, Turbonilla, and Tathrella, do kill tridacnids in aquaria with some regularity. These snails use a trunk-like snout called a proboscis to puncture a tridacnid’s mantle near the shell margin and then feed on the victim’s body fluids, and if too many attack a tridacnid, it can weaken or even kill it. These pyramidellid snails, better known as pyrams to hobbyists, are well-covered by Cumming (1988) and Boglio & Lucas (1997), but I’ll give you the basics.
They reach a maximum size of just a few millimeters, are light colored, usually hide under a tridacnid or in the substrate during the day, and are thus very difficult to spot. So, you have to look very carefully for them, usually at night, if you can’t remove a tridacnid to inspect it up close. They can also move from one clam to another if they choose, and they can reproduce very rapidly if left alone.
Once mature, these little snails will produce small, gelatinous, egg-filled globs on the shells of tridacnids every few days, which are transparent and particularly hard to spot. And from these globs can spring up to a couple of hundred offspring within just a few days. As if that’s not bad enough, the offspring can feed on a clam, become sexually mature, and start to lay egg masses themselves in as little as a few weeks.
Without any natural predators to keep them in check in an aquarium, you can imagine how quickly a clam could succumb to their numbers. So, if pyrams or their egg masses are found on a clam, they must be scrubbed off with a stiff toothbrush immediately, and you should watch carefully for any signs of re-infestation. Of course, these could certainly lead to a mysterious clam death, as the snails and their eggs are hard to spot if you’re not looking, and an infested clam will typically look fine until it gets so weak that it begins to gape. Note: You can find much more information about these snails and what to do about them online by taking a look at Fatherree (2006b).
Worms
There are several types of worms that may also pose a threat to the health to tridacnids. One of these is Urastoma cyprinae, better known as the oyster gill-worm, which is a type of turbellarian flatworm. These small worms are sometimes found in the mantle cavity, often on the gills, and in the digestive tract of Tridacna gigas and T. maxima, as reported by Goggin & Cannon (1989). However, they don’t seem to have any adverse affects on the health of these clams in the wild. Still, in a closed aquarium system and/or under stressful conditions, I’d imagine they may become problematic. Humphrey et al. (1987) reported that some clams carried an unidentified turbellarian flatworm and the nematode roundworm Syringolaimus, but no specific problems were mentioned. So, yet again, they may not be anything to worry about in the sea, but they may also become a problem in aquariums. And, Newman et al. (1993) reported consistently finding the polyclad flatworm Stylochus matatasi associated with the death of Tridacna gigas in one study of clam mortalities, which, unlike the others, may actually be large enough to see, as they can reach a full size of about 6cm. However, while they may be spotted at times on the mantle, they can also do their damage inside the mantle cavity where you more than likely wouldn’t spot them until after a clam is dead and removed. So, again, any of these may have the potential to cause a mysterious clam death, as there no distinctive symptoms will appear until a clam is weakened to the point of gaping.
There are also a number of errant polychaetes, commonly known as bristle worms, which have the potential to kill a clam. A few can get ridiculously large, with a handful of species reaching lengths of one or more meters, and some are indeed carnivorous. However, these relatively large and potentially dangerous species are actually very, very uncommon in aquariums, and they represent only a tiny percent of the hundreds of species of bristle worms that can be found in the seas. To the contrary, the overwhelming majority of species are quite harmless, and are actually beneficial in aquariums, as many are scavengers and/or detritus eaters.
However, there is the remote possibility that you may unintentionally introduce a carnivorous species to your tank, maybe hidden in a piece of live rock, so you should watch out for any worm that is obviously attacking a tridacnid. Likewise, there is at least one species (Oenone fulgida) that can apparently bore a hole through a clam’s shell and then chew on its tissues (Delbeek & Sprung 1994), so watch out for anything like that, too. It shouldn’t be difficult to spot, as this worm may be only about 2.5mm in diameter, but can reach a length of around 30cm and is bright orange in color.
Still, I personally have never seen any evidence whatsoever of a bristle worm attacking a healthy clam in any way. Yet what I have seen is scavenging worms coming out and feeding on the tissues of a very unhealthy or dead clam. So, if you happen to lose a tridacnid and then discover that there are some bristle worms crawling around inside it, don’t assume that the worms were the cause of death. In other words, there’s a chance that a mysterious clam death may be brought about by a hungry bristle worm, but it’s a slim chance.
Microbial Problems
A tremendous number of bacteria are found in seawater, but they typically aren’t a problem for healthy tridacnids. However, if a clam is stressed and its ability to defend itself is reduced, these same bacteria can become deadly. And, to make matters worse, bacterial populations can be much larger in aquariums, as they are closed systems with relatively high nutrient levels. In fact, Fitt et al. (1992) wrote that even small amounts of organic material added to seawater can lead to a 1000-fold increase in bacterial populations within a matter of hours in some situations. Thus, anything from excessive changes in temperature, salinity, lighting, etc., or something like a physical injury (like damage to the byssal organ during a relocation or a nip from a fish, for example) can compromise a clam’s immune system and in turn lead to a quick death in an aquarium.
Humphrey et al. (1987) listed off quite a few of these potentially dangerous bacteria, including various species of Vibrio, Acinetobacter, Enterobacter, and Pseudomonas, and Knop (1996) added Xeromonas and Plesoimonas, too. Then there’s a rickettsiales-like bacteria, that forms cysts on the gills of Hippopus hippopus, which was reported by Norton et al. (1993a), and an unidentified bacteria found on the gills of Tridacna gigas, reported by Norton et al. (1993b), too. Apparently any of these have the ability to kill a clam, and again, the only symptom of trouble that you might see is gaping, and possibly some necrotic mantle tissue.
Likewise, a protozoan by the name of Perkinsus olseni has been found in the digestive tract of Tridacna crocea, T. maxima, and T. gigas (Goggin & Lester 1987 and Goggin 1996), which can possibly lead to a clam’s death. But, as you’d expect by now, there are no diagnostic symptoms that you can identify, and there’s no recommended treatment even if you could.
An unidentified protozoan was also found along with Perkinsus in dead and dying specimens of Tridacna maxima, T. gigas, and Hippopus hippopus in some areas of the Great Barrier Reef and reported by Alder & Braley (1988). They were looking for the possible cause(s) of a great number of relatively sudden deaths of many clams when they found these two protozoans, but could not determine if one or both were the actual cause of the deaths. There were no specific symptoms of trouble in this case either, as the only indication that something was wrong was the death of the clams.

This specimen of T. crocea is suffering from a severe case of pinched mantle. The edges of the mantle are curled and literally look pinched.
Marteilia spp. is a protozoan known to attack the digestive tract of oysters, and Norton et al. (1993c) reported a Marteilia-like protozoan that attacked the kidneys of Tridacna maxima, too. Again, there are no outwards symptoms of trouble, infection can lead to death, and there is no recommended treatment.
Another unidentified protozoan has also been found in the blood of many specimens of Tridacna crocea, as reported by Nakayama et al. (1998). In fact, in one survey they found it present in 77 of 99 clams tested. They didn’t report any noteworthy effects, but the things are still there and I would guess they could become a problem in some situations.
And, yet another unidentified protozoan can invade the mantle tissue of Tridacna gigas and consume the zooxanthellae according to Humphrey (1988). However, its effects are poorly understood, too.
Knop (1996) also reported that an unidentified protozoan similar to Perkinsus was reported to attack Tridacna crocea, T. maxima, and T. squamosa. The organism produced small white cysts on the upper and lower surfaces of the mantle and around the byssal opening, and was 100% fatal when left untreated. So, this one actually has signs of trouble that you can see, and Knop gave it the appropriate name “whitespot disease”. Unfortunately, it is also apparently very contagious and leads to death in a few weeks to a few months time.
However, there is one disease called “pinched mantle” that can be easily recognized, which is also thought to be caused by yet another unidentified protozoan (Barry Neigut, pers. comm.). This condition can be spotted when the smooth, curving edges of the mantle are pinched and contorted. The clam may look like it is doing its best to stretch out the mantle tissue, but the margins just won’t extend fully the way they should. So, this wouldn’t be mysterious, either.

This T. squamosa is also suffering from pinched mantle. It was healthy until an infected clam was added to the same tank.
According to Neigut, it affects Tridacna crocea most often, with the other species being more resistant, but not immune. And, it can spread to other clams at times, being nearly 100% fatal, usually within a week or two of the first signs. Pinched mantle can be treated by giving an infected clam a freshwater dip for approximately 30 minutes, and Neigut has had good results with the use of the medication metronidazole, too. Note: You can also find much more information about pinched mantle and what to do about it online by taking a look at Fatherree (2006b).
As far as what’s in the scientific literature that can affect tridacnids, that’s all I can find regarding microbes. However, it’s important to note that there may be many, many more sorts of things from viruses, to bacteria, to worms that might affect tridacnids. Again, I haven’t found anything other than what I’ve presented above, but you can take a look at numerous sources, such as Paillard et al. (2004), Bower (2001), Bower & McGladdery (2001), Hine & Thorne (2000) and Dungan et al. (1989), and see just how many known infectious diseases and parasites affect various other sorts of clams. Many clams that are aquacultured for food have been given far more attention than tridacnids have, and after seeing all of the things that researches have come up with that affect food clams, I can’t help but think that tridacnids likely have far more tiny enemies than have been documented.

A good indicator of good health is the presence of a clean band of new shell material at the shell’s margins. It may be bigger or smaller than these depending on a clam’s growth rate, but there should always be at least a little new material.
Lighting Problems
Corals are particularly simple as far as animals go, and they require relatively few calories to get through the day. On the other hand, tridacnids have a complete digestive system, gills, kidneys, a heart, blood, etc. and burn up much, much more energy than corals. So, many tridacnids require more light than many corals do. Each species of giant clam is different though, and some certainly need more light than others in order to thrive. For example, Tridacna crocea is found only in very shallow waters in the wild and needs more light than the other tridacnid species. In fact, I’m quite certain I’ve never seen one living more than about 10 feet down. Conversely, I’ve found numerous specimens of T. gigas and T. squamosa living at well more than twice that depth, and T. derasa can often be found living even deeper. Regardless, don’t make the mistake of automatically thinking that if a given lighting system is bright enough to keep various types of corals alive and well that it’s also automatically bright enough to keep various types of tridacnids alive and well. Maybe it’s not.
Personal experience and the stories of others’ have convinced me that there’s a great deal of variability amongst clams of the same species when it comes to lighting needs, too. In other words, if a given lighting system is bright enough to keep one specimen of Tridacna crocea alive, that doesn’t necessarily mean it’s bright enough for all specimens of T. crocea. In any given batch of T. crocea, there will be some that grow faster than the others, some that get bigger than others, some that are more resistant to bleaching/disease than the others, some that are more brightly colored the others, and some that can thrive under less light than the others. Get the idea? It’s simply due to genetic diversity within the species, and that’s the reason that I generally make a conservative recommendation of placing any specimen of any species under intense metal halide lighting, a lot of high-output fluorescent bulbs, or something comparable like a good L.E.D. fixture.

Here you can see that there is no new shell material at all, and the margins have been overgrown by algae. This gaping T. crocea has been in poor health for some time now, likely due to insufficient lighting.
With that said, it’s time to say that I think the vast majority of mysterious clam deaths are simply due to insufficient lighting. Certainly not all cases, but most of them. The problem is that too many hobbyists do exactly what I warned against above. They have a lighting system that seems to be perfectly fine for keeping a number of corals, so they assume they can add in a tridacnid, too. Unfortunately, Tridacna crocea, the most light-demanding of the bunch, is also often the most attractive choice, and most often picked, at that. Then the clam slowly begins to starve.
The bad part is that it can take several weeks or even a few months for a tridacnid to finally starve to death, and they’ll typically look just fine until they have reached the point of no return and start gaping. This is why so many hobbyists tell the same story, that their corals are fine, but their clam looked great for some months, and then up and died for no apparent reason – mysteriously. In reality, the deceased may have been in the process of dying since the day it was put in the tank. Look at it this way, if a specimen is getting 95% of the light it needs, then it won’t just croak overnight. Instead, it will very slowly waste away. Of course, if it’s getting only 5% of what it needs, then yes, it may take only a few days before problems start.
On many occasions I’ve talked to hobbyists that have lost a tridacnid, and a few times have seen their tank and the shell of the deceased, and one thing keeps coming up – a lack of growth. In so many cases, I learned that while corals and such were growing fine in the same tank, the clam extended its mantle and looked normal (no signs of pinched mantle, bleaching, etc.) but never grew at all. And in just about all of these cases, I also find that they have relatively low-output lighting for a reef aquarium.

This is more “mysterious”, as the shell margin of this gaping T. crocea is very clean, indicating that it has been in good health for some time and then became seriously ill relatively quickly. Lighting was not an issue in this tank, and everything else seemed fine. Yet, the clam started gaping and later died for no apparent reason
The key to preventing such a death is to regularly look for new shell growth. Assuming water quality is where it should be and that other skeleton-building organisms are thriving, if a tridacnid is not adding on new material to the edge of its shell, there’s a problem. While there are uncommon specimens that have a yellowish/orangish/pinkish shell margin, the overwhelming majority should have a clean white one. It should never look “dirty”, greenish, brownish, etc., which is the result of a lack of shell growth and the encroachment of algae right up to the mantle. In fact, small to medium-sized clams of any species should have a clearly visible clean white band of new shell material all the way around the shell margin, as they typically have much higher growth rates than larger, older clams. So, if you don’t see a clean white shell margin and a clean band of new shell material on a small to medium-sized specimen, the problem is very likely insufficient lighting, and it’ll need to be moved up closer to the lights, taken out of the tank, or rescued with a new lighting system.

I didn’t mention it above, but be aware that cleaner shrimps and algae-eating hermits will not attack a healthy tridacnid. However, if they sense that one is very near death (or dead) they will oftentimes start scavenging on the remains.
References/Sources for More Information
- Alder, J. and R.D. Braley. 1988. Mass mortalities of giant clams on the Great Barrier Reef. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific. ACIAR Monograph Number 9, Canberra. 274pp.
- Asada, K. and M. Takahashi. 1987. Production and scavenging of active oxygen in photosynthesis. In: Kyle, D.J., C.B. Osmond, and C.J. Arntzen (eds.) Photoinhibition. Elsevier, Amsterdam. 307pp.
- Boglio, E. and J.S. Lucas. 1997. Impacts of ectoparasitic gastropods on growth, survival, and physiology of juvenile giant clams (Tridacna gigas), including a simulation model of mortality and reduced growth rate. Aquaculture 150: 25-43.
- Bower, S.M. 2001. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Hinge Ligament Disease of Juvenile Oysters: http://www-sci.pac.dfo-mpo.gc.ca/shelldis/pages/hldjoy_e.htm
- Bower, S.M. and S.E. McGladdery. 2001. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Table of Contents: http://www-sci.pac.dfo-mpo.gc.ca/shelldis/toc_e.htm
- Cumming, R.L. 1988. Pyramidellid parasites in giant clam mariculture systems. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific. ACIAR Monograph Number 9, Canberra. 274pp.
- Delbeek, J.C. and J. Sprung. 1994. The Reef Aquarium: Volume One. Ricordea Publishing, Coconut Grove, FL. 544pp.
- Dungan, C.F., R.A. Elston, and M.H. Schiewe. 1989. Evidence for colonization and destruction of hinge ligaments in cultured juvenile Pacific oysters (Crassostrea gigas) by cytophaga-like bacteria. Applied Environmental Microbiology 55(5):1128-1135.
- Fatherree, J.W. 2006a. Giant Clams in the Sea and the Aquarium. Liquid Medium, Tampa. 227pp.
- Fatherree, J.W. 2006b. Troubles with Tridacnids: a Look at Two Common Problems. Reefkeeping: www.reefkeeping.com/issues/2006-09/jf/index.php
- Fitt, W.K., G.A. Heslinga, and T.C. Watson. 1992. Use of antibiotics in the mariculture of giant clams (F. Tridacnidae). Aquaculture 104:1-10.
- Goggin, C.L. 1996. Effect of Perkinsus olseni (Protozoa, Apicomplexa) on the weight of Tridacna crocea (Mollusca, Bivalvia) from Lizard Island, Great Barrier Reef. Aquaculture 141:25-30.
- Goggin, C. L. and R.J.G. Lester. 1987. Occurrence of Perkinsus species (Protozoa, Apicomplexa) in bivalves from the Great Barrier Reef. Diseases of Aquatic Organisms 3:113-117.
- Goggin, C.L. and L.R.G. Cannon. 1989. Occurrence of a turbellarian from Australian tridacnid clams. International Journal for Parasitology 19:345-346.
- Govan, H. 1995. Cymatium muricinum and other ranellid gastropods: major predators of cultured tridacnid clams. ICLARM Technical Report 49, ICLARM, Manila, 136pp.
- Hine, P.M. and T. Thorne. 2000. A survey of some parasites and diseases of several species of bivalve mollusc in northern Western Australia. Diseases of Aquatic Organisms, 40.
- Humphrey, J.D. 1988. Disease risks associated with translocation of shellfish, with special reference to the giant clam Tridacna gigas. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific. ACIAR Monograph Number 9, Canberra. 274pp.
- Humphrey, J.D., N. Gudkovs, and J.S. Langdon. 1987. Report on Health Certification of Cultured Giant Clams Tridacna gigas Proposed for Export. Australian Fish Health Reference Laboratory. Geelong, Australia. 13pp.
- Knop, D. 1996. Giant Clams: A Comprehensive Guide to the Identification and Care of Tridacnid Clams. Dahne Verlag, Ettlingen, Germany. 255pp.
- Nakayama, K., M. Nishijima, and T. Maruyama. 1998. Parasitism by a protozoan in the hemolymph of the giant clam, Tridacna crocea. Journal of Invertebrate Pathology 71:193-198.
- Neigut, B. 2005. Owner of Clams Direct. personal communication.
- Newman, L.J., L.R.G. Cannon, and H. Govan. 1993. Stylochus (Imogene) matatasi n. sp. (Platyhelminthes, Polycladida): pest of cultured giant clams and pearl oysters from Solomon Islands. Hydrobiologia 257(3):185-189.
- Norton, J.H., M.A. Shepherd, M.R. Abdon-Naguit, and S. Lindsay. 1993a. Mortalities in the giant clam Hippopus hippopus associated with Rickettsiales-like organisms. Journal of Invertebrate Pathology 62:207-209.
- Norton, J.H., M.A. Shepherd, and H.C. Prior. 1993b. Intercellular bacteria associated with winter mortality in juvenile giant clams, Tridacna gigas. Journal of Invertebrate Pathology 62:204-206.
- Norton, J.H., F.P. Perkins, and E. Ledua. 1993c. Marteilia-like infection in a giant clam, Tridacna maxima, in Fiji. Journal of Invertebrate Pathology 61:328-330.
- Osmond, C.B. 1981. Photorespiration and photoinhibition; some implications for the energetics of photosynthesis. Biochemica Biophysica Acta 639:77-98.
- Paillard, C., F. Le Roux, and J.J. Borrego. 2004. Bacterial disease in marine bivalves, a review of recent studies: Trends and evolution. Aquatic Living Resources, 17.




Hi James greeting from Tonga fisheries…please I have some desease in T gigas that need to disscuss with you regard how to manage this to spread out.
some photo will send to your email when you get back to my mail.thanks
Hi James. Greeting from Fiji. We have a Tridacna squamosa nursery in subt-tidal zone which is raised above the subsrate. We clean the cage and the juvenile clams everyday. We are having issues with clams gaping. Could it be us brushing the clams that may be stressing them out and resulting in gaping?