For many advanced aquarists, successfully maintaining an array of coral species in captivity is no longer a true challenge. A few dedicated hobbyists are now searching for the keys in unlocking the mysteries of the ultimate challenge in the reef aquarium hobby – that of closing the life cycles of corals and other invertebrates in captivity, where the offspring of captive invertebrates grow, thrive and reproduce in order to begin the cycle anew.
Just 16 years ago, an article in Audubon magazine (Derr, 1992) bashed the marine aquarium hobby over the collection of live rock in Florida waters and (in its view) the dismal rate of survival of captive corals (Quote: “The big commercial aquariums are not much more successful than the home hobbyist in keeping stony coral alive.”). Further, Derr chose to ignore scattered reports of coral reproduction in captivity (according to those interviewed for the story). If that article was lop-sided and biased in its reporting, it remains a fact that reports of successful sexual broadcast spawnings of corals resulting in settlement and colonization in aquaria are few and far between.
There are many reports of captive corals reproducing asexually via vegetative means including programmed fragmentation of various sorts, including “dripping” and “popping” and others. Some corals bear live young in a process called “brooding” and this is often seen in the stony coral Pocillopora damicornis and the azooxanthellate “sun” corals, Tubastraea spp.. Hobbyists can take pride when an animal reproduces in an aquarium – it is a rewarding experience and is a demonstration of the aquarist’s skills in creating an artificial environment conducive to long-term maintenance of captive animals. Still, reports of successful recruitment of broadcast spawners are scarce. What are we doing wrong (or right)?
This series of articles will examine factors that can encourage, or inhibit, sexual spawnings of aquarium corals. These include salinity, temperature, proper lighting, the effects of ultraviolet radiation, intentional fragmentation, how the tank’s life support system impacts reproduction (and vice versa) and water motion. We’ll also examine coral colony size and how it relates to sexual maturity, coral life spans, gonochoric sex ratios, egg size, techniques to artificially fertilize eggs, planula larvae competency, and even how to induce coral spawnings. Post-spawning requirements such as substrate preference, light intensity, water motion, resistance to effects of ultraviolet radiation and temperature will also be discussed.
Before beginning, we should ask why we would want to spawn corals in captivity. At first glance, it seems a lofty and worthwhile goal. We can take comfort that we are, even in a small way, conserving natural reefs. Perhaps it will simply be the challenge that motivates a dedicated hobbyist – the “because it is there” mind set. It is doubtful that there would be much financial reward, even if successfully-bred corals were to be a “limited edition”, where high prices are asked, and paid. And this will be the situation until collection and importation of corals is restricted or banned (there are serious attempts by various groups to do so every few years). Commercial ventures located near natural coral reefs and having access to clean seawater, abundant sunlight and spawning corals (where planula larvae can be collected from coral spawn slicks) have a better chance of being financially viable (See Goldman, 2006 & 2007). However, GBRMPA (Great Barrier Reef Marine Park
Authority) examined the issue of cost-effectiveness in captive coral breeding and concluded that time frames involved would lessen the appeal to commercial operations.
Fret not, this and following articles will barely touch upon intentional fragmentation which is by far the most common method of propagating corals in captivity. Though difficult to imagine, this technique was cutting-edge technology in the 1990s. It is extremely easy to do and can yield outstanding results with Acropora, Montipora, Stylophora and many other taxa. However, fragmentation does not lend itself to some corals.
The hobbyist consistently successful in providing proper environmental conditions to induce captive coral reproduction will not approach it in a haphazard manner. As we shall see, almost every detail of the captive breeding system must be deliberately engineered to consistently and reliably obtain the desired results. We will begin by examining the current state of captive coral reproduction.

Figure 1. Unidentified soft coral ‘twigs’ dropped from the adjacent parent colony. This form of reproduction is sometimes called vegetative reproduction. Photo by the author.
Asexual Reproduction
Asexual reproduction is essentially a cloning of the adult colony. This cloning involves several modes known as parthenogenesis, as well as accidental fragmentation and programmed fragmentation (including vegetative propagation, and phenomena called “dripping”, “popping” and “budding”).
Some corals are capable of self-fertilization (where a colony or even individual polyps contain male and female sex organs) and this is sometimes referred to as asexual reproduction. Little information on asexual coral reproduction exists in hobby literature, but several authors have devoted at least a few pages of their works to the examination of this phenomenon in aquaria (see Delbeek and Sprung, 1994 (emphasis on stony corals); Sprung and Delbeek, 1997 (emphasis on soft corals, zoanthids, anemones and gorgonians); Borneman, 2001; and Calfo, 2001).
Parthenogenesis
For our purposes, parthenogenic reproduction involves the development of an unfertilized egg with the potential to mature into a new coral colony. Parthenogenesis is common among some insects but occurs infrequently in some anemones (Bell, 1981), and has been inferred to occur in at least one soft coral (Alcyonium sp., Hartnoll, 1977; McFadden et al., 2001), a gorgonian (Plexaura sp.; Brazeau and Lasker, 1989), and several stony corals (Pocillopora damicornis (Permata et al., 2000); Fungia scutaria (Krupp, 1983), Porites lobata and Porites lutea – Fadlallah, 1983). Parthenogenesis is also known to occur in a few reptile and fish species. This process would have advantages when individuals of reproductive size are scarce, but has a major disadvantage in that parthenogenically produced colonies are simply clones (female) and lack genetic diversity.
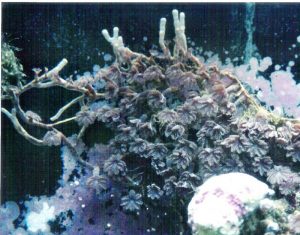
Figure 2.”Runners” called stolons, could eventually detach from the parent colony and become a new soft coral colony. Photo by the author.
Vegetative Propagation
This term often refers to asexual reproduction by plants but applies to corals as well. It is not uncommon, especially in soft corals and gorgonians, for small but complete colonies to detach from the maternal colony (See Figure 1). Another example of vegetative reproduction is that of stoloniferous growth and detachment (see Figure 2).
Programmed Fragmentation – Diaseris spp.
Programmed fragmentation, in the case of Diaseris species, is a means of reproduction that differs from accidental fragmentation such as that by storms or surge, and intentional fragmentation by hobbyists. With Diaseris spp., programmed fragmentation involves slit formation with associated skeletal dissolution, resulting in small, roughly tear-drop shaped colonies dropping away from the parent colony (See Figure 3 and 4). These daughter colonies eventually grow a new mouth and mature quickly to functional adults. Colley et al., 2000 describe the frequency of this occurrence as ‘high rate’ on natural reefs.

Figure 3. Daughter colonies of Diaseris. Aquarist Larry Jackson reports he has given away about 150 colonies that were asexually produced within his aquaria. Photo courtesy of Larry Jackson.

Figure 4. The underside of an adult Diaseris showing its developing fragmentation lines. Portions of the colony will soon break away. Photo courtesy Larry Jackson.
“Dripping”
Dripping is a term used to describe a slow detachment and separation of viable tissues from an adult coral colony. The separation is in essence a cloning of the adult which allows colonization of an area immediately surrounding the adult. The new colony may, or may not, contain a skeleton.
Figures 8 through 10 show “dripping” of several Caribbean coral taxa. These photographs were taken in a refugium of an aquarium maintained by Bill Hoffman of the Smithsonian Marine Station in Fort Pierce, Florida. The photographer (Julian Sprung) relayed his observations of these corals. Sprung noticed the developing offspring tended to be localized and speculates that the causative agent of these “bubbles” might be viral, or perhaps a response to environmental conditions such as high light intensity or ultraviolet radiation. In any case, the corals are not bleached and seem to suffer no permanent damage.
Figures 11 and 12 are of “satellite” or “daughter” colonies of the stony coral Goniopora. These corals are also known to reproduce sexually, with broadcasting of gametes from separately-sexed (gonochoric) parent colonies. At least one Goniopora species is known to be a brooder.

Figure 6. Euphyllia clones that dripped from the parent colony have settled in an aquarium of Gary Moss.

Figure 7. Offspring “dripping” from a stony coral (Favia?). From an aquarium maintained by Steve Poleo, The Aquarium, Tempe, Arizona.

Figure 8. Dendrogyra cylindrus forming tissue bubbles that will eventually develop a skeleton and drop off. Photographed in a refugium at the Smithsonian Marine Station, Ft. Pierce, Florida. Photo courtesy of Julian Sprung.

Figure 9. Montastrea cavernosa adjacent to the Dendrogyra in the same tank, forming the same type of tissue bubbles. Note advanced development. Photo courtesy of Julian Sprung.

Figure 10. Mycetophyllia aliciae (adjacent to the corals in Figures 8 and 9) has some tissue buds forming along the side facing them. Note settled daughter colony of M. cavernosa (out of focus) between the corals. Photo courtesy of Julian Sprung.

Figure 11. This Goniopora stony coral is producing at least two “daughter” or “satellite” offspring (whiles others, already detached, are to left and behind). Photo courtesy Larry Jackson.

Figure 12. A Goniopora daughter colony isolated in a spawning basket. From one of the author”s aquaria in the early 1990’s.

Figure 15. Sub-adult Elegance corals (Catalaphyllia jardineri; at lower right) produced asexually in an aquarium maintained by aquarist Richard Durso. Photo courtesy of R. Durso.

Figure 16. Lobophyllia sp. forming a bud on its surface in the “big aquarium” – the Pacific Ocean. Photographed in the Solomon Islands by Julian Sprung.
“Popping”
“Popping”, as described by Kramarsky-Winter (1997) is another asexual reproductive mode where new colonies arise from a maternal colony (or sometimes the remains of an adult colony). These new colonies eventually break away. This reproductive mode is seen in stony corals (including genera Fungia, Trachyphyllia, Turbinaria, Euphyllia, Psammocora and others) and soft corals.

Figure 17. Small colonies “pop” from the remains of an adult Trachyphyllia colony. Photos courtesy Steven Pro.
Goemans (2000) reported similar “popping” of a captive coral identified as Turbinaria peltata and a soft coral (Sarcophyton elegans, commonly called Yellow Fiji leather coral). Calfo (2001) also reports similar “popping” of a Turbinaria specimen.

Figure 18. These mushroom-shaped colonies are “popping” from a Turbinaria reniformiscoral. There are at least 8 of them. Photo by the author.

Figure 19. Many growths are popping above the prominent “twig” on this Turbinaria specimen. Photo courtesy Julian Sprung.

Figure 21. A Euphyllia ancora (Anchor coral) producing a twig-like growth. This will eventually break off and possibly produce a new colony. Courtesy Gary Moss.
“Brooding” Corals
Brooding is the act of retaining fertilized eggs either internally or upon a coral”s surface. This reproductive technique bears young (planula larvae) of relatively large size, though in fewer numbers than broadcast spawners. The advantage of advanced development upon release from the parent colony increases chances of survival.

Figure 23. This Tubastraea specimen settled in the overflow of a reef tank where it is exposed to turbulent water motion – and food. Photo by the author.
There are at least two brooding corals that routinely reproduce in aquaria, and most reports seem to include the stony coral Pocillopora damicornis and the “sun” coral (Tubastraea species). Reproduction by these corals requires little effort on the part of the hobbyist (other than routine husbandry chores) and it is possible for many larvae to settle and attach to substrata.

Figure 25. This Pocillopora damicornis has settled on the back wall of an aquarium at Sea World Ohio. Photo by the author.

Figure 26. These three Pocillopora damicornis specimens could have been brooded by the simultaneous hermaphroditic parent colony. For reasons unknown, the planula larvae preferred to settle on the powerhead and its electrical cord. From an aquarium of Joe Ranney; photo by the author.
Anemones
Anemones reproduce sexually and asexually. All too many hobbyists are familiar with asexual pedal budding (or pedal laceration) of “pest” anemones such as Aiptasia and Boloceroides. On the other hand, some desirable species also reproduce asexually in aquaria through means known as longitudinal fission (“splitting down the middle”), budding, and possible cloning via parthenogenesis (Walker and Bull, 1983).

Figure 27. Baskets of young Bubble-tipped anemones are growing out in the aquarium of Bob Lemke. Photo courtesy Bob Lemke.
Acknowledgements
The author is indebted to those answering a request for coral reproduction photographs, as well as those who allowed me into their businesses and homes across the country and kindly granted permission to document their animals’ activities. For this installment, these persons are:
Joe Ranney (then of Cleveland Ohio); Eric Amadio of Phoenix, Arizona; Steve Poleo, Tempe, Arizona; Rich Durso; Pete Mohan then of Sea World Ohio; Steven Pro, Pittsburgh, Pennsylvania; Julian Sprung of Two Little Fishies, Coconut Grove, Florida; Larry Jackson, San Angelo, Texas; Steve Ruddy of Coral Reef Ecosystems, Guerneville, California; Bob Lemke of Canton, Georgia; Gary Moss of Baltimore, Maryland; Walter Bobe, Canton, Georgia; and The Fish Store and More, Atlanta, Georgia.

Figure 28. Anemones reproduce sexually as well, as this bubble-tip anemone is believed to have been. Photo courtesy Steve Ruddy.
Invariably, I have misplaced notes of some photographs, or the markings on slides have become illegible over the years. To those individuals, I apologize but salute you for your dedication.
Several factors influenced my decision to dedicate the time and effort to write this series of articles. A major consideration was preservation of some of the photographs and transparencies I have accumulated over the years. Some photographs were stored in a shoebox while some slides were showing signs of deterioration in the warm, humid climate of Hawaii. Many of the photographs in this article were taken when consumer level digital photography was unknown, when we made do with cameras with an extremely limited number of possible photographs. We did not have the luxury of instant photograph review then, which resulted in many pictures of poor and unusable quality. Some of my photographs are testaments to the tribulations of silver-based photography. However, I felt it better to share them than have them degrade and become lost forever.
A complete reference list will be presented at the close of this series.
Questions? Comments? Reach me at: [email protected]









0 Comments