
Iodine was the focus of the last editorial while herein we illuminate the role of strontium, boron, and aluminum which appear to persist by various means.
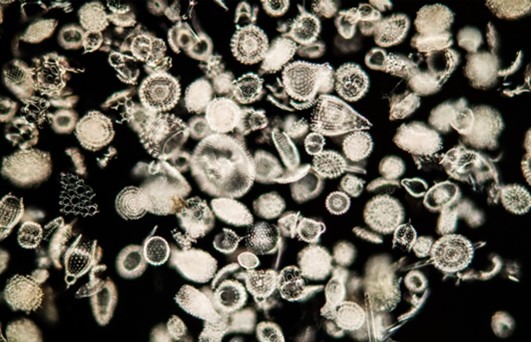
Fig 1. Zooxanthellate marine chromists of the phylum Radiozoa and class Acantharia build their shells from crystalline strontium sulphate (SrSO4).
The common nonradioactive isotopes of strontium are 84Sr, 86Sr, 87Sr, and 88Sr with a mean atomic mass of 87.62 daltons (Da). Strontium occurs in group two of the periodic table meaning it has two valence electrons in its outer orbital available to form covalent bonds with those of other atoms with opposing spin (Aldred et al. 2009). Strontium therefore loses these electrons in aqueous solution to form the divalent cation Sr2+ like the other familiar group two elements: calcium (Ca2+) and magnesium (Mg2+). Nevertheless, their alikeness means that all are prone to competition at intracellular binding sites which may exert unpredictable outcomes.
~8 mg l-1 (ppm) Sr2+ are evident in seawater, while these ions are 13 and 70 percent larger than Ca2+ and Mg2+ (Holmes-Farley 2003a). Luckily size and velocity matter when it comes to intracellular molecular binding and cascades, which tend to be inversely proportional.
~90 and ~10 percent of strontium occurs as Sr2+ and strontium sulphate (SrSO4) whilst the remainder forms traces of strontium bicarbonate (SrHCO3+); strontium borate (SrB(OH)4+); strontium carbonate (SrCO3); strontium fluoride (SrF+), and strontium hydroxide (SrOH+; Holmes-Farley 2003a). The radioactive isotope strontium 90Sr was inoculated in the wild during nuclear weapons testing and its decay and prevalence are simple to trace.
Strontium carbonate (SrCO3; strontianite), calcium carbonate (CaCO3), and magnesium carbonate (MgCO3) are the least to the most soluble where SrCO3 remains the predominant crystalline form. Blooms of strontium sulphate (SrSO4)-utilising zooxanthellate chromists of the class Acantharia deplete surface waters, while they are sufficiently large to consume all plankton and are absent from the fossil record because their frameworks descend and dissolve postmortem (Fig 1.). Acantharians inhabit the euphotic zone of tropical and subtropical waters yet their vestiges enrich strontium at depth (Holmes-Farley 2003a; Boltovskoy & Correa 2010).
Swarms of free-swimming acantharian larvae each containing a crystal of strontium sulphate are propelled by tiny microtubule (whip-like) appendages called flagella. Numerous marine microorganisms exploit flagella like the mastigotes of dinoflagellate zooxanthellae which use them to seek a host (Fig 2.; LaJeunesse et al. 2012).
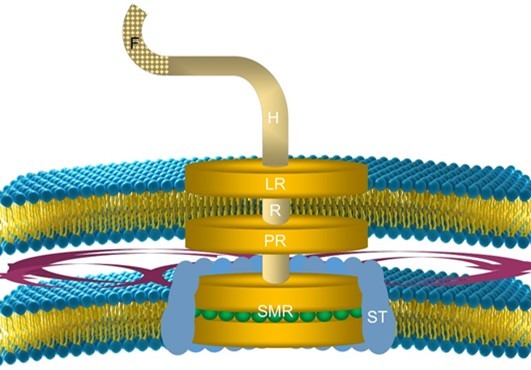
Fig 2. An illustration of the transmembrane flagellum motor of a gram negative bacterium: [F] flagellum base; [H] hook; [LR] L-ring; [PR] P-ring; [R] rod; [SMR] S-M-ring assembly, and [ST] stator.
Malfunctional statocysts and abnormal shells develop during strontium limitation (Bidwell et al. 1986; Wiederhold et al. 1989; Holmes-Farley 2003a).
Octopuses, squid, cuttlefish, and nautiluses belong to the class Cephalopoda where the statocysts of developing larval cephalopods lack statoliths throughout strontium dearth (Fig 4.; Fig 6.). Hence they cannot coordinate their swimming in order to catch their prey.
Statoliths are formed from multiple laminations deposited after pH manipulation of a secreted mineral-rich liquor called endolymph. Lighter-coloured calcite is deposited in the evening, whereas morning veneers consist of darker polysaccharides, phospho- and glyco-proteins. Granule polymorphs are influenced by numerous inorganic and organic inclusions where strontium is concentrated in the wing (Hanlon & Bidwell 1989; Bettencourt & Guerra 2000).
Aragonite cuttlefish bones comprise ~50 and ~30 percent β-chitin and protein fashioned into multifaceted chambers where strontium is required for precise mineral deposition via self-organising liquid crystallisation and pillar-forming viscous fingering (Checa et al. 2015).
The ratio of strontium to calcium (Sr:Ca) in aragonite such as the skeletons of SPS corals typically reflect those of seawater (~1:103 at 25oC; Cohen et al. 2002) with escalated ratios occurring in colder water.
Sr:Ca in ancient coral skeletons reveals prehistoric water temperatures, where corals of the genus Porites must be sampled through their major vertical axis (Marshall & McCulloch 2002). Numerous factors influence strontium inclusions (Cohen et al. 2002; Marshall & McCulloch 2002; Holmes-Farley 2003a) while present day sea temperatures influence species and reef zone-specific ratios of Sr:Ca or lithium to magnesium (Li:Mg; Fowell et al. 2016).

Fig 3. Californian sea hares (Aplysia californica; Mollusca: Gastropoda) require strontium for their larval development (Holmes-Farley 2003a).
Aragonite skeletons contain nominal Sr2+ and thus Ca2+ could be inadvertently replaced, albeit additions can exceed those of seawater, appear skeletal zone- and inter-specific, and may be influenced by circadian rhythm. Such findings suggest defined mechanisms where seawater Sr2+ and symbionts impact skeletal composition (Cohen et al. 2002; Holmes-Farley 2003a). Doctor Holmes-Farley proposed that corals may exploit mineral deposition as a toxic metal purgative (Holmes-Farley 2003a).

Fig 4. The European cuttlefish (Sepia officinalis; Mollusca: Cephalopoda).
SPS Coral Requirements
Growth was elevated and Sr2+ accretion increased when seawater was enriched 37.5-fold (Delbeek & Sprung 1994; Ferrier-Pagès et al. 2003). Inclusions of Sr2+ tend to cease when calcification pathways are blocked, where elevated Ca2+ reduce Sr2+ uptake which suggests their substitutions are incidental (Ferrier-Pagès et al. 2003). Ca2+ and Sr2+ may compete beneath the calicoblastic ectoderm, where aragonite polymorphs are not disrupted by their exchange (Fig 5.; Ruiz-Hernandez et al. 2010). Elevated Mg2+ impedes skeletal deposition which is accelerated during Ca2+/Sr2+ co-enrichment (Swart 1981).
Sr2+ may neutralise the mucin glycoproteins of coral mucus insofar as they malfunction when charged which may forestall Ca2+ uptake, albeit mucosal enrichment typically exceeds ambient. Mucin glycoproteins are short-chain sulphated sugars, where SO42- repel and prohibit granule formation. Sr2+/SO42- ionic bonds form neutral and comparatively insoluble intramucosal SrSO4 (Clode & Marshall 2002; Holmes-Farley 2003a).
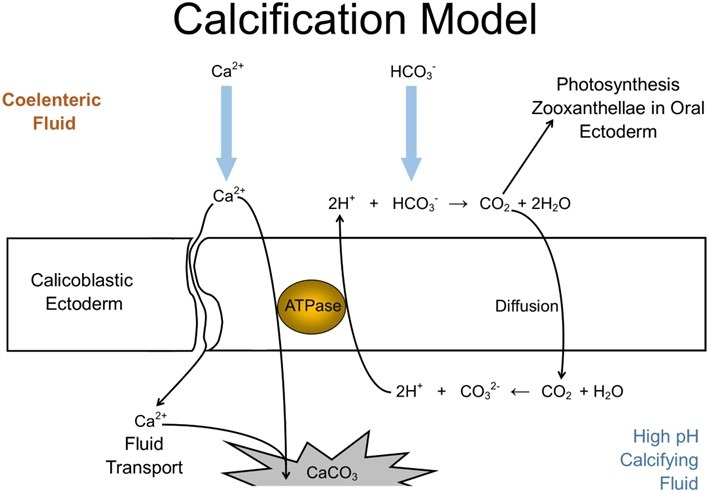
Fig 5. The proposed scleractinian skeletal accretion model occurring beneath the calicoblastic epithelium. CO2 diffusion and proton (H+) and calcium ion (Ca2+) active symport elevate the alkalinity of the calcifying fluid which shifts the equilibrium towards the deposition of carbonate (CO32-), while coelenteron protons react with bicarbonate (HCO3-) to form water and CO2 for photosynthesis. Adapted from Sinclair and Risk 2006, Jokiel et al. 2016, and Nakamura et al. 2017.
Notwithstanding significant ingress originating from feeds, water changes, and fortifications of calcium/magnesium/carbonate/bicarbonate, co-supplementation with Mg2+ may prove advantageous (Holmes-Farley 2003a; Holmes-Farley 2003b).

Fig 6. A nautilus of the phylum Mollusca and class Cephalopoda.
Comparative assays performed on established reef aquaria are of little value because limitless variables confound objective investigations. Sr2+ ranged from 4 to 10 mg l-1 (ppm) in the 23 aquaria investigated by Doctor Shimek in 2002, while Doctor Holmes-Farley’s system contained 15 mg l-1 of Sr2+ and he had not supplemented strontium for years (Holmes-Farley 2003a).
Sr2+ might be sustained by coral skeleton and SrSO4 dissolution within deep euxinic substrate, which acts as a PO43- “sponge” that proton abundance likely amplifies with increasing depth (Brooks et al. 1968; Borneman 2008).
Sr2+, Ca2+, and Mg2+ are found in the oxic and suboxic REDOX seals of plenums and DSBs yet they appear scarce at greater depth. Ample protons within sulphide-rich anaerobic zones likely liberate aragonite-bound PO43-, Ca2+, HCO3– and CO32- (Brooks et al. 1968). Whether bedbound erosion of strontianite/aragonite evolves Sr2+ remains enigmatic, yet it likely contributes to ionic budgets (Baker & Bloomer 1988).
Median lethal concentration 50 (LC50) analyses ascertain the strength of toxins that instigate mortalities in 50 percent of populations, which are typically carried out in replicate aquaria.
Sr2+ are more toxic to the inhabitants of freshwater (McPherson et al. 2014), while they poison adult and larval European shore crabs (Carcinus maenas) at a LC50 of 38 mg l-1 (ppm) after 216 hours, which is merely 30 ppm greater than natural seawater (Fig 7.; Amiard 1976; Holmes-Farley 2003a). Exercise caution when supplementing Sr2+ using weak solutions of strontium chloride (SrCl); however, less than 10 percent water changes are less hazardous and more beneficial.
Sr2+ and PO43- are elevated in some marine salts and the SPS enthusiast must have complete control of dissolved inorganic ions. Ideally Sr2+ should not exceed 8 mg l-1, whereas reclaimed salts may comprise wild microcontaminants and heat-tolerant cysts.

Fig 7. The green or European shore crab (Carcinus maenas).
Doctor Holmes-Farley’s (2003a) estimates of annual mean aquarium influxes ranged from 0.8 to 9 mg l-1 (ppm) Sr2+ where the latter quasi-equates to natural seawater, and none of the aquaria analysed by Doctor Shimek were remarkably depleted (Shimek 2002; Holmes-Farley 2003a). Annual feed-derived estimates ranged from 0.01 to 0.7 mg l-1 (Shimek, cited in Holmes-Farley 2003a) so strontium persists in marine systems by hitherto unexplained means; therefore, all findings appear to render Sr2+ supplementation moot.
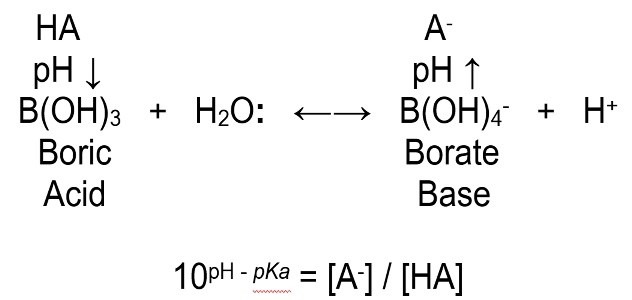
where square brackets represent concentration
pKa seawater = 8.6
[A-] / [HA] 2/3 at pH 8.4
(Brockington et al. 1981; Holmes-Farley 2002; Kochkodan et al. 2015)
Non-metallic boron (B3+) confers ~23 percent of seawater buffering when it complexes like other cations with ligands such as three hydroxyls (OH–). It is a group 13 element of the periodic table that has merely one electron () in one of its three 2p subshells with a lone pair in 2s, yet when ungrounded, this pair is severed and one is promoted to a vacant 2p to create its three unpaired valence electrons 2s 2p 2p, 2p0. Consequently, orthoboric acid (H3BO3) behaves like a Lewis acid and accepts a lone pair of electrons into its degenerate 2p from a Lewis base donor like water (H2O:), to form a coordinate covalent bond where it behaves like a strong monoprotonic acid like Non-metallic boron (B3+) confers ~23 percent of seawater buffering when it complexes like other cations with ligands such as three hydroxyls (OH–). It is a group 13 element of the periodic table that has merely one electron () in one of its three 2p subshells with a lone pair in 2s, yet when ungrounded, this pair is severed and one is promoted to a vacant 2p to create its three unpaired valence electrons 2s 2p 2p, 2p0. Consequently, orthoboric acid (H3BO3) behaves like a Lewis acid and accepts a lone pair of electrons into its degenerate 2p from a Lewis base donor like water (H2O:), to form a coordinate covalent bond where it behaves like a strong monoprotonic acid like Non-metallic boron (B3+) confers ~23 percent of seawater buffering when it complexes like other cations with ligands such as three hydroxyls (OH–). It is a group 13 element of the periodic table that has merely one electron () in one of its three 2p subshells with a lone pair in 2s, yet when ungrounded, this pair is severed and one is promoted to a vacant 2p to create its three unpaired valence electrons 2s 2p 2p, 2p0. Consequently, orthoboric acid (H3BO3) behaves like a Lewis acid and accepts a lone pair of electrons into its degenerate 2p from a Lewis base donor like water (H2O:), to form a coordinate covalent bond where it behaves like a strong monoprotonic acid like hydrochloric (HCl; Brockington et al. 1981).
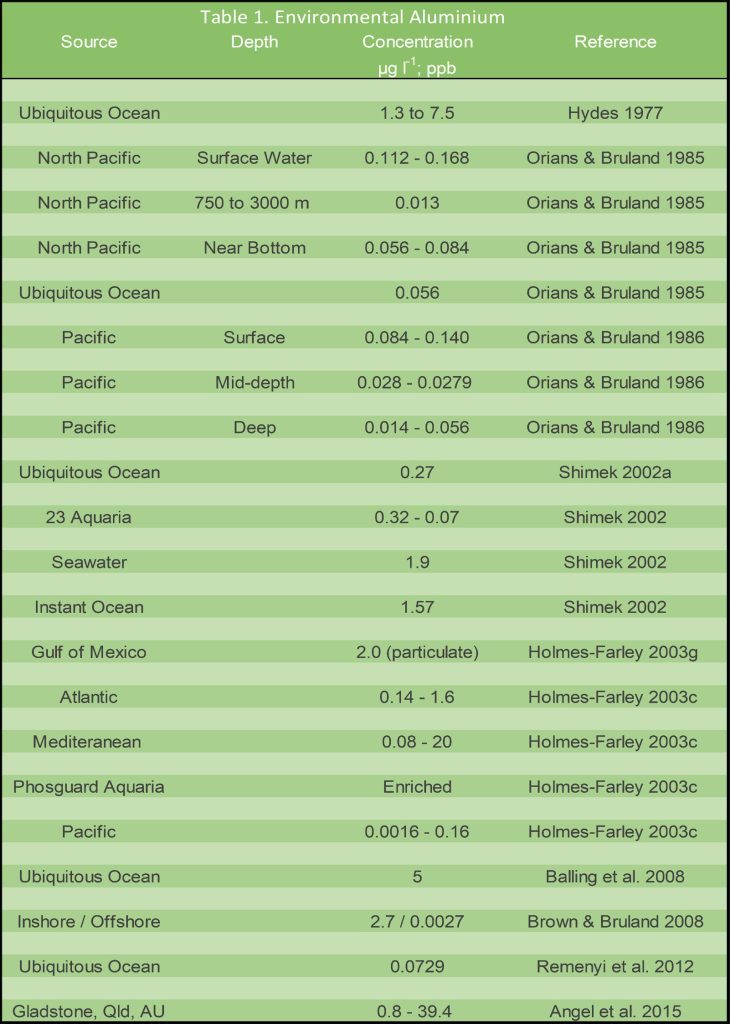
Table 1. Denoised evidence evaluating total dissolved inorganic aluminium (DIA) in natural seawater, reconstituted salt formulations, and aquaria expressed in µg l-1 (ppb).
Consult: Part VI. Boron prevails at ~4.4 mg l-1 (ppm) in seawater which is utilised by micro- and macro-algae including diatoms, whereas coral skeletons comprise 50 to 100 mg kg-1. Calcium reactor effluent typically fortifies Boron, and synthetic marine salts use it to enhance buffering by raising its occurrence above that found in the wild. Test everything. Salifert® Profi test kits are available, but the wealth of organic and inorganic kinds means their results will be unrepresentative of the species gamut (Noakes & Hood 1953). All findings suggest that boron persists in recirculating systems while it is harmful when enriched (Holmes-Farley 2002). Water changes more than adequately sustain its presence at near-natural ionic strengths.
The metal aluminium is another group 13 trivalent element with a 3s2 and 3p1 configuration and like most cations, it complexes with several types of contrasting ligands including water. The electrons of each water molecule are asymmetrically distributed (2HO) where Al3+ becomes octahedrally coordinated by six such ligands to form the complex [Al(H2O)6]3+ which adopts the net charge of its centralised cation. Complexed aluminium attracts each oxygen’s electrons which reinforces oxygen’s electronic draw (2HO®Al3+; Brockington et al. 1981).
Aluminium is exceedingly toxic that putatively instigates mass fish kills in Scandinavian lakes and rivers (Timbrell 2002). African dust most likely deposits the majority of Al3+, where numerous oceanic forms include the inorganic complexes aluminium hydroxide (Al(OH)3) and tetrahydroxy aluminate (Al(OH)4) together with alumina-silica, and organoaluminium complexes prone to protein skimmer export (Holmes-Farley 2003c). The abundances of dissolved inorganic and organic aluminium (DIA; DOA) vary greatly because surface and near-bottom waters may be enriched although diminished at hundreds to thousands of metres (Orians & Bruland 1985; Orians & Bruland 1986). Concentrations are 40 times scarcer in the Pacific compared with the Atlantic, while heavily industrialised and contaminated inshore ecosystems contrast with the purer pelagic zone (Orians & Bruland 1985; Holmes-Farley 2003c; Angel et al. 2015).
Archaic methodologies, miscalculations, and artefacts generated atypical findings because several waters would be devoid of life if the data was believed. Table 1. thus conveys a more reliable representation of total dissolved inorganic aluminium (DIA).
Al3+ are exceedingly toxic to fish and somewhat to invertebrates where inordinate ingress can trigger asphyxiation, while intra-organismal lessens environmental DIA due to contest at Ca2+ and Mg2+ molecular binding sites (Ganrot 1986; Yokel & Golub 1997; Santore et al. 2018). The paracellular routes of marine finfish gill epithelia are less constricted than those of freshwater teleosts which are no barrier to charged metallic cations. Both ionic and particulate forms of aluminium remain harmful (Golding et al. 2015) where ingress is determined by dissolved inorganic/organic speciation, alkalinity, and pH (Santore et al. 2018).
Fish, invertebrate, and algal intoxications are reliably predicted by the biotic ligand model (BLM) framework (Santore et al. 2018). 50 percent of red threadworms (Ctenodrilus species) and the copepod Nitokra spinipes subspecies spinipes expired when exposed to 96 hours of 0.48 and 10 mg l-1 (ppm) of aluminium chloride (AlCl3; Petrich & Reish 1979) while Al3+ begins to limit diatomaceous growth at 18 ppb (µg l-1), and it perturbs mussel and oyster embryonic development at 0.25 to 0.41 mg l-1 (ppm; Brooks & White 1995). Furthermore, 14.453 µg l-1 (ppb) of AlCl3 is lethal to 50 percent of Artemia parthenogenetica after 96 hours, and thus some natural environments are virtually hostile to life according to the conservative data from table 1.
Synthetic salt formulations and feeds contribute markedly to systemic DIA. One study proposed brine shrimp contained as much as 120 ppm (mg l-1; Shimek 2001, cited in Holmes-Farley 2003c) which if performed on thawed diets, likely reflects the polluted water within which they were frozen because living flesh cannot bear >8,300 times more than the above LC50. Part IV recommended that pouches of Gamma® frozen brine and mysis shrimp be rapidly defrosted, washed in copious amounts of cold R/O water, and promptly refrozen. See: Part IV for complete clarification.
50 to 75 grams of pre-cleansed brine or mysis shrimp was administered in a Reef Ranch fish-only system each day for over half a decade. All animals thrived where our losses were fewer than one fish every three months. The only purification was a weekly 10 percent water change, seawater replenishment for packaged livestock, and 50 mg of ozone (O3) each quarter. However, >1 mg l-1 (ppm) of PO43- supported an algal bloom, where phosphate readily complexes Al3+, albeit a diverse assortment of marine phytoplankton experience mortality from 16 µg l-1 to 6.8 mg l-1 DIA (Sparling & Lowe 1996; Golding et al. 2015) which is anecdotal yet compelling evidence.
Several detoxification strategies have evolved where the gut epithelial enterocytes of freshwater and terrestrial snails precipitate and excrete granules of Al, S, P, and Si (Brooks & White 1995; Elangovan et al. 2000). Silicon may thus be implicated in toxic metal excretion (Holmes-Farley 2003c).
Calcium hydroxide may contain significant aluminium despite kalkwasser sequestering toxic metals as insoluble hydroxides (Holmes-Farley 2003b; Holmes-Farley 2003c) yet profound aluminium-derived poisonings of humans may force revealing analyses of pulverised food grade Ca(OH)2 (Exley 2016).
Aluminium-based Seachem® PhosGuard is a phosphate absorption medium where Doctor Holmes-Farley’s experiments concluded it raised ionic and particulate aluminium to potentially toxic concentrations (Homes-Farley 2003c). Leather corals closed and mushrooms shrank, and pH diminished by 0.1 following three sequential administrations of 0.5 ppm (mg l-1) of AlCl3 (Holmes-Farley 2003c) where minor shifts in pH reflect a profound change in proton availability (Aslett 2024).
Next we learn how to optimally and successfully fabricate branching and plating SPS propagules (ramets; “frags”).
For more information, visit THIS website.
References
Aldred, E., M., Buck, C. & Vall, K. (2009) Free radicals. Aldred, E., M., Buck, C. & Vall, K. (eds.). Pharmacology, A Handbook for Complementary Healthcare Professionals. Churchill Livingstone. pp 41-52.
Amiard, J., C. (1976) Experimental study of acute toxicity of cobalt, antimony, strontium and silver salts in some crustaceans and their larvae and in some teleosteans. Revue Internationale d’Oceanographie Medicale. pp 79-95.
Angel, B., Apte, S., Batley, G. & Golding, L. (2015) Geochemical controls on aluminium concentrations in coastal waters. Environmental Chemistry. 13(1), 111-118.
Aslett, C., G. (2024) The Complete Reef Aquarist, for hobbyists, the trade and academics – A Conservation Manual. Aslett, C., G. (ed.). Reef Ranch Publishing Ltd, Leeds, West Yorkshire, UK available worldwide.
Baker, P., A. & Bloomer, S., H. (1988) The origin of celestite in deep-sea carbonate sediments. Geochimica et Cosmochimica Acta. 52(2), 335-339.
Balling, H., W., Janse, M. & Sondervan, P. (2008) Trace elements, functions, sinks and replenishment in reef aquaria. Advances in coral husbandry in public aquariums. Leewis, R., J. & Janse, M. (eds.). Burgers’ Zoo, Arnhem, The Netherlands. pp 143-156.
Bettencourt, V. & Guerra, A. (2000) Growth increments and biomineralization process in cephalopod statoliths. Journal of Experimental Marine Biology and Ecology. 248(2), 191-205.
Bidwell, J., Paige, J. & Kuzirian, A. (1986) Effects of Strontium on the Embryonic Development of Aplysia californica. Biological Bulletin. 170(1), 75-90.
Boltovskoy, D. & Correa, N., M. (2010) Acantharia. The Tree of Life Web Project. TolWeb.org. http://tolweb.org/Acantharia/2385
Borneman, E. (2008) Applications of Sand in Reef Aquariums: Theory and Practice. ReefKeeping.com. http://reefkeeping.com/issues/2007-02/eb/index.php
Brockington, J., Stamper, P., J., Browning, D., R. & Skinner, A., C. (1981) Combined Chemistry. Brockington, J., Stamper, P., J., Browning, D., R. & Skinner, A., C. (eds.). Longman Group Limited, Essex, UK. p 115; p 302 pp 364-369; p 621.
Brooks, A., W. & White, K., N. (1995) The localization of aluminium in the digestive gland of the terrestrial snail Helix aspersa. Tissue & cell. 27(1), 61-72.
Brooks, R., K., Presley, B., J. & Kaplan, I., R. (1968) Trace elements in the interstitial waters of marine sediments. Geochimica et Cosmochimica Acta. 32(4), 397-414.
Brown, M. & Bruland, K. (2008) An improved flow‐injection analysis method for the determination of dissolved aluminum in seawater. Limnology and Oceanography: Methods. 6(1), 87-95.
Checa, A., G., Cartwright, J., H., Sánchez-Almazo, I., Andrade, J., P. & Ruiz-Raya, F. (2015) The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Scientific reports. 5, 11513.
Clode, P., L. & Marshall, A., T. (2002) Low temperature X-ray microanalysis of calcium in a scleractinian coral: evidence of active transport mechanisms. Journal of Experimental Biology. 205(22), 3543-3552.
Cohen, A., L., Owens, K., E., Layne, G., D. & Shimizu, N. (2002) The Effect of Algal Symbionts on the Accuracy of Sr/Ca Paleotemperatures from Coral. Science. 296(5566), 331-333.
Delbeek, C. & Sprung, J. (1994) The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates (Volume 1). Two Little Fishies. p 115.
Elangovan, R., McCrohan, C., R., Ballance, S., Powell, J., J. & White, K., N. (2000) Localization and fate of aluminium in the digestive gland of the freshwater snail Lymnaea stagnalis. Tissue and Cell. 32(1), 79-87.
Exley, C. (2016) The toxicity of aluminium in humans. Morphologie: bulletin de l’Association des anatomistes. 100(329), 51-55.
Ferrier-Pagès, C., Boisson, F., Allemand, D. & Tambutté, E. (2003) Kinetics of strontium uptake in the scleractinian coral Stylophora pistillata. Marine Ecology Progress Series. 1, 14374.
Fowell, S., E., Sandford, K., Stewart, J., A., Castillo, K., D., Ries, J., B. & Foster, G., L. (2016) Intrareef variations in Li/Mg and Sr/Ca sea surface temperature proxies in the Caribbean reef‐building coral Siderastrea siderea. Paleoceanography. 31, 1315-1329.
Ganrot, P. (1986) Metabolism and Possible Health Effects of Aluminum. Environmental Health Perspectives. 65, 363-441.
Golding, L., Angel, B., Batley, G., Apte, S., Krassoi, R. & Doyle, C. (2015) Derivation of a water quality guideline for aluminium in marine waters. Environmental Toxicology & Chemistry. 34(1), 141-151.
Hanlon, R. & Bidwell, J. (1989) Strontium is required for statolith development and thus normal swimming behaviour of hatchling cephalopods. The Journal of experimental biology. 141,187-95.
Holmes-Farley, R. (2002) Chemistry and The Aquarium: Boron in A Reef Tank. AdvancedAquarist.com. https://www.advancedaquarist.com/2002/12/chemistry
Holmes-Farley, R. (2003a) Aquarium Chemistry: Strontium and the Reef Aquarium. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/11/chemistry
Holmes-Farley, R. (2003b) Aquarium Chemistry: Magnesium and Strontium in Limewater. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/12/chemistry
Holmes-Farley, R. (2003c) Chemistry and The Aquarium: Aluminium in The Reef Aquarium. AdvancedAquarist.com. https://www.advancedaquarist.com/2003/7/chemistry
Holmes-Farley, R. (2003d) Aquaria with low soluble metals. ReefKeeping.com. Volume 4. http://reefkeeping.com/issues/2003-04/rhf/feature/index.htm
Hydes, D., J. (1977) Dissolved aluminium concentration in sea water. Nature. 268(5616),136-137.
Jokiel, P., Jury, C. & Kuffner, I. (2016) Coral Calcification and Ocean Acidification. Coral Reefs of the World Series Volume 6. Riegl, B. & Dodge, R., E. (eds.). Springer. pp 7-45.
Kochkodan, V., Darwish, N., B., Hilal, N., Kabay, N., Bryjak, M. & Hilal, N. (2015) Chapter 2 – The Chemistry of Boron in Water. Boron Separation Processes. Elsevier, Amsterdam. pp 35-63.
LaJeunesse, T., Parkinson, J., E. & Trench, R., K. (2012) Symbiodinium. http://tolweb.org/Symbiodinium/126705
Marshall, J. & McCulloch, M. (2002) An assessment of the Sr/Ca ratio in shallow water hermatypic corals as a proxy for sea surface temperature. Geochimica et Cosmochimica Acta. 66(18), 3263-3280.
Nakamura, T., Nadaoka, K., Watanabe, A., Yamamoto, T., Miyajima, T. & Blanco, A. (2017) Reef-scale modeling of coral calcification responses to ocean acidification and sea-level rise. Coral Reefs. 37(1), 37-53.
Noakes, J., E. & Hood, D., W. (1953) Boron-boric acid complexes in sea-water. Deep Sea Research. 8(2), 121-129.
Orians, K. & Bruland, K. (1985) Dissolved aluminium in the central North Pacific. Nature. 316, 427-429.
Orians, K., J. & Bruland, K., W. (1986) The biogeochemistry of aluminum in the Pacific Ocean. Earth and Planetary Science Letters. 78, 397-410.
Petrich, S., M. & Reish, D., J. (1979) Effects of aluminium and nickel on survival and reproduction in polychaetous annelids. Bull. Environ. Contam. Toxicol. 23, 698.
Remenyi, T., Nesterenko, P., Bowie, A., Butler, E. & Haddad, P. (2012) Reversed phase high performance liquid chromatographic determination of dissolved aluminium in open ocean seawater. Limnology and Oceanography: Methods. 10(11), 832-839.
Ruiz-Hernandez, S., Grau-Crespo, R., Ruiz-Salvador, A. & De Leeuw, N. (2010) Thermochemistry of strontium incorporation in aragonite from atomistic simulations. Geochimica et Cosmochimica Acta. 74(4), 1320-1328.
Santore, R., Ryan, A., Kroglund, F., Rodriguez, P., Stubblefield, W., Cardwell, A., Adams, W. & Nordheim, E. (2018) Development and application of a biotic ligand model for predicting the chronic toxicity of dissolved and precipitated aluminum to aquatic organisms. Environmental Toxicology & Chemistry. 37(1), 70-79.
Shimek, R., L. (2001) Necessary Nutrition, Foods and Supplements, A Preliminary Investigation. Aquarium Fish Magazine. 13, 42-53.
Shimek, R., L. (2002) It’s (In) The Water. Reefkeeping.com http://reefkeeping.com/issues/2002-02/rs/feature/index.htm
Shimek, R., L. (2002a) Down the Drain: Exports from Reef Aquaria. Reefkeeping.com http://reefkeeping.com/issues/2002-12/rs/feature/index.htm
Sinclair, D. & Risk, M. (2006) A numerical model of trace-element coprecipitation in a physicochemical calcification system: Application to coral biomineralization and trace-element ‘vital effects’. Geochimica et Cosmochimica Acta. 70(15), 3855-3868.
Sparling, D., W. & Lowe, T., P. (1996) Environmental hazards of aluminum to plants, invertebrates, fish, and wildlife. Rev Environ Contam Toxicol. 145(1), 127.
Swart, P., K. (1981) The strontium, magnesium and sodium composition of recent scleractinian coral skeletons as standards for palaeoenvironmental analysis. Palaeogeography, Palaeoclimatology, Palaeoecology. 34, 115-136.
Timbrell, J., (2002) Introduction to Toxicology, Third Edition. Timbrell, J. (ed.). Taylor & Francis, New York, US. p 127.
Wiederhold, M., L., Sheridan, C., E. & Smith, N., K., R. (1989) Function of Molluscan Statocysts. Origin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals. Crick, R., E. (ed.). Springer, Boston, MA. pp 393-408.
WoRMS (2022) Gastropoda. MarinesSpecies.org. https://www.marinespecies.org/aphia.php?p=taxdetails&id=101
Yokel, R., A. & Golub, M., S. (1997) Ecotoxicology of aluminum to fish and wildlife. Research Issues in Aluminum Toxicity. Yokel, R., A., Mari, S. & Golub, M., S. (eds.). Taylor & Francis, Washington, DC. pp 47-68




0 Comments