Most reefkeepers know they need to measure alkalinity, and most know it has something to do with carbonate. But what is alkalinity exactly? Why is it important? How is it measured? What can confound alkalinity tests? This article will answer these questions and will hopefully give you all of the information that you need to more fully understand one of the most important chemical parameters of our tanks.
What is alkalinity?
Alkalinity is defined in different ways for different applications. In the chemistry of natural waters, there are several types of alkalinity that are encountered. Each of these is a measure of how much acid (H+) is required to lower the pH to a specific level. I’ll come back to some of
the other types of alkalinity later, but for now we will confine our discussion to the “total alkalinity.” frequently referred to as TA.
TA is defined as the amount of acid required to lower the pH of the sample to the point where all of the bicarbonate [HCO3–] and carbonate [CO3—] could be converted to carbonic acid [H2CO3]. This is called the carbonic acid equivalence point or the carbonic acid endpoint. These equations show what happens to carbonate and bicarbonate as acid is added:
(1) H+ + CO3 ==> HCO3–
(2) H+ + HCO3– ==> H2CO3
I say “could be converted” because regardless of the pH, there will always be some bicarbonate and carbonate present, but at some pH there are enough protons (H+) in solution that if they were combined with the bicarbonate and carbonate present, it would all be converted to carbonic acid.
The precise endpoint of a total alkalinity titration isn’t always the same pH, but rather depends a bit on the nature of the sample (both its ionic strength and its alkalinity). For normal seawater, this endpoint is about pH = 4.2. In freshwater it depends strongly on the alkalinity, with an endpoint of pH = 4.5 for an alkalinity of 2.2 meq/L, and pH = 5.2 for an alkalinity of 0.1 meq/L.
Consequently, total alkalinity tests have been invented that determine how much acid is required to lower the pH into the 4-5 range. Later in this article I’ll describe how these tests kits are measuring alkalinity.
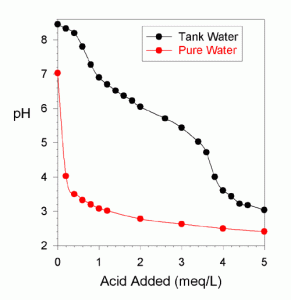
Figure 1 shows a pH titration of water from a reef tank (mine). The water starts off at pH 8.45 and as acid is added, the pH drops. As can be seen in Figure 1, it takes about 3.4 meq/L of base to drop the pH to 5, and 3.8 meq/L to drop the pH to 4.0. Figure 1 also shows the same pH titration of pure water. In that case, the pH immediately drops from pH 7 (or thereabouts; the pH of pure water drifts around since it has no buffering) to pH 4 with only 0.2 meq/L of acid added.
We can, however, get more from these types of graphs than the total alkalinity. In order to do so, however, we must understand what alkalinity is on a chemical level.
Chemical Nature of Alkalinity
Based on the definition of total alkalinity given above, it is clear that anything that absorbs protons when the pH is dropped from normal levels to about 4-5 will be counted toward alkalinity. In seawater there are a variety of things that contribute, and in reef tanks the list is even longer. Equation 3 is the defining equation for total alkalinity in normal seawater.
TA = [HCO3–] + 2[CO3—] + [B(OH)4–] + [OH–] + [Si(OH)3O–] + [MgOH+] + [HPO4—] + 2[PO4—] – [H+]
The reason for the 2 in front of the carbonate and phosphate concentrations is that they take up two protons as the pH is dropped down to pH 4. All of the other ions just take up a single proton (except protons themselves which must be subtracted).
The main chemical species that contribute to alkalinity in seawater (and the reason it is useful to reefkeepers) are bicarbonate and carbonate (equations 1 and 2). The table below (from “Chemical Oceanography” by Frank Millero; 1996) shows the contribution to alkalinity from the major contributors in seawater at pH 8. If you start at higher pH, the relative contribution of bicarbonate will go down relative the others.
| Chemical Species | Relative Contribution To Alkalinity |
|---|---|
| HCO3– (bicarbonate) | 89.8 |
| CO3— (carbonate) | 6.7 |
| B(OH)4– (borate) | 2.9 |
| SiO(OH)3– (silicate) | 0.2 |
| MgOH+ (magnesium monohydroxylate) | 0.1 |
| OH– (hydroxide) | 0.1 |
| HPO4— and PO4— (phosphate) |
0.1 |
Other species can also contribute measurably to alkalinity in seawater in certain situations, such as anoxic regions. These would include NH4+ and HS– .
In reef tanks, some of these species can be present in substantially higher concentrations than in seawater. For example, a reef tank with a phosphate concentration of 0.5 ppm will have a higher contribution from phosphate (2.5 times the value shown in the table).
Even more concerning is the tendency of some salt mixes to greatly boost the borate concentration. Seachem intentionally adds extra borate to a level of about
5 mM. This increases the borate contribution by more than a factor of 10 over seawater, and makes it a significant factor in alkalinity measurements (and interpretations).
Step by Step Acidification
Here’s a blow-by-blow description of what’s happening during an alkalinity titration, either with a pH meter or with a test kit.
At the start (say, pH = 8.2), we have the following constituents where the ions in red predominate, ions in blue have smaller relative concentrations, and ions in black have much lower relative concentrations:
H2CO3
HCO3–
CO3—
B(OH)3
B(OH)4–
Si(OH)4
Si(OH)3O–
Mg++ MgOH+
H2O
OH–
H3PO4
H2PO4–
HPO4—
PO4—
As the pH drops from 8.2 to about 7.5, the most important thing happening is that the carbonate is converted into bicarbonate (equation 1). In figures 1 and 2 this part of the titration can be seen to take about 0.6 meq/L in my tank, and represents about 17% of the total alkalinity, in line with expectations for a tank that starts at a relatively high pH (8.45). All of the other minor contributors also get protonated at this point, and we see a shift to:
H2CO3
HCO3–
CO3—
B(OH)3
B(OH)4–
Si(OH)4
Si(OH)3O–
Mg++ MgOH+
H2O OH–
H3PO4
H2PO4–
HPO4—
PO4—
As the pH drops to about 6, the main thing happening is that bicarbonate is getting converted into carbonic acid. Also in this range, phosphate continues to take up protons:
H2CO3HCO
3– CO3—
B(OH)3
B(OH)4–
Si(OH)4
Si(OH)3O–
Mg++ MgOH+
H2O OH–
H3PO4
H2PO4–
HPO4—
PO4—
As the pH drops to about 4, the bicarbonate becomes fully converted into carbonic acid. Also in this range, phosphate continues to take up protons and ends up as mostly H2PO4–, but very little phosphoric acid itself forms.
H2CO3
HCO3– CO3—
B(OH)3
B(OH)4–
Si(OH)4
Si(OH)3O–
Mg++ MgOH+
H2O OH–
H3PO4
H2PO4–
HPO4—
PO4—
Alkalinity using Test Kits
Of course, most reefkeepers measure alkalinity with a test kit, not with a pH titration. How does that work?
Well, in effect test kits do a pH endpoint titration. They all include pH indicating dyes (providing a color change) and an acid (frequently dilute sulfuric acid) to lower the pH. You typically add acid until the dyes turn color. Since these dyes are selected to have a color change in the pH = 4 to 5 range, what you get is a measurement of how much acid it takes to lower the pH to that range. This color change is used to approximate the endpoint of the titration.
Interestingly, many test kits use more than one pH indicating dye. Using more than one dye at the same time permits the endpoint to be sharper. For example, bromcresol green has a broad color transition between pH 3.8 (yellow) and 5.4 (blue-green) and methyl red has a broad transition between pH 4.4 (red) and 6.2 (yellow). A mixture of the two (used in the Hach alkalinity kit) has a sharp transition (orange to blue-green) around pH 5.1 in fresh water (which may be slightly different in salt water).
Five point 1 you say? Based on the discussion above, is that low enough? Well, the Hach kit was designed for use in fresh water where the pKa of the bicarbonate is much higher than in seawater, and in that situation, it is appropriate. In seawater, however, it is marginal. My tank water took 3.4 meq/L to get down to pH = 5.03, and then an additional 0.4 meq/L to get down to pH 4.00. Consequently, this kit (and others with a similar dye mix) may be missing out on 10% of the alkalinity simply because it isn’t titrating low enough. This difference obviously isn’t significant to most reef keepers, but is something to keep in mind when doing such things as comparing test kits to standards (in seawater) or to each other.
Some test kits also provide a different dye for a different measure of alkalinity. Frequently, this other dye is phenolphthalein. This dye has a color change between pH 8.2 and pH 9.8. In fresh water, carbonate is almost completely converted into bicarbonate at pH 8.3, and that is the purpose of phenolphthalein titrations: to determine alkalinity in freshwater due to carbonate only (discussed in detail below). This test serves no purpose in a reef tank or seawater for two reasons: 1) the water is probably already more acidic than the endpoint of this dye, and 2) the carbonate in seawater is not completely converted into bicarbonate at this pH anyway. That is, even if the pH were higher than 8.3 (say, 8.6), titrating down to the phenolphthalein endpoint will not effectively “count” all of the carbonate because in saltwater there will still be substantial carbonate present at the phenolphthalein endpoint.
Why is Alkalinity Important?
Now that we know what alkalinity is, we can understand why it is an important measure for reef tanks. Corals and other organisms deposit calcium carbonate in their skeletons and other body parts. In order to do this they must generate calcium and carbonate at the surface of the growing calcium carbonate crystal. While it is far beyond the scope of this paper to describe this process, it is readily apparent that if corals deposit these chemicals, they are using them up from the water that they inhabit. So, if that’s the case, why not just measure carbonate as we do calcium?
Well, there are two answers. The first is that there is no simply way to measure carbonate with a kit without doing a pH titration as an alkalinity test kit does. Second, corals may actually use bicarbonate instead of carbonate as their ultimate source of carbonate (which they split into H+ and CO3—). If we could easily measure bicarbonate, we’d probably be doing just that. Unfortunately, we can’t do either of those things easily.
So what we are doing is using a very simple alkalinity test as a surrogate measure for bicarbonate and carbonate. Since these two substances comprise the great majority of alkalinity in seawater, it is safe for most people to equate alkalinity with “availability of bicarbonate and carbonate for my corals”.
There are, however, some important caveats to that equation. Some of these were described above, such as salt mixes that have excessive borate. Such complications make it difficult to know how much of the measured alkalinity is bicarbonate and carbonate, and thus it is difficult to know if you are satisfying the needs of the corals [Hence the unusually high alkalinity recommendations by Seachem].
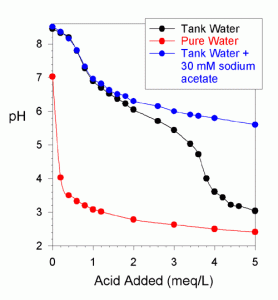
Figure 2. A pH titration of pure water, water from the author’s reef tank, and water from the author’s reef tank with 30 mM of sodium acetate added (titrated with 0.1 N HCl).
Unusual Contributors
Reef tanks can also have contributors to the total alkalinity that are simply not present in seawater at any appreciable concentration. This result comes from the fact that we have a closed system in which organics (e.g., acetate, polygluconate, EDTA; citric acid) and other ions may be unusually high.
As an example, consider those people who are dosing limewater with organic acids such as vinegar. Acetic acid is a complication to an alkalinity test that may or may not be significant to people using it, but the more vinegar that is used, the more confounding it may become. Ultimately, the acetate that is added in this fashion will be oxidized into CO2 and OH– (equation 4), with the OH– providing alkalinity in the same fashion that the original limewater would have.
(4) 2 O2 + CH3COO– ==> H2O + 2 CO2 + OH–
The issue at hand is how fast this conversion takes place, or alternatively, how much acetate is present in such a system when one measures the alkalinity. Since I’ve seen no studies of acetate levels in reef tanks, the question remains unanswered (at least to me).
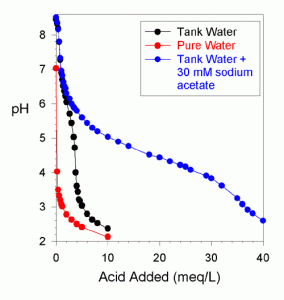
Figure 3. An extended pH titration of pure water, water from the author’s reef tank, and water from the author’s reef tank with 30 mM of sodium acetate added (titrated with 0.1 N HCl).
The potential for a problem comes about because acetate is partially “counted” in a total alkalinity titration of tank water. The extent to which it is counted will depend upon what pH is being used as the titration endpoint. Figures 2 and 3 show the pH titration of tank water with a huge excess of acetate added (30 mM). This excessively large amount was added not because a reef tank would contain such a large amount (after all, the measured total alkalinity is about 20 meq/L), but because it makes the acetate titration clearly visible in the presence of carbonate and bicarbonate. If the endpoint of the alkalinity titration is at pH 5, then about 25% of the acetate is counted. With the endpoint at pH 4, about 80% is counted.
Consequently, if a tank has marginal alkalinity and some substantial portion of this alkalinity is acetate (or some other organic), then the availability of bicarbonate and carbonate may be less than optimal for corals and other calcifying organisms. Note that the acetate does not impact the titration of carbonate between the native pH and about 7.3. If one is using large amounts of vinegar, it might be worthwhile to titrate the carbonate down to 7.3 to verify that the total alkalinity is not being dominated by acetate (by observing at least 0.2-0.4 meq/L alkalinity down to pH = 7.3. My tank water without acetate had 0.6 meq/L for this titration (Figure 1) and the same when a large amount of acetate was added (Figures 2 and 3).
Alkalinity Facts
There are several facts about total alkalinity that follow directly from the definition. Unfortunately, some of these have been misunderstood by some hobby authors.
One of these facts is termed The Principle of Conservation of Alkalinity by Pankow (“Aquatic Chemistry Concepts”, 1991). He shows mathematically that the total alkalinity of a sample CANNOT be changed by adding or subtracting CO2. Unfortunately, there is an article available on line, which claims otherwise, and encourages people to “lower alkalinity” by adding CO2 in the form of seltzer water. This is simply incorrect.
Forgetting the math for the moment, it is easy to see how this must be the case. If carbonic acid is added to any aqueous sample with a measurable alkalinity, what can happen?
Well, the carbonic acid can release protons by reversing equations 1 and 2:
(5) H2CO3 ==> H+ + HCO3–
(6) HCO3– ==> H+ + CO3—
These protons can go on to reduce alkalinity by combining with something that is in the sample that provides alkalinity (carbonate, bicarbonate, borate, phosphate, etc). However, for every proton that leaves the carbonic acid and reduces alkalinity, a new bicarbonate or carbonate ion is formed that adds to alkalinity, and the net change in total alkalinity is exactly zero. The pH will change, and the speciation of the things contributing to alkalinity will change, but not the total alkalinity.
This is not true for strong acids, however. If you add hydrochloric, sulfuric or phosphoric acids (or any acid with a pKa lower than the carbonic acid endpoint), there will be a reduction in the alkalinity.
Another interesting result of the Principle of Conservation of Alkalinity is the equation for determining the total alkalinity when two different aqueous solutions are mixed together. If you mix (a) parts of a solution with total alkalinity A with (b) parts of a solution of total alkalinity B, the resulting alkalinity is just the weighted average of the two samples:
TAmix = [a(A) + b(B)]/[a + b]
Equation 7 can be used to calculate changes in TA for water changes in a tank, for additions of limewater, for dilution of tank water with pure water, and a host of other situations where you might want to know what the final alkalinity will be. It can also be used for calculating reductions in alkalinity caused by strong acids, where the alkalinity of the acid is just the normal strength of the acid as a negative number.
Other Definitions of Alkalinity
Any definition of alkalinity other than the total alkalinity seems to lead to confusion. For example, Millero defines the carbonate alkalinity (AC) as the alkalinity coming from just bicarbonate and carbonate (equation 8). Some test kits use this definition as well.
(8) AC = [HCO3–] + 2[CO3—]
Unfortunately, another leading author, Pankow, defines carbonate alkalinity (CO3— – Alk) as the total alkalinity down to the pH where all carbonate is converted into bicarbonate (the bicarbonate equivalence point or endpoint; about pH 8.3 in fresh water; about pH 7.3 in seawater). Consequently, it doesn’t count bicarbonate at all, and does count borate and other ions that take up acid above the carbonate endpoint. For freshwater, this type of alkalinity is represented by the phenolphthalein endpoint used in the Hach and other kits.
Others define carbonate alkalinity as just that portion of total alkalinity down to the carbonic acid endpoint that comes from carbonate ions, exclusive of bicarbonate, hydroxide, borate, etc. And there are still other definitions of alkalinity. The hydroxide alkalinity (OH– – Alk), sometimes called the caustic alkalinity, is defined by some as the total alkalinity down to the carbonate equivalence point (about pH 10.7 in fresh water).
One test kit (Seachem) provides a test for borate and hydroxide alkalinity. I have not tested this kit to know whether it is effective or not.
Because of these potential points of confusion, in any discussion of alkalinity other than the total alkalinity, one needs to be very clear about the definitions being used.
Units of Alkalinity
The various units used for alkalinity are themselves cause for confusion. The clearest unit, and that used by most scientists is milliequivalents per L (meq/L). For a 1 millimolar solution of bicarbonate, the alkalinity is 1 meq/L. Since carbonate takes up two protons for each molecule of carbonate, it “counts” twice, and a 1 millimolar solution of carbonate has an alkalinity of 2 meq/L.

Figure 4. Two Trachyphyllia geoffroyi in the refugium of the author’s tank. The pH of the refugium is often higher than the tank due to the photosynthetic processes reducing the CO2, but the alkalinity is largely unchanged as described by the Principle of Conservation of Alkalinity.
A unit that is used by many kits and some industries involves representing alkalinity in terms of the amount of calcium carbonate that would need to be dissolved in fresh water to give the same alkalinity. Typically, it is reported as ppm calcium carbonate. Of course, it has nothing to do with calcium, and there may be no carbonate in the water at all. Nevertheless, it is frequently used. Since calcium carbonate weighs 100 grams/mole (100 mg/mmole), then a solution that has an alkalinity of 100 ppm calcium carbonate equivalent contains 100 mg/L calcium carbonate divided by 100 mg/mmole calcium carbonate = 1 mmol/L calcium carbonate equivalent. Since carbonate has 2 equivalents per mole, this 100 ppm of alkalinity is equivalent to 2 meq/L. So to convert an alkalinity expressed as ppm CaCO3 to meq/L, divide by 50.
Finally there is the German term dKH (degrees of carbonate hardness), or just KH (carbonate hardness).Strictly speaking, it is the same as the carbonate alkalinity (AC in equation 8). Unfortunately, it is a very confusing term, as it has nothing to do with hardness. Further, it has been corrupted by the marine aquarium hobby to mean the same as total alkalinity, and every test kit that tests for dKH with a single titration is giving total alkalinity. The only kit that I am aware of that even makes a distinction between carbonate alkalinity and total alkalinity is one of the Seachem kits (Reef Status: Magnesium, Carbonate, & Borate) and it thankfully doesn’t use the term dKH at all. Consequently, most hobbyists should think of dKH as simply another measure of total alkalinity. The results obtained with such a kit (dKH) can be divided by 2.8 to yield the alkalinity in meq/L.
For those who are mathematically challenged, here is an alkalinity conversion table for all three units.
Conclusion
I hope this article provides a detailed understanding of alkalinity, from what it is and how it is measured, to why it is important in coral reef tanks. I also hope that it serves to clear up some of the confusion about alkalinity and how it is impacted by carbon dioxide and other acids.



0 Comments