Phosphorus is one of the basic building blocks of living matter. It is present in every living creature, and in the water of every reef tank. Unfortunately, it is present in excess in many reef tanks, and that excess has the potential to cause two big problems for reef keepers. The first is that is can drive excessive growth of undesirable algae. The second is that it can directly inhibit calcification by corals and coralline algae. Since most reef keepers don’t want either of these things to happen, they strive to keep phosphorus levels under control.
Fortunately, there are some effective ways of keeping phosphorus concentrations to acceptable levels. Unfortunately, the means for testing for total phosphorus are not trivial. One can readily test for one of the common forms of phosphorus in reef tanks, inorganic orthophosphate, but testing for organic phosphorus compounds is considerably more tedious. Moreover, if there is an algae “problem”, then the algae may be consuming the phosphate as fast as it enters the water, masking the issue. Consequently, reef keepers may not recognize that they have a phosphorus problem, only that they have an algae problem.
This article describes some of the issues around phosphorus in reef tanks, including the forms that it takes, its origins, ways to test for it, and most importantly, ways to export it.
Phosphate in Seawater
The “simplest” form of phosphorus in reef tanks is inorganic orthophosphate (sometimes called Pi by biologists). It is also present in natural seawater, although other forms do exist there as well. Its concentration in seawater varies greatly from place to place, and also with depth and with the time of day. Surface waters are greatly depleted in phosphate, relative to deeper waters, due to biological activities that serve to sequester phosphate in organisms. Typical phosphate ocean surface concentrations are very low by reef keeping standards, sometimes as low as 0.005 ppm.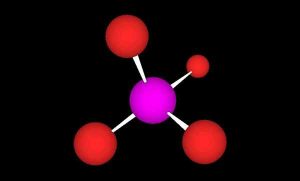
The structure of orthophosphate, with a central phosphorus atom (purple) and four oxygen atoms (red) arranged in a tetrahedron.
At concentrations below about 0.03 ppm, the growth rate of many species of phytoplankton is dependent on the phosphate concentration (assuming that something else is not limiting growth, such as nitrogen or iron). Above this level, the growth rate is independent of phosphate concentration for many organisms. So if you want to deter algae growth by controlling phosphate, you need to keep phosphate levels quite low.
In order to best understand how to maintain appropriate phosphate levels, we must first understand our quarry. Orthophosphate consists of a central phosphorus atom surrounded by four oxygen atoms in a tetrahedron (Figure 1). Orthophosphate exists in various forms in seawater, depending on the pH. At pH 8.1, seawater contains 0.5% H2PO4–, 79 % HPO42-, and 20% PO43-. At higher pH the equilibrium shifts toward more PO43- and less HPO42-.
The shift in distribution with pH may seem esoteric, but it actually has important implications for such things as the binding of phosphate to rock and sand. It may also surprise some people that so much of the phosphate is present as PO43- while in fresh water only 0.1% is present in that form at the same pH. There are a number of reasons for this difference between salt water and fresh water that involve the effects of other ions in the seawater on the phosphate (such as calcium and magnesium ion-pairs), and these have been described previously.
Other Forms of Inorganic Phosphate
Phosphorus can also take other inorganic forms, such as the polyphosphates which are rings and chains of phosphate ions strung together by P-O-P bonds. While these are not significant in natural seawater, they can be present in things that get added to our tanks. There are many of these compounds, but most will likely break down into orthophosphate when added to a reef tank.
Polyphosphates are used industrially to bind metals, such as in some laundry detergents. In that application, they form soluble complexes with calcium and magnesium, softening the water and permitting better cleaning action. The amount of phosphate getting into natural waterways from laundry detergents, however, is high enough that algae blooms sometimes result, and the practice is now illegal in many places.
Organic Phosphates
Unfortunately for reef keepers, the world of organic phosphorus compounds is far more complex than inorganic phosphates. Many common biochemicals contain phosphate esters. Every living cell contains some. Molecules such as DNA, ATP, phospholipids (lecithin), and many proteins contain phosphate groups. In these molecules, the basic phosphate structure is covalently attached to the remainder of the organic molecule through one or more phosphate ester bonds to a carbon atom.
These bonds are stable for some period of time in water, but will eventually break down to release inorganic orthophosphate from the organic part of the molecule, a process that can be sped up through the action of enzymes in a reef tank. Many of these organic phosphate compounds will be readily removed from a tank by skimming. Export of organic phosphates is the major way that skimming can result in reduced inorganic orthophosphate levels in a tank. Orthophosphate ions themselves are not significantly removed via skimmate (since they do not adsorb onto an air/water interface), but organic phosphates can be removed before they are converted into inorganic orthophosphate.
An important point about organic phosphates is that they will mostly not be impacted by phosphate-binding materials sold to the aquarium hobby. Consequently, while these products may do a fine job of reducing inorganic orthophosphate, they may not help an algae problem that is caused primarily by organic phosphates.
A final point is that organic phosphates will not be detected by most test kits. Those that do detect organic phosphates (e.g., Hach PO-24) break the phosphate off of the organic compound and thereby convert it into inorganic orthophosphate prior to testing. However, these kits are tedious and expensive, and not for every hobbyist.
Phosphate Sources in Reef Tanks
Organic phosphorus compounds, as well as orthophosphate, are so prevalent that any natural food will contain significant concentrations of phosphorus. Flake fish food is typically about 1% phosphorus (3% phosphate equivalent) by weight. Consequently, if 5 grams of flake food is added to a 100 gallon tank, there is the potential for the inorganic orthophosphate level to be raised by 0.4 ppm in that SINGLE FEEDING. That fact can be a significant issue for reef keepers: what to do with all of that phosphorus?
If the food is completely converted into tissue mass then there will be no excess phosphate. But much of the food that any organism consumes goes to provide energy, leaving a residue of CO2, phosphate, and a variety of nitrogen-containing compounds. A fish, whether it is an adult or a growing juvenile will consequently excrete much of the phosphorus that it takes in with food as phosphate in its waste. Of course, overfeeding will result in more delivery of phosphate than will lower feeding levels.
Additionally, many types of seafood available at the grocery store have various inorganic phosphate salts intentionally added to them as preservatives. These foods include canned and frozen seafood, as evidenced by the label, and even some fresh seafood. In these cases, rinsing the food before using it may help reduce the phosphate load added to the tank.
Finally, tap water can be a significant source of phosphate. The tap water supplied by the Massachusetts Water Resources Authority to me is acceptably low in phosphate, or at least it was the last time that I measured it a few years ago (I use RO/DI due to excessive silica in it). In other water supplies, however, phosphate levels can be too high. I’d recommend anyone with an algae problem who uses tap water to test to see if phosphate in the water is a possible issue.
On the other side of the issue are those tanks without fish. Since phosphorus is required for growing tissue, it is mandatory that there be some phosphorus source for corals growing in a reef tank. Finding a source is trivial if there are fish in the tank that require feeding, but in tanks without fish, reef keepers must somehow add phosphorus. The answer to this question is rather easy: either add fish food even though there are no fish, or add a source of phosphorus such as a plant fertilizer (and don’t forget about a source of nitrogen as well).
How to Export Phosphate
So now that we know where phosphate comes from, and how much, we can proceed to ask where it goes and how to maximize those export processes. Certainly, some phosphorus goes into the bodies of growing organisms, including bacteria, algae, corals, and fish. Some of these organisms stay permanently in the tank, and others may be removed by harvesting of algae, skimming of small organisms, and even pruning of corals.
A less frequently discussed mechanism for phosphate reduction may simply be the precipitation of calcium phosphate, Ca3(PO4)2. The water in many reef tanks will be supersaturated with respect to this material, as the equilibrium saturation concentration in normal seawater is only 0.002 ppm phosphate. As with CaCO3, the precipitation of Ca3(PO4)2in seawater may be limited by kinetic factors more than equilibrium factors, so it is impossible to say how much might precipitate under reef tank conditions (without, of course, somehow determining it experimentally). This precipitation may be especially likely where calcium and high pH additives (like limewater) enter the tank water. The locally high pH converts much of the HPO42- to PO43-. Combined with the locally high calcium, the locally high PO43- may push the supersaturation of Ca3(PO4)2 to unstable levels, causing precipitation.
Likewise, phosphate can precipitate onto the surface of calcium carbonate, such as onto live rock and sand. The absorption of phosphate from seawater onto aragonite is pH dependent, with the maximum binding taking place around pH 8.4 and with less binding at lower and higher pH values. If the calcium carbonate crystal is static (not growing), then this process is reversible, and the aragonite can act as a reservoir for phosphate. This reservoir can make it difficult to completely remove excess phosphate from a tank that has experienced very high phosphate levels, and may permit algae to continue to thrive despite cutting off all external phosphate sources. In such cases, removal of the substrate may even be required.
The relationship of calcium carbonate to the phosphate cycle has been studied by Frank Millero in the Florida Bay ecosystem (click here for Millero’s studies). If aragonite crystals are growing, as they often are in some parts of our systems, then I’d expect some of this phosphate to get buried and locked into the aragonite crystals.
A side effect of the adsorption of phosphate onto aragonite may well be the reported impact of phosphate on the calcification of corals. The presence of phosphate may inhibit the formation of calcium carbonate crystals via surface adsorption, and this effect may very well be the factor that inhibits calcification of corals at high phosphate levels.
Many reef keepers accept the concept that limewater addition reduces phosphate levels. This may be true, but the mechanism remains to be demonstrated. Craig Bingman has done a variety of experiments related to this hypothesis, and published them in Aquarium Frontiers. While many may not care what the mechanism is, knowing it would help to understand the limits to this method, and how it might best be employed.
Habib Sekha (the owner of Salifert) has pointed out that limewater additions may lead to substantial precipitation of calcium carbonate in reef tanks. This idea makes perfect sense. After all, it is certainly not the case that large numbers of reef tanks will exactly balance calcification needs by replacing all evaporated water with saturated limewater. And yet, many find that calcium and alkalinity levels are stable over long periods with just that scenario. The only way that can be true is if such additions typically dump excess calcium and alkalinity into the tank that is subsequently removed by precipitation of calcium carbonate (such as on heaters).
It is this ongoing precipitation of calcium carbonate, then, that may reduce the phosphate levels: phosphate binds to these growing surfaces, and becomes part of the solid precipitate. If true, this mechanism may be attained with other high pH additive systems (like some of the two-part additives such as the original B-ionic) if enough is added. However, it will not be as readily attained with low pH systems, such as calcium carbonate/carbon dioxide reactors because the low pH inhibits the precipitation of excess calcium and alkalinity.
Uptake of Phosphate by Organisms
How organisms obtain phosphate is, in nearly all cases, poorly understood. Even the absorption mechanisms used by humans are still the subject of intense research (one of my research areas involves drugs to modify this absorption, such as Renagel). It’s not surprising then that phosphate absorption mechanisms in coral reef creatures would also be poorly understood.
One frequently hears that limiting phosphate will limit algae growth in reef tanks. That is almost certainly true, but some species of microalgae thrive more readily under phosphate limitation than others (click here for phosphate limitation studies). Some species of microalgae can, in fact, significantly regulate their inorganic phosphate transport capabilities to deal with variable phosphate levels (click here for Upregulation of Phosphate Transport).
Finally, one must also consider organic phosphates. Many organisms can enzymatically break down organic phosphates prior to absorption. Consequently, we are left not having a very good understanding of what organisms in our tanks use what forms and concentrations of phosphorus. Further complicating matters, our tanks are usually greatly skewed from natural seawater in terms of other nutrients (e.g., nitrogen and iron), so one cannot readily extrapolate from phosphate studies in seawater to our tanks.
Nevertheless, growing and harvesting macroalgae (Figure 2) remains one of the best ways to reduce phosphate levels in reef tanks (along with other nutrients). Tanks with large amounts of thriving macroalgae rarely have microalgae problems or excessive phosphate levels that might inhibit calcification of corals. Whether the reduction in phosphate is the cause of the microalgae reduction is not obvious; other nutrients can also become limiting. But in a certain sense it makes no difference. If rapidly growing macroalgae absorb enough phosphorus to keep the orthophosphate concentrations in the water column acceptably low, and at the same time keep microalgae under control, most reef keepers will be satisfied.
For those interested in knowing how much phosphorus is being exported by macroalgae, this free pdf article in the journal Marine Biology has some important information. It gives the phosphorus and nitrogen content for 9 different species of macroalgae, including many that reefkeepers maintain. For example, Caulerpa racemosa collected off Hawaii contains about 0.08 % by dry weight phosphorus and 5.6% nitrogen. If one were to harvest 10 grams (dry weight) of this macroalgae from a tank, it would be the equivalent of removing 24 mg of phosphate. That amount is the equivalent of reducing the phosphate concentration from 0.2 ppm to 0.1 ppm in a 67 gallon tank. All of the other species tested gave similar results (plus or minus a factor of 2). Interestingly, using nitrogen data in the same paper, it would also be equivalent to reducing the nitrate content by 2.5 grams, or 10 ppm in that same tank.
Commercial Products
There are, of course, many commercial products for reducing phosphate concentrations. Typically, these only reduce inorganic orthophosphate, but they can do that effectively, if not inexpensively. Two of the main types are those based on aluminum oxide (such as Seachem’s Phosguard) and those based on iron oxides and hydroxides (such as Rowaphos). Many people have successfully used these products (including myself), but others claim problems from the aluminum products that they blame on aluminum toxicity. I’ve seen no basis for these claims in my own tank, but I did not use them long term.
My advice on these products is that they can be used successfully, but that there may be better, and certainly less expensive and more interesting ways to reduce phosphate levels (such as setting up a refugium with macroalgae in it).
Summary of Phosphate Reduction Methods
Here is a list of ways that people can reduce phosphate levels. They are listed in order of preference that I have for addressing these issues in my own system:
- The big winner is macroalgae growth. Not only does it do a good job of reducing phosphate levels, but it reduces other nutrients as well (e.g., nitrogen compounds). It is also inexpensive and may benefit the tank in other ways, such as a haven for the growth of small life forms that help feed and diversify the tank. It is also fun to watch. I’d also include in this category the growth of any organism that you routinely harvest, whether corals or something else.
- Skimming is another big winner, in my opinion. Not only does it reduce organic forms of phosphate, but it reduces other nutrients and increases gas exchange. Gas exchange is an issue that many people don’t recognize, but that can contribute to pH problems.
- The use of limewater, and possibly other high pH alkalinity supplements, is also a good choice. It can be very inexpensive, and it solves two other big issues for reef keepers: maintaining calcium and alkalinity.
- Commercial phosphate binding agents clearly are effective.
- Simply keeping the pH high in a reef tank (8.4) may help keep phosphate that binds to rock and sand from reentering the water column. Allowing the pH to drop into the 7’s, especially if it drops low enough to dissolve some of the aragonite, may serve to deliver phosphate to the water column. In such systems (typically those with carbon dioxide reactors), raising the pH may help control soluble phosphate.
Summary
Issues involving phosphorus can be among the most difficult to diagnose in a reef tank, especially if the live rock and sand have been exposed to very high phosphate levels and may be acting as a phosphate reservoir. Fortunately, there are steps that can be taken even in the absence of any algae problem that will benefit reef tanks in a variety of ways, not the least of which is reduction of phosphate levels. All reef keepers, and especially those designing new systems, should have a clear idea in mind about how they expect phosphorus to be exported from their system. If allowed to find its own way out, it will more than likely end up in microalgae that the reef keeper is constantly battling.




0 Comments