Additional content from Chris Aslett is available HERE.
Mere survival in the nutrient-poor ecosystems peculiar to pristine wild reefs demands a profundity of nutrient recycling, yet these conditions support the greatest productivity and diversity. Our competence as reef aquarists is therefore founded on an appreciation of the interdependency, metabolism, and food webs of the micro- and macro-biota within our captive reefs and how this delicate balance can be accomplished or inadvertently destabilised.
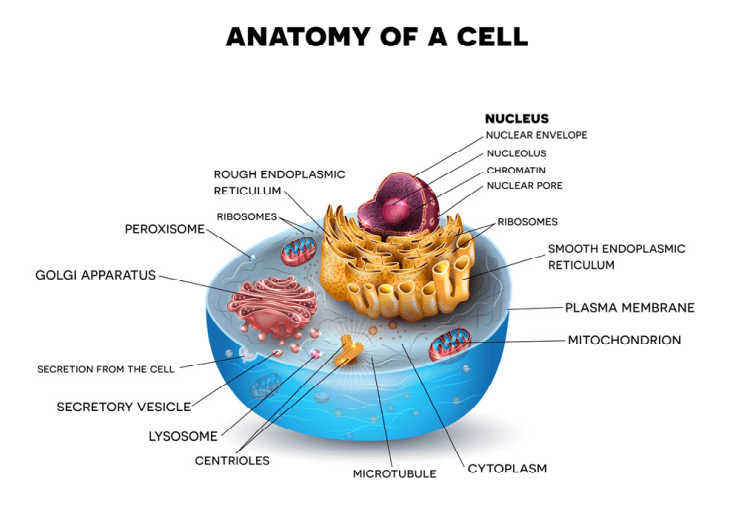
Fig 1. A diagram of a eukaryotic animal cell.
Eutrophic waters contain elevated concentrations of dissolved inorganic nitrogen (DIN) and phosphorus (DIP) whilst oligotrophy refers to water with few contaminants. Many hermatypic stony corals (zooxanthellate Scleractinia) are intolerant of raised temperatures and enriched phosphate (PO43-) and nitrate (NO3-) while resilience is conferred inter- and intra-specifically and according to symbiont lineage. Hence we must optimise conditions for all reef-forming corals because hardiness cannot be discerned visually (phenotypically), albeit a growing genomic database may yet identify biomarkers that distinguish robust lineages (Zoccola et al. 2020). Nevertheless the stability and efficacy of captive life support is not influenced merely by the hardiness of its ornamental species, but by the inertia and wellbeing of the entire ecosystem which proposes the paradigm of holosystemics.
Prokaryotic and eukaryotic cells are primitive and evolved to the degree that the former are conventionally freeliving or colonial and parasitic, symbiotic, or commensals that lack a nuclear membrane and specialised organelles common to eukaryotes which can be free-living unicellular, colonial and/or multicellular plants and animals (Fig 1.; Fig 2.). Although the dinoflagellate symbionts (zooxanthellae) of hermatypes have a nucleus with permanently condensed chromosomes called a mesokaryon which represents an intermediate phase of evolutionary development (Fig 3.; Guiry 2019).
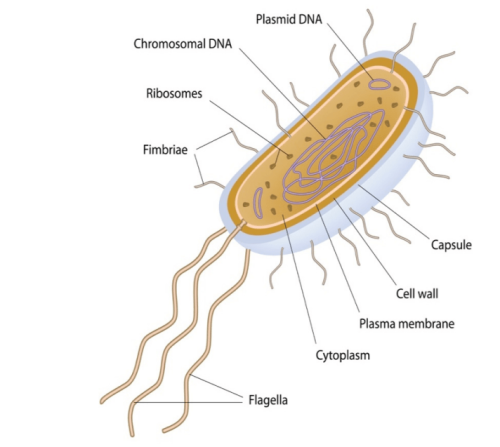
Fig 2. An illustration of a prokaryotic bacterium which lacks a nuclear membrane and complex organelles, yet it comprises ribosomes, flagella, a short genomic DNA chromosome, and plasmid DNA.
Virions are the most abundant particles in seawater which are either specific for bacteria (bacteriophage) or they are viruses of mesokaryotes and eukaryotes which undergo a lytic or lysogenic phase (Weinbauer et al. 2011). The former life cycle causes disease (pathology) and generates progeny virions that bud from or burst (lyse) the cell, while the latter instigates dormancy which frequently confers genetic attributes that aid environmental persistence and compliance (Wessner 2010). Such are the conspicuous virulence-conferring prophage of Vibrio species of bacteria several of which are prominent parasites of corals (Oakey & Owens 2000; Oakey et al. 2002). Lysogeny contributes to bleaching inasmuch as heat stress routinely induces the lytic cycle in zooxanthellae (Munn 2019).
Interdependent organisms with predestined prey, predators, parasites, commensals, and symbionts are adapted to the prevailing conditions within their conventional ecological niche. All affiliates are proportionally represented and undertake their roles within functional uncontaminated and abundantly diverse ecosystems. The infinite dilution offered by nature provides stability to which reef organisms are suited, yet the epigenotype and plastic phenotype of eukaryotes means they are highly adaptable. There is a band of purity at which all organisms thrive and nuisance algae are abated insofar as the toxicity of dissolved inorganic forms of nitrogen (N) and aggression compounds are determined by husbandry and the composition and abundance of animals and “algae”.
Autotrophs can assimilate (fix) inorganic sources of carbon into their structures such as carbon dioxide (CO2) or carbonate/bicarbonate (CO32-/HCO3-) while heterotrophs must gain carbon for growth from autotrophs or other heterotrophs. Autotrophs are thus the primary producers many of which a photosynthetic upon which all other lifeforms depend, while mixotrophs can utilise both inorganic and organic carbons sources. Hermatypic corals and their zooxanthellae are heterotrophs and photoautotrophs.
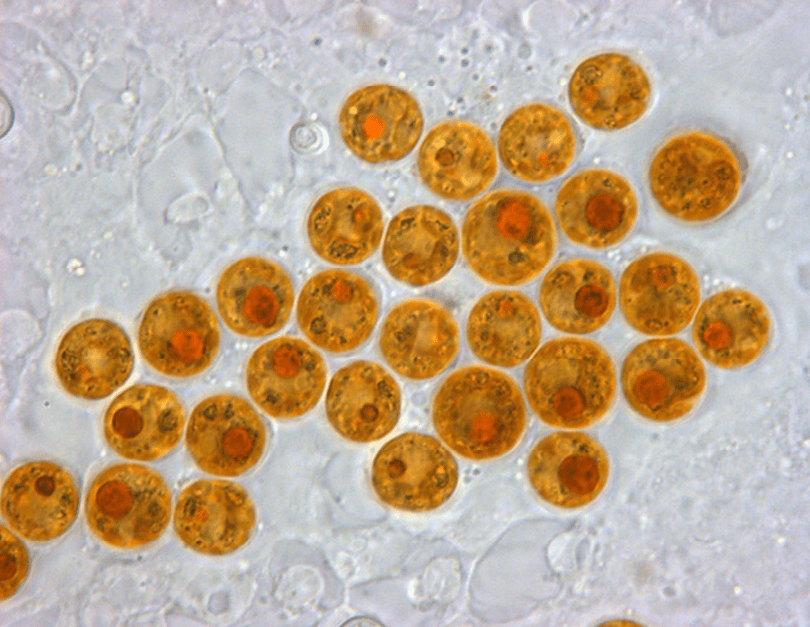
Fig 3. A micrograph of the coccoid form of reclassified Symbiodinium “fitti” found in Caribbean acroporids and giant clams. Endangered Acropora palmata, A. cervicornis, and their hybrid A. prolifera maintain an enduring association with clade A3 S. “fitti” (Pinzón et al. 2010; Reich et al. 2021). Image courtesy of Todd C. LaJeunesse©.
Nutrient limitation refers to a concentration at which an essential element becomes nonlife-sustaining, which is as harmful as enrichment inasmuch as it stifles productivity and diversity, therefore the aquarist’s primary objective is to maintain DIN and DIP within a critical range. 0.0372 and 0.019 mg l-1 of NO3- and PO43- were observed in 90 percent of wild reef locations (Kleypas et al. 1999) which is PO43-limited, yet wild reefs obtain their nutrients intermittently (Delbeek & Sprung 1994). Sensible captive targets are thus 0.065 mg l-1 PO43- and ~1 mg l-1 NO3- (Aslett 2023a) which is specified by a mere hint of pink after three minutes on a Salifert® Profi NO3 test.
Iron (Fe) is necessary for all life which is sequestered by the immune defences of several animals such as humans and fish because its absence constrains microorganisms (Butler 1998) including biofilter consortia (Keuter 2017). Most oceanic dissolved inorganic Fe (DIFe) is supplied by airborne dust, estuarine discharge, nutrient-rich upwellings, delugederived land runoff, and cross-shelf transport (Karl et al. 2004; Jiang et al. 2018) however significant DIFe originates from captive feeds.
Marine prokaryotes liberate iron binding siderophores that are later scavenged and internalised (Hopkinson & Morel 2009; Butler & Theisen 2010; Sanchez et al. 2018). Vegetation likely converts bound ferric Fe3+ to rare and transient free ferrous (Fe2+) before uptake (Hopkinson & Morel 2009) because oceanic iron is oxidised yet a minor component remains as soluble ferric hydroxide (Fe(OH)3). DIFe absorbance may rely upon DIP, and the phosphate binding medium granular ferric oxide (GFO) liberates microparticulate Fe (PIFe) which is biologically unavailable (Holmes-Farley 2002) yet it may be ingested by corals and acidic calcium reactor effluent solubilises some before coating each granule in calcite (Kuma et al. 1996; Millero 1998) that is removed with citric acid before recharge.

Fig 4. A scanning electron micrograph (SEM) of toxigenic dinoflagellate Prorocentrum hoffmannianum. Image courtesy of The Florida Fish and Wildlife Commission©.
Some reefs cope with enriched DIP which may be DINlimited. The only hope for many is immediate DIP sequestration expedited by Kent Marine Phosphate Sponge and a spurt of macroalgal growth supported by cautious supplementation of Fe2+, manganese (Mn2+), and iodide (I-) which is cycled to iodate (IO3-) by UV sterilisation (HolmesFarley 2003). Small polyp stony (SPS) corals turn brown and stop growing >1.75 mg l-1 NO3- but their development may be normalised by increasing carbonate hardness to 13.45 dKH (4.804 meq l-1; 241 mg l-1 CaCO3; Fig 6.; Hylkema 2014).
Coil denitrifyers consisting of 100 feet of ¼” John Guest® tube cable tied in a wide arc are relatively safe when a fast dribble of effluent is extracted using a peristaltic pump mounted over water. That said, they take up to three months to fully mature and are hazardous when they slow or occlude so their throughput must be checked twice daily (Fig 7).
Macroalgae stabilise an upsurge of N and P, while many like Caulerpa and Chaetomorpha species degrade and liberate potentially detrimental concentrations of pollutants during nutrient limitation. The former undergo a spawning stress response, yet these pulses which initially appear harmful provide a welcome respite for malnourished zooxanthellae. Rhodophyta like red grape (Botryocladia pseudodichotoma: Rhodymeniaceae) and red bamboo (Solieria species: Solieriaceae) acquire their coloration from phycobilins (Bonacolta et al. 2023) and endure nutrient limitation and are thus a better choice for refugia insofar as they are not considered invasive when they become naturalised in new wild territories (Fig 8.; Boudouresque & Verlaque 2002).
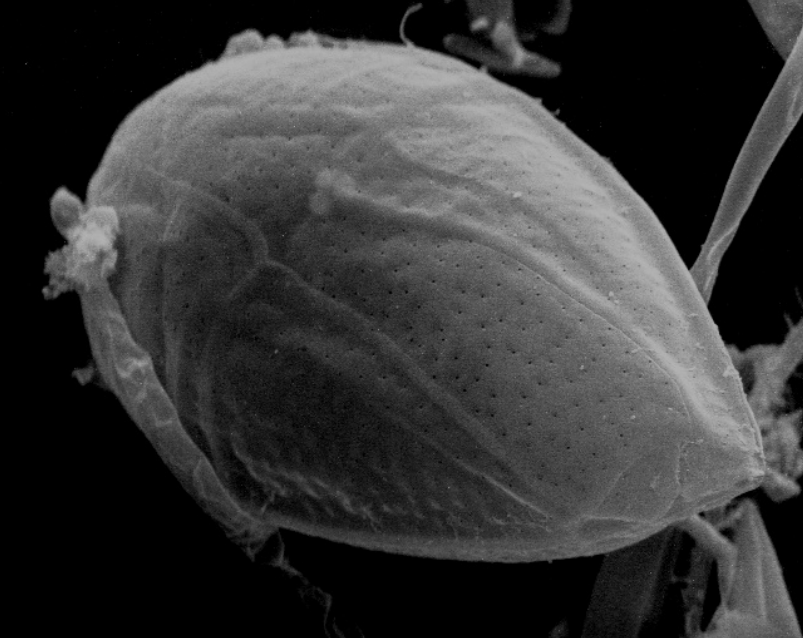
Fig 5. A SEM of Ostreopsis species (Myzozoa: Dinophyceae) of toxigenic dinoflagellate which can inhabit reef aquaria or induce catastrophic mortalities in fish-only recirculating systems. Image courtesy of The Florida Fish and Wildlife Commission©.
The Redfield hypothesis proposes a C to N to P ratio of 106:16:1 is assimilated into algal structures (Codispoti 2007) which is released upon complete microbial digestion (biomineralisation; Tyrrell & Steele 2001). This ratio is considered optimal for algal growth however reef water ranges from 4:3:1 to 7:2:1 (Rosset et al. 2017). The uptake ratios of marine vegetation remain unconserved amongst taxa, yet the Redfield hypothesis is substantiated when we consider the absorption rates of the gamut of photosynthetic organisms including nitrogen fixers (Mills & Arrigo 2010).
Nutrients within reef systems are insufficient to sustain a waterborne bloom of microscopic “algae” (phytoplankton) that are common to eutrophic fish-only export and retail systems, and thus never contaminate captive reefs with water from your livestock supplier. Organisms that make up wild phytoplankton and suspensions of small animals (zooplankton) remain associated with surfaces in reef aquaria. Nano- and pico-plankton comprise tiny chromists or bacteria which must be kept to a minimum when housing SPS corals (Kline et al. 2006; Feldman et al. 2011)

Fig 6. The feeding scars of the corallivorous flatworm Prosthiostomum acroporae where brown and a lack of polyp extension are indicative of enriched NO3-, zooxanthellae overabundance, and poor health (Balling et al. 2008). Image courtesy of Beau Henschen, https://www.CoralRX.com/,
Phillip Root©.

Fig 7. A coil denitrifyer. Aslett©.

Fig 8. Left – red grape (Botryocladia pseudodichotoma; Rhodophyta: Rhodymeniaceae) does not degrade when trace elements attain growth-limiting concentrations and is therefore an intuitive choice for a refugium.
Chemolithotrophs produce energy from inorganic molecules containing elements such as Fe, sulphur (S), and N which assume a vast range of ionic charges (oxidation states) making them ideal electron carriers (REDOX partners; Aslett 2023b).
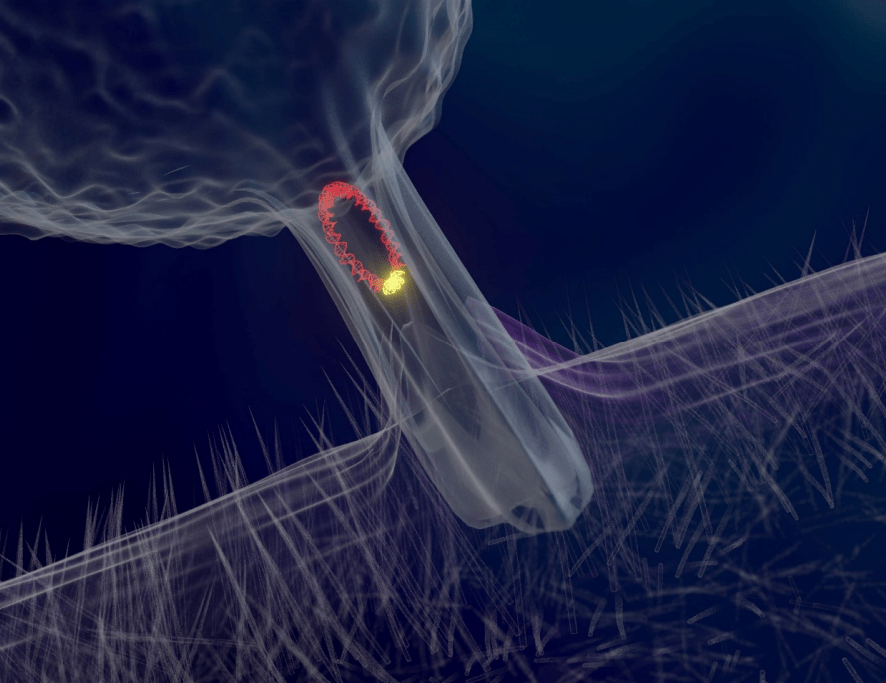
Fig 9. A representation of a conjugation tube distributing plasmid DNA.
Biofilms are “slimy” matrices of exopolymers excreted on wet surfaces by microorganisms (Fig 10.; Sehar & Naz 2016) that facilitate the adherence and recruitment of vast interdependent communities analogous to a coral’s mucosal microbiome. Most of these consortia rapidly respond to an everchanging environment due to their elevated rates of mutation and brief doubling (interfission), however marine organisms must expend resources on osmoregulation because water is predisposed to leaving their cells via osmosis. Moreover autotrophic metabolism only generates moderate energy which extends “true” nitrifier interfission to 30 to 40 hours instead of minutes (Hovanec, personal communication 2019). Nevertheless the biofilms of saltwater recirculating systems retain a propensity for adaptation while they frequently become reservoirs of antibiotic resistance, which is contagious to neighbouring microbes on a circular DNA molecule called a plasmid via an intercellular conjugation tube (Fig 9.; Li et al. 2017).
Aerobic and anaerobic microorganisms survive in environments where oxygen is present (oxic) or absent (anoxic) while facultative microbes switch metabolism. Hence obligate aerobes and anaerobes die when exposed to anoxia or dissolved oxygen (DO), and facultative anaerobes are primarily aerobic. Furthermore Fe and S remain the preferred electron carriers for anaerobic metabolism an intensification of which can collapse life support (Aslett 2023a).
Slow growing “true” nitrifiers are aerobic chemolithoautotrophs that generate energy from ammonia (NH3) and nitrite (NO2-) that inhabit merely the external 100 microns (micrometres; μm) of a biofilm. Heterotrophic bacteria are fast growing versatile exploiters of NH3, NO2-, and NO3- which outcompete and overgrow “true” nitrifiers during detrital fouling. Detecting NO2- thus indicates biofilter stress (Hovanec, personal communication 2019). Hence we must purge and protein skim particulate and dissolved organic matter (POM; DOM) and exercise caution when administering heterotrophic bacteria from bottles. Ammonia oxidisers can withstand its dearth whereas starved nitrite oxidisers appear to decline.
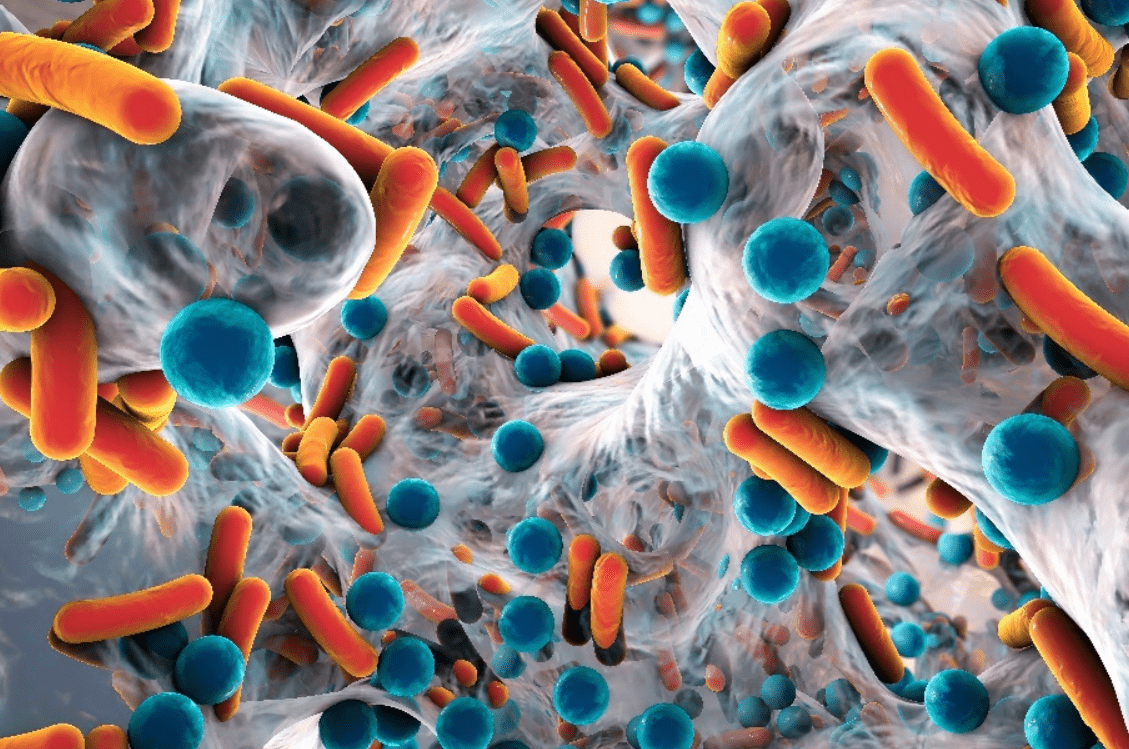
Fig 10. An artist’s impression of a biofilm embedded with single-celled interdependent microorganisms like those within biofilters. Biofilm matrices are composed of excreted biopolymers (exopolymers), and their inhabitants gain nutrition from molecules to which they are regularly exposed, including the metabolic by-products of their neighbours.

Fig 11. A sawdust-like neurotoxigenic cyanobacterial bloom (“red tide”) of Trichodesmium species. Image courtesy of The Florida Fish and Wildlife Commission©.
Cyanobacteria are photosynthetic and gram negative with a comparatively fortified peptidoglycan layer which are recognised symbionts of some corals and sponges, yet their blooms create green or maroon slime algae that warn of unoptimised DO (Fig 12.; Aslett 2023a; Aslett 2023b). They utilise the enzyme nitrogenase that forms the intermediate ammonium (NH4+) from dissolved dinitrogen (DN2) which contributes to potentially detrimental NO3- (DIN). However these processes are essentially natural and diazotrophic, which are dominated by vast subtropical and tropical harmful algal blooms (HABs) of the cyanobacterial genus Trichodesmium (Fig 11.; Dupouy 1988; Capone 2021). Chemolithoautotrophs, heterotrophs, and phototrophs also fix nitrogen (Capone 2021), whereas the system only malfunctions when “pollution” elicits nosedives in diversity from which a few resistant species arise, however discernible Cyanobacteria remain absent in “wholesome” reefs.
Microscopic evaluation of glass scrapes indicates diversity and assists the diagnosis of potentially harmful toxigenic “algae” (Fig 4.; Fig 5.; Fig 11.).
Amphipods, copepods, and heterotrophic protists create waterborne suspensions when they disrupt cells and faeces, which is biomineralised by microbes and fixed by phototrophy (Strom et al. 1997). Likewise viral lytic cycles elevate environmental POM and DOM which is exploited by Archaea (synonym: archaebacteria), heterotrophic microbes, and unicellular “algae” that are devoured by protists which are consumed by small arthropods that nutrify corals and fish (Weinbauer et al. 2011).
Captive benthic organisms that structure wild phytoplankton include phototrophic prokaryotes and mixotrophic eukaryotes (Zhang et al. 2018) and photoheterotrophs that respire oxygen (Fenchel 2001). Yet it remains undetermined if such heterogeneity is present in aquaria, but coral holobionts comprise organisms from every kingdom which includes microeukaryotes that may serve as significant inoculums. For instance skeletal chlorophytes of the genus Ostreobium exchange C and N with the coral and use chlorophyll b that absorbs wavelengths unutilised by other members of the holobiont (Bonacolta et al. 2023).

Fig 12. A burgundy mat of Cyanobacteria. Image courtesy of Arvind©; a Reef2Reef.com forum contributor.
Bacteria are prey for heterotrophic flagellates that are sustenance for microzooplankton including phagotrophic chromists, whereupon large zooplankton consume flagellates, microzooplankton, and phytoplankton which are eaten by corals and fish (Azam et al. 1983).
Microbe-rich deep-water sponges biomineralise POM and DOM including that derived from coral mucus to DIC, DIN, and DIP (Maldonado et al. 2012) that re-enter the food chain via autotrophy. The sponge loop is an indispensable component of benthic-pelagic coupling, which occurs in our aquaria when small animal ecoengineers form burrows in deep substrate that supplies DO and waterborne pollutants to anoxic zones (Dornhoffer 2014). Nevertheless sponges cannot be sustained in aquaria for reasons yet obscure, so POM and DOM are incompletely biomineralised by heterotrophic and mixotrophic microbes and exported by protein skimming.
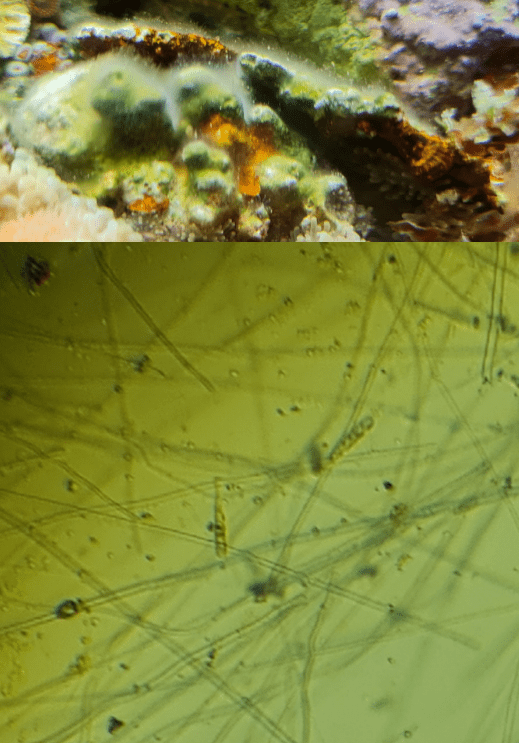
Fig 13. Top – resilient floccose growth on rocks that resists toothbrushing and the attentions of small hermit crabs, yet it is bioremediated by large hermits with shells greater than an inch. Bottom – a micrograph of the organism which has fungallike hyphae and macroconidia-like spores (cf. mitospores). Aslett©.
Nutrient recycling renders POM nutritionally poor because N and P are utilised which amplifies C (Benner & Amon 2015). Much of the microparticulate oceanic carbon is biodegradation resistant (refractory; recalcitrant) which is partly explained by the size-reactivity continuum model in that its dimensions are beneath the uptake threshold (Benner & Amon 2015; Zhang et al. 2018).
Eutrophy curtails diversity in several ways. NO3- is only mildly toxic to most sea life yet echinoderms and arthropods which include sea urchins, copepods, and amphipods are susceptible (Holmes-Farley 2005). The contaminants may therefore attain toxic thresholds which may kill a vast crosssection of the ecosystem. However elevated DIN and DIP fuel the disproportionate proliferation of a few species of animals and “algae”. Acoelomorphan flatworms, Exaiptasia species of anemone, and vermetid snails proliferate uncontrollably which stings, smothers, poisons and starves corals and other system inhabitants. Many “algae” amass iodine to inhibit predation (Holmes-Farley 2003) and liberate allelopathic compounds and toxins that influence the growth and life cycle of competing organisms. They inhibit the settlement of coral larvae and cause coral mortality by destabilising their mucosal microbiome (Birrell et al. 2008). Nevertheless water changes and the periodic use of granular activated carbon (GAC) diminishes their concentrations to below lethal limits within functional reefs. Moreover periodic manual removal (“sea weeding”) of macroalgae in the wild increases coral cover by up to 47 percent within three years (Smith et al. 2023).
Nutrient-derived malfunction promotes the logarithmic growth of one type of photosynthetic organism such as freeliving dinoflagellates or skeletal-eroding green filamentous “hair” algae, which overgrow all surfaces and substratum including corals. Dinoflagellates are often inedible and toxigenic which further curtails diversity, however Ostreopsis species form resting cysts at 21oC (70oF) that germinate at 25oC (77oF; Fig 5.; Fig 14.; Accoroni & Totti 2016). Cooler water carries more DO and reef performance is exemplary at 23oC (73.4oF).
The solubility of calcium phosphate (Ca3(PO4)2) often exceeds saturation in alkaline reefs which precipitates as an insoluble residuum (NCBI 2004). Dinoflagellates create pH instability which may cleave the ionic bond of Ca3(PO4)2 and liberate supplementary phosphate. Small animals and palatable vegetation are constrained which increases the nutritional demand of corals and fish, that require supplementary feeds which intensifies contamination. The algae restore oligotrophy but the pollutants are manifested in their biomass and thus remain system-bound. The solution is to identify and obviate future phosphate enrichment and physically remove the “algae” from the rocks with a brush in a bucket of aged seawater. Cease adding “potions” and phytoplankton and test all consumables and water for phosphate.
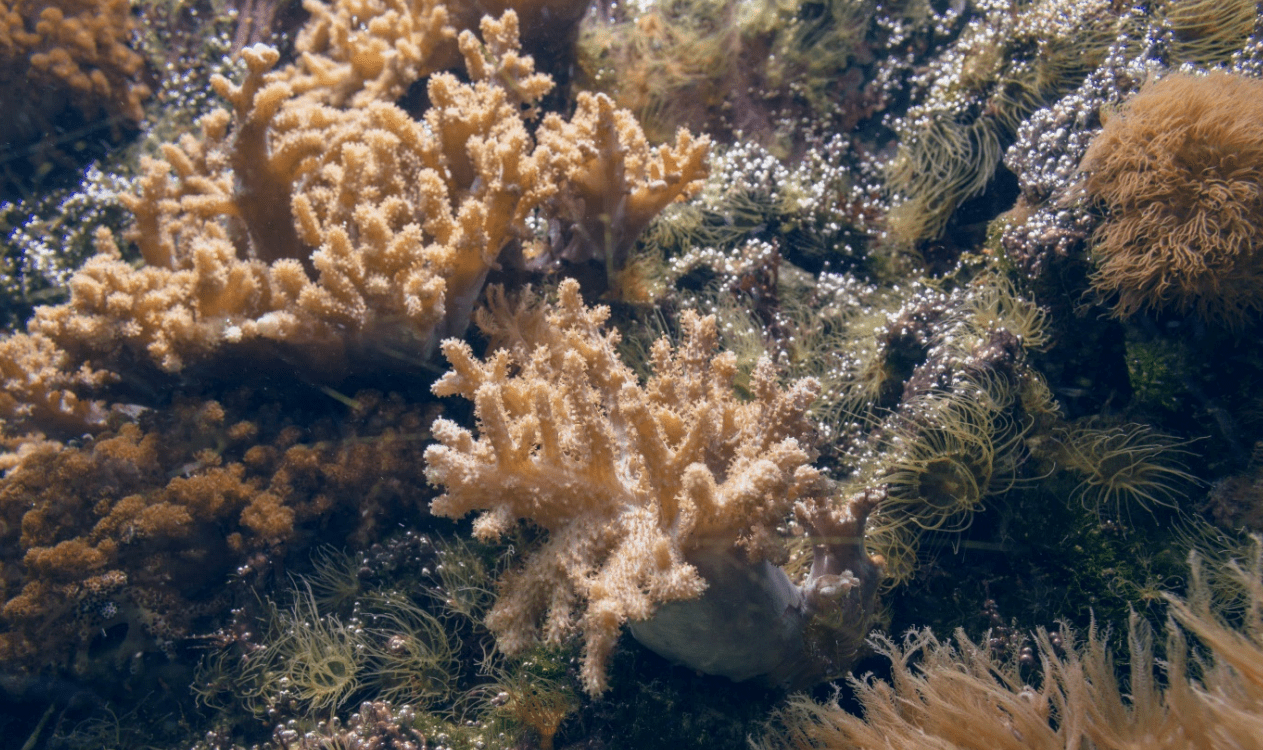
Fig 14. A malfunctioning system infested with dinoflagellates and nuisance anemones (Exaiptasia species) which are redolent of frequent and excessive inputs of DIP, where mucus and polysaccharide-bound bubbles are peculiar to free-living dinoflagellates (Fig 4.; Fig 5.; Durán-Riveroll et al. 2019).
Nonetheless it is challenging to reinstate these reefs insofar as restoring water quality does not re-establish diversity. Hence several vacant ecological niches are exploited by atypical organisms (Fig 13.) albeit a comparatively risk-free inoculum of substrate may be acquired from functional reefs housing established tangs and surgeonfish, which is likely more efficacious than organisms from a bottle.
The diets of the inhabitants need merely supplementing in functional reefs abounding in diversity, whereas malfunction is initiated and reinforced by overfeeding and degrading specimens.
Next we begin to explore the microbial network of hermatypic corals which buffers environmental stress by stabilising the host-symbiont association.
The PDF version of this article, as well as lots of additional related content, is available HERE.
References
- Accoroni, S. & Totti, C. (2016) The toxic benthic dinoflagellates of the genus Ostreopsis in temperate areas: a review. Advances in Oceanography and Limnology. 7(1), 1-15.
- Aslett, C., G. (2023a) The Complete Reef Aquarist, for hobbyists, the trade and academics. A Conservation Manual. Aslett, C., G. (ed.). Reef Ranch Publishing Ltd, Leeds, UK. In Press. https://www.ReefRanch.co.uk/
- Aslett, C., G. (2023b) Reef Digress: Saltwater REDOX. https://www.ReefRanch.co.uk/
- Azam, F., Fenchel, T., Field, J., Gray, J., S., Meyer-Reil, L., A. & Thingstad, T., F. (1983) The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257-263.
- Balling, H., W., Janse, M. & Sondervan, P. (2008) Trace elements, functions, sinks and replenishment in reef aquaria. Advances in coral husbandry in public aquariums. Leewis, R., J. & Janse, M. (eds.). Burgers’ Zoo, Arnhem, The Netherlands. pp 143-156.
- Benner, R. & Amon, R. (2015) The Size-Reactivity Continuum of Major Bioelements in the Ocean. Annual Review of Marine Science. 7, 185-205.
- Birrell, C., Mccook, L., Willis, B. & Diaz-Pulido, G. (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanography and marine biology. 46,.
- Bonacolta, A., M., Weiler, B., A., Porta‐Fitó, T., Sweet, M., Keeling, P., del Campo, J. (2023) Beyond the Symbiodiniaceae: diversity and role of microeukaryotic coral symbionts. Coral Reefs. 42, 567–577. https://doi.org/10.1007/s00338-023-02352-0
- Boudouresque, C., F. & Verlaque, M. (2002) Biological pollution in the Mediterranean Sea: invasive versus introduced macrophytes. Marine pollution bulletin. 44(1), 32-38.
- Butler, A. (1998) Acquisition and Utilization of Transition Metal Ions by Marine Organisms. Science. 281(5374), 207-209.
- Butler, A. & Theisen, R. (2010) Iron(III)–siderophore coordination chemistry: Reactivity of marine siderophores. Coordination Chemistry Reviews. 254(3), 288-296.
- Capone D., G. (2021) Coming full circle on diazotrophy in the marine cyanobacterium Trichodesmium. Proceedings of the National Academy of Sciences of the United States of America. 118(47), e2117967118.
- Codispoti, L., A. (2007) An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences. 4, 233-253.
- Delbeek, C. & Sprung, J. (1994) The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates (Volume 1). Two Little Fishies. p 30.
- Dornhoffer, T. (2014) Nitrogen Cycling Revisited: Sand, critters, carbon, and why you may be under-feeding your tank. AdvancedAquarist.com. https://www.advancedaquarist.com/2014/5/chemistry
- Dupouy, C., Petit, M. & Dandonneau, Y. (1988) Satellite detected cyanobacteria bloom in the southwestern tropical Pacific Implication for oceanic nitrogen fixation. International Journal of Remote Sensing. 9(3), 389-396.
- Durán-Riveroll, L., M., Cembella, A., D. & Okolodkov, Y., B. (2019) A Review on the Biodiversity and Biogeography of Toxigenic Benthic Marine Dinoflagellates of the Coasts of Latin America. Frontiers in Marine Science. 6, 148.
- Feldman, K., S., Place, A., A., Joshi, S. & White, G. (2011) Water: Baseline Values and Modulation by Carbon Dosing, Protein Skimming, and Granular Activated Carbon Filtration. AdvancedAquarist.com. https://www.advancedaquarist.com/2011/3/aafeature
- Fenchel, T. (2001) Marine Bugs and Carbon Flow. Science. 292(5526), 2444-2445.
- Guiry, M., D. (2019) Dinophyta (Pyrrophyta): Dinoflagellates. The Seaweed Site: information on marine algae. http://www.seaweed.ie/algae/dinophyta.php
- Holmes-Farley, R. (2002) Chemistry And The Aquarium: Iron In A Reef Tank. AdvancedAquarist.com. https://www.advancedaquarist.com/2002/8/chemistry
- Holmes-Farley, R. (2003) Chemistry And The Aquarium: Iodine in Marine Aquaria: Part I. AdvancesAquarist.com. https://www.advancedaquarist.com/2003/3/chemistry
- Holmes-Farley, R. (2005) Reef Alchemy: Nitrite and the Reef Aquarium. ReefKeeping.com. https://reefkeeping.com/issues/2005-06/rhf/index.php
- Hopkinson, B. & Morel, F. (2009) The role of siderophores in iron acquisition by photosynthetic marine microorganisms. BioMetals. 22(4), 659-669.
- Hovanec, T. (2019) Dr. How to harness bacteria to cycle your saltwater tank quickly! | MACNA 2019. BrsTV. https://www.youtube.com/watch?v=zDI7sxqC-ss
- Hylkema, A. (2014) Decreased growth of Stylophora pistillata with nutrient-driven elevated zooxanthellae density is largely explained by DIC limitation. Reefs.com. https://reefs.com/magazine/decreased-growth-of-stylophora-pistillata-with-nutrient-driven-elevated-zooxanthellae-density-is-largely-explained-by-dic-limitation/
- Jiang, L., Dong, C. & Yin, L. (2018) Cross-shelf transport induced by coastal trapped waves along the coast of East China Sea. Chinese Journal of Oceanology and Limnology. 36(3), 630-640.
- Karl, D., Michaels, A., Bergman, B., Capone, D., Carpenter, E., Letelier, R., Lipschultz, F., Paerl, H., Sigman, D. & Stal, L. (2004) Dinitrogen fixation in the world’s oceans. Biogeochemistry. 58(1), 47-98.
- Keuter, S. (2017) Longterm Monitoring of Nitrification and Nitrifying Communities during Biofilter Activation of Two Marine Recirculation Aquaculture Systems (RAS). International Journal of Aquaculture and Fishery Sciences. 10.17352/2455-8400.000029.
- Kleypas, J., A., McManus, J., W., McManus, I., Lambert, A. & Menezdf, A., B. (1999) Environmental Limits to Coral Reef Development: Where Do We Draw the Line? Amer. Zool. 39, 146-159.
- Kline, D., I., Kuntz, N., M., Breitbart, M., Knowlton, N. & Rohwer, F. (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Marine Ecology Progress Series. 314, 119-125.
- Kuma, K., Nishioka, J. & Matsunaga, K. (1996) Controls on iron (III) hydroxide solubility in seawater: The influence of pH and natural organic chelators. Limnol. Oceanogr. 41(3), 396-407.
- Li, S., Zhang, S., Ye, C., Lin, W., Zhang, M., Chen, L., Li, J. & Yu, X. (2017) Biofilm processes in treating mariculture wastewater may be a reservoir of antibiotic resistance genes. Marine pollution bulletin. 118(1-2), 289-296.
- Maldonado, M., Ribes, M. & Duyl, F. (2012) Nutrient Fluxes Through Sponges. Biology, Budgets, and Ecological Implications. Advances in marine biology. 62, 83-113.
- Millero, F. (1998) Solubility of Fe(III) in seawater. Earth and Planetary Science Letters. 154(1), 323-329.
- Mills, M. & Arrigo, K. (2010) Magnitude of oceanic nitrogen fixation influenced by the nutrient uptake ratio of phytoplankton. Nature Geoscience. 3,.
- Munn, C., B. (2019) Marine Microbiology: Ecology & Applications, Third Edition. Munn, C., B. (ed.). CRC Press, Taylor & Francis Group, London. pp 273-326.
- NCBI (2004) National Centre for Biotechnology Information. PubChem Cofound Database; CID=24456. https://pubchem.ncbi.nlm.nih.gov/compound/24456
- Oakey, H., Cullen, B. & Owens, L. (2002) The complete nucleotide sequence of the Vibrio harveyi bacteriophage VHML. Journal of Applied Microbiology, 93,1089-1098.
- Oakey, H., J. & Owens, L. (2000) A new bacteriophage, VHML, isolated from a toxin-producing strain of Vibrio harveyi in tropical Australia. Journal of Applied Microbiology. 89, 702-709.
- Pinzón, J., Devlin-Durante, M., Weber, M., Baums, I. & LaJeunesse, T. (2010) Microsatellite loci for Symbiodinium A3 (S. fitti) a common algal symbiont among Caribbean Acropora (stony corals) and Indo-Pacific giant clams (Tridacna). Conservation Genetics Resources. 3(1), 45-47.
- Reich, H., Kitchen, S., Stankiewicz, K., Devlin‐Durante, M., Fogarty, N. & Baums, I. (2021) Genomic variation of an endosymbiotic dinoflagellate (Symbiodinium ‘fitti’) among closely related coral hosts. Molecular Ecology. 30(14), 3500-3514.
- Rosset, S., Wiedenmann, J., Reed, A., J. & D’Angelo, C. (2017) Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Marine pollution bulletin. 118(1-2), 180-187.
- Sanchez, N., Brown, E., Olsen, Y., Vadstein, O., Iriarte, J., Gonzalez, H. & Ardelan, M. (2018) Effect of Siderophore on Iron Availability in a Diatom and a Dinoflagellate Species: Contrasting Response in Associated Bacteria. Frontiers in Marine Science. 5,.
- Sehar, S. & Naz, I. (2016) Role of the Biofilms in Wastewater Treatment. Microbial Biofilms. Dhanasekaran, D. & Thajuddin, N (eds.). IntechOpen.com. https://www.intechopen.com/books/microbial-biofilms-importance-and-applications/role-of-the-biofilms-in-wastewater-treatment
- Smith, H., A., Fulton, S., E., McLeod. I., M. & Bourne, D., G. (2023) Sea-weeding: Manual removal of macroalgae facilitates rapid coral recovery. Journal of Applied Ecology. 00, 1-13. DOI: 10.1111/1365-2664.14502
- Strom, S., L., Benner, R., Ziegler, S. & Dagg, M., J. (1997) Planktonic grazers are a potentially important source of marine dissolved organic carbon. Limnology and Oceanography. 42(6), 1364-1374.
- Tyrrell, T. & Steele, J., H. (2001) Redfield Ratio. Encyclopedia of Ocean Sciences. Steele, J., H. (ed.). Academic Press, Oxford. pp 2377-2387.
- Weinbauer, M., Chen, F. & Wilhelm, S. (2011) Virus-mediated redistribution and partitioning of carbon in the global oceans. Jiao, N., Azam, F. & Sanders, S. (eds.). SCIENCE / AAAS. pp 54-56.
- Wessner, D., R. (2010) The Origins of Viruses. Nature Education. 3(9), 37.
- Zhang, C., Dang, H., Azam, F., Benner, R., Legendre, L., Passow, U., Polimene, L., Robinson, C., Suttle, C., A. & Jiao, N. (2018) Evolving paradigms in biological carbon cycling in the ocean. National Science Review. 5(4), 481-499.
- Zoccola, D., Ounais, N., Barthelemy, D., Calcagno, R., Gaill, F., Henard, S., Hoegh-Guldberg, O., Janse, M., Jaubert, J., Putnam, H., Salvat, B., Voolstra, C. & Allemand, D. (2020) The World Coral Conservatory (WCC): A Noah’s ark for corals to support survival of reef ecosystems. PLoS Biology. 18(9),.



0 Comments