For the past few months we have examined the staples of the home fish breeders: the culture of phytoplankton species and the use of rotifers are fish fry’s first foods. Also we discussed the fact that a number of fish fry (such as Gobisomas sp., Centropyge sp. angels, Scorpaenopsis sp. and Dascyllus sp. Chromis sp. damsels) are too small to utilize rotifers as a primary food item and therefore we needed to consider the culture of helper food items to assist in the development of the fish larvae. One such helper organism is ciliates (this species and its home culture is described in detail below). While the culture of ciliates is not traditional for the home breeders they do appear to have useful applications, in so far as ciliates seem to have potential as a larval food or a bridging food item for marine fish and also as a planktonic food for some invertebrates. However, this optimism must be tempered with a dose of reality: rotifers adequately serve the basic needs of most commercial aquaculture programs and therefore serious research into ciliate culture has been neglected. Additionally, a species of ciliate that is suitable as a first food for small marine larvae may or may not be found, but it seems to be worthwhile to look for one. So why would we even consider writing a column on raising a food item which may not be useful to the home breeder? The answer is that in nature, ciliates are a critical (helper) food item. Ciliates are important in the transfer of nutrient material through coastal food webs, as these organisms act as a link between small phytoplankton and larger zooplanktons (Reid et al. 1991). Ciliates graze between 30 – 50 % of primary production in many marine systems and may be the dominant group (up to 100 %) of micro zooplankton in temperate coastal waters (Pierce & Turner 1992).
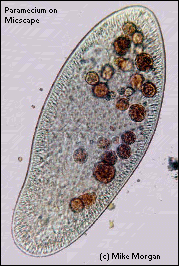
Photomicrographs of ciliates. Close up of a single ciliate (a paramecium) with a number of dark staining food vacuoles (photo courtesy of Mike Morgan http://ebiomedia.com/gall/ciliates/)
The quantitative importance of protozoa as a food source of zooplankton is well established, and studies indicate that qualitative aspects of a protozoan diet may enhance survival and fecundity of some zooplankton species. Hopefully from this description, you can see the value of ciliate culture for the home fish breeder and understand why we dedicated this month’s column to them.
-Introduction by Frank Marini, Ph.D.
The Perfect Food
The perfect food for marine larval fish has the following requirements:
- It is just the right size for capture and ingestion by the larval fish.
- It displays the proper behavior to stimulate larval fish to feed upon it.
- All larval marine fish will avidly feed on this organism.
- It can be cultured with little effort in great numbers in small containers.
- Its reproductive cycle is completed in only a few days so that immense numbers are quickly attained.
- It contains, or can be enriched to contain, all the proper nutrients for strong and healthy larval development.
- The variations in size of the food organism are great enough to adequately feed a wide size range during development of the larval fish.
- It can be maintained with a simple media that does not require extensive algae culture.
The perfect food organism for larval marine fish has not yet been found. At least I don’t know about it if it has. The rotifer, Brachionus plicatilis, is the closest that fish breeders have come to this ideal. It fulfils, for the most part, requirements 1, 2, 4, 5, 6, and to some extent 8, but it isn’t perfect. Many first feeding larval marine fish are too small to take rotifers, the larvae of some species of fish will not feed on rotifers (although they are large enough to take them), and most larval fish outgrow the size range of rotifers before they no longer require a planktonic food organism. Culture of a substantial algal base as well as the vast numbers of food organisms required to feed even modest numbers of marine fish larvae can also be problematic with rotifers, especially for small hobbyist’s hatcheries. Brine shrimp, Artemia, are historically the quintessential food organism for larval fish and invertebrates. For some species, especially freshwater fish, Artemia fulfils all the above requirements, but for many species of marine fish it falls woefully short. Artemia nauplii are notoriously too large for the early larvae of most species of marine fish and the nutritive content of the nauplii are often not compatible with the requirements for normal larval development. For many species, however, rotifers followed by brine shrimp is a feeding protocol that can be made to work with nutritional enrichment and this is currently the paradigm for feeding marine fish larvae.

A photomicrograph of brine shrimp nauplii, about 250 microns long, rotifers, about 125 microns long, and oyster larvae (veliger, bivalve mollusk larvae), about 20 – 30 microns long. The oyster larvae are in the size range of many ciliates. This photo is useful to compare the size of various food organisms suitable for various larval fish.
Obvious to anyone that has reared, or tried to rear, the larvae of a number of species of marine fish, it is highly unlikely that any “perfect” food organism exists. Probably the closest one can come is some species of copepod since copepods have many desirable characteristics, especially wide size range and excellent nutritional qualities. But the long reproductive cycle is a formidable barrier in copepod culture. Because of the slow reproductive cycle, about 25 days, a relatively small culture vessel cannot produce enough copepods to satisfy the demands of very many fish larvae. A single species of copepod may have a size range from about 50 to 70 microns from the early instar to about 700 microns or more in the adult. But even at 50 microns the smallest copepod nauplii may be just a bit too large for some species of marine fish. Although most marine fish larvae select first prey organisms in the 50 to 100 micron range, many marine fish species, pygmy angels, tangs, some wrasses, parrot fish, butterfly fish, some damsels, and others have eggs that are between about 500 and 800 microns, and these small eggs produce small first feeding larvae that seem to require a first food organism in the 20 to 30 micron range, a bit below a copepod instar, quite a bit smaller than a rotifer at 100 microns, and very much smaller than a brine shrimp at 250 microns wide and 400 microns long. There are other issues in rearing these small-egged species of marine fish, but providing an acceptable first food organism, of the proper size and nutrition, and available in acceptable numbers, is the biggest bear in the woods.

A range of sieves suitable for sizing food organisms for larval fish and various invertebrates. The sieves range from 25 microns to about 1000 microns in mesh size. A functional sieve with ample water volume above the mesh for concentrating organisms of the desired size can be made from various plastic containers by cutting off the bottom, cutting out the center of the screw on lid, and then fastening the lid back on the container with the mesh cloth between the lid and the container.
All about Ciliates
Now it is quite possible for any marine aquarist to easily rear a marine organism much smaller than rotifers in incredibly vast numbers. These would be ciliates. There are about 8,000 named species in the Phylum Ciliophora, Kingdom Protoctista, and many more still unknown. The name Ciliophora means “bearing eyelashes” and this is a good description of the tiny, short, whip shaped flagella that cover most species of ciliates. These short, threadlike cilia function in feeding and locomotion. Typically, ciliates feed on bacteria and small algal cells, as well as take up nutrients from the surrounding aquatic environment. Most are free living, a relative few are parasitic or commensal. Most ciliates reproduce by transverse binary fission dividing along the shorter width of the cell, although stalked ciliates that attach to a substrate usually reproduce by budding. Ciliates are among the most complex of the eukaryotic single celled microorganisms. Ciliates have even developed a method for exchange of genetic material called conjugation. Two cells attach together, sometimes for several hours, and exchange micronuclei, which results in two individuals with essentially the same genetic complement. A free living ciliate, rather than a stalked species, has the greater potential as a first food organism for the smallest of marine fish larvae. The hypotrichid ciliate, Euplotes sp. so often found in rotifer cultures measures about 20 by 40 microns, a size that seems to be in the range of many small fish larvae. Dinoflagellates are also a potential food organism. Ciliates are animals and Dinoflagellates are classed as algae, but their rRNA relates these diverse groups. Many dinoflagellates are in the same size range as ciliates and are photosynthetic, but dinoflagellates may be more difficult to culture and some species are quite toxic, which could be a problem.
Both ciliates and dinoflagellates are part of the “microbial loop” in the marine food web. The microbial loop is relatively new concept developed to explain and explore the interactions of the smallest elements of life in the sea, essential minerals, viruses, bacteria, small phytoplankton, etc., that are too small to be consumed by copepods, but are actively consumed by ciliates and flagellates. This cyclic food web at the foundation of the food chain supports the copepods that fuel the classical food chain. The point is that the sea is full of organisms that are below the average size of copepod instars and that these organisms may form a food base for the early larvae of the smallest egged fish. And on an elemental basis, the understanding of this microbial loop in the food web of the sea may have some bearing on the basic functions of marine aquariums, but I digress.
There are many species of ciliates capable of living in the marine environment, both planktonic and benthic, and some, particularly in the genera Tintinnopsis and Euplotes, have potential as food organisms for very small fish larvae and perhaps invertebrates as well. One of the key requirements of any good larval food organism is that it must be capable of rapid reproduction, and must be able to sustain dense cultures in order to supply the quantity of food required to feed large numbers of larvae. Ciliates reproduce by division and so in the proper culture environment, reproduction can be very rapid. Other requirements, however, such as nutritive value and acceptability by larval fish as food organisms, are not as encouraging.

The vegetable juice formula for culturing ciliates and rotifers can be handled much like a rotifer culture based on a phytoplankton food source. Instead of feeding the phytoplankton, the vegetable juice based formula is added periodically. In the photo, the upper jars are phytoplankton cultures and the two lower jars are young, about 3 days old, vegetable juice rotifer culture; and an old, about 2 weeks, vegetable juice culture. These vegetable juice cultures are generally useful for about two weeks.
Whether ciliates would work as an initial food to rear the tiniest of marine fish larva, or various invertebrates, is entirely another story. I have not had success with ciliates as a first food for marine fish larvae and I don’t know of anyone else that has had success with them, but this certainly does not mean that no one has been successful with ciliates or that it is not possible to utilize ciliates as a first food. There are many variables. It may not be possible under relaxed culture conditions to maintain a specific species of ciliate. Contamination from other species may reduce or eliminate the target species in the culture, a species of the proper size or nutritive value may not develop in the culture, and ciliate cultures, just as rotifer cultures, can crash for no apparent reason. And even if the ciliates cultured are of the right size and have adequate nutrition and are actually consumed by the larvae, bacterial and/or fungal contamination of the ciliates may destroy the ciliate culture and/or the larva within a day or two. But if a species of ciliate is found that can serve as a first food organism for small fish larvae, all these difficulties can be resolved.
I don’t think that a current culture of an acceptable ciliate species exists. As far as I know, aquaculture labs do not have a useful species of ciliate (or dinoflagellate) under culture that is shared or researched as is rotifers. To this point, most aquacultured species do not require a food organism smaller than a rotifer and thus not much effort has been expended in finding and developing a smaller version of the rotifer. Marine ornamental breeders may have to find a suitable species of small food organism and develop the culture techniques for this species with little help from the commercial food fish and scientific sectors if the small-egged ornamental fish are to be widely bred. A small organism is needed that will thrive within the nutritional, temperature, and salinity parameters of a captive marine breeding system. So it makes sense to use these parameters as the foundation for efforts to find and maintain such an organisms. For the most part, ciliate culture is very
similar to rotifer culture.
This reminds me of an incident that occurred at the Aqualife Research Corporation facility at Walker’s Cay in the Bahamas some years ago. We were culturing macro algae in some of the 300 gallon fiberglass grow-out tanks and on one morning when I was scheduled to return to Ft. Lauderdale for the weekend, I observed something interesting in one tank that had been scheduled for cleaning and had remained several days with aeration, but no water exchange. It was swarming with a tiny creature, apparently a ciliate, about half the size of a rotifer. The plane was warming up on the runway so all I had time to do was to leave strict instructions that the tank was not to be touched and to leave a sign on the tank, “Do Not Clean.” All weekend I thought of those little creatures and wondered if they could be the “Holy Grail” of small fish larval culture. Of course you know what I found when I returned in a few days. The tank was clean, the sign was still on the tank, and no one knew who cleaned the tank. I never saw that organism again, even though I tried a few times to replicate the situation that had developed that culture.
This is a clue, however, as to how to go about finding a microorganism that might, just might, fill that gap before rotifers or copepods. I ran across another potential piece to this puzzle during the time that I was working with culture of the orchid dottyback (Moe, 1997). I cultured this species, Pseudochromis fridmani, as a hobbyist would, in a small, modified bathroom in a house far from the sea (OK, just 20 miles). I started with the typical phytoplankton cultures for rotifers but early on, as do many hobbyists with limited time and facilities, I had difficulty maintaining the quality and quantity of phytoplankton cultures required to produce the vast numbers of rotifers that hordes of hungry larval dottybacks required. So during that project I developed a formula based on a popular commercial vegetable juice that I used to feed and maintain rotifer populations without, or at least greatly reducing, dependency on phytoplankton cultures. The formula for this vegetable
juice based rotifer food is reproduced below with permission from the publisher of my dottyback book (Barbara).
Preparing the rotifer feeding formula
- Take one 11.5 oz. (340 ml) can of XX juice (I suppose any brand of vegetable juice would be acceptable) and strain it through a 500 micron sieve. Typical window screening is 1000 microns and those little stainless steel strainers you can buy in the supermarket are about 500 microns. This straining removes the larger particles that do not help the culture.
- Dilute the strained juice with about one quart (950 ml) of cold fresh water. It is easier to strain the juice if it is diluted first or during the straining process.
- Add two teaspoons of bakers yeast. The yeast is optional, it is mainly a feeding supplement to the juice particles, but I find that the culture is more stable in that food remains in suspension longer and this helps the rotifers maintain high population levels, and reduces the need for more frequent feedings. The amount, or even the use of yeast is a subject for future experimentation.
- I then add several drops of an omega-3 fatty acid supplement (Super Selco, another type of fish food supplement or even an Omega-3 or fish oil supplement from a health food store) to the juice solution and also add a pre dissolved B vitamin complex tablet and a vitamin C tablet. Put the top tightly on the container and shake very well. It may well be that different supplements or different amounts of these supplements will produce a better rotifer food. Much experimentation remains to be done.
This mixture is then kept in the refrigerator and a portion is fed to the rotifer cultures each day in an amount fitting to the purpose of the culture. I feed about 30 to 50 ml per day to each gallon jar of rotifers to maintain rotifer populations at low levels during periods between breeding projects. High production would require at least two, perhaps three similar feedings each day. Stir the formula well before feeding.”
One of the good news/bad news developments in working with this rotifer formula was that it was a superb media for ciliates, several different species, and several different sizes. One was approximately 10 microns and one was about 30 microns with some in-between and they occasionally flourished in vast numbers. I had to develop methods for screening out the rotifers and beginning new cultures when the rotifers begin to diminish. Allowing the culture to settle, siphoning off the rotifer/ciliate mix above the sediment and then passing the culture through a mesh of 53 microns separated the rotifers and ciliates quite well. (An interesting aside is that some aquaculture interests in Japan use ciliates to enhance the health of rotifer cultures since the ciliates feed on the bacteria in the cultures.)
This gives us a tool to use in the search for a ciliate that may be useful in culture of some marine fish larvae. Other organic preparations, potatoes, straw, fruit juice, algae, etc., could also be used and there may well be a better base, but I would start with the vegetable juice formula above just because it worked well before.
After preparation of the vegetable juice formula, the next step would be to make up several gallon jars of the formula and add light aeration to keep the mixture suspended and oxygenated. Only 30 to 50 ml of the mix is needed in each jar of salt water. Now all we have to do is to find a source for a species of ciliate that may be useful. Some ciliate species may be available from commercial educational cultures, such as Didinium, Paramecium, and Euplotes, and these can be tried, but a better possibility for a marine species may be a natural source. These experimental cultures can be seeded with live sand, live rock, or even water from a natural marine source. A bit of live sand and/or rock from an old established reef tank could also be tried. Experimentation with different salinities, temperatures, and sources of potential ciliates will probably result in a wide variety of cultured ciliates, which can be selected for the larger species. A microscope will be a most useful tool for this work, but a 10x loop might be adequate.
Once a possible candidate species is found, right size, large numbers, one should try to develop a pure culture of that species. Seeding a new culture with a pure sample of only that organism should be attempted. However, without good laboratory technique, this may not be possible. In fact, it may be that ciliate cultures do better when some rotifers are present in the culture. Under primitive conditions, sometimes the best one can do is to start a new culture with as massive an inoculation of the target organism as is possible and hope that the head start given to the desired species will be enough to out grow the competition, at least initially.
Keep the culture rolling gently with an air stone and watch it for a week or so. I’m sure you will get a wild culture of ciliates (who knows what species). Whether they will work as a successful larval food is another story. It is not difficult these days to keep a breeding pair or harem of pigmy angelfish, damselfish, occasionally mandarinfish, maybe a wrasse species or two, and some of the small egged gobies. These species, and others, can provide plenty of larvae for experimental first feeding trials. Add the food organisms at about 3 per ml to the larval tank maybe a day or the night before first feeding is expected. This is about the time the yolk sac on the demersally spawned larva is absorbed and about three days after pelagically spawned larvae hatch. At the time first feeding begins, two things should happen. The larval fish should have a full gut at all times except first thing in the morning, and the larval fish should grow noticeably in two or three days after feeding
begins. Again, a 10X loop or better still, a dissecting microscope is very important. If these two things happen then the fish larvae are able to take the food organism and the food organism is at least nutritionally adequate. It is then time to break out the Champagne.
- Amazing ciliate photomicrographs: http://ebiomedia.com/gall/ciliates/
- Pictures of paramecium: http://www.microscopy-uk.org.uk/mag/wimsmall/cilidr.html
- Nice diagrams of paramecium-this is a free resource: http://www.microscopy-uk.org.uk/mag/articles/param1.html
- Excellent resource on cilates (homepage of ciliate researcher denis Lynn, Department of Zoology, University of Guelph Guelph, ON, CANADA): http://www.uoguelph.ca/%7Eciliates/
- Closeup photomicrographs of ciliates: http://www.microscopy-uk.org.uk/mag/artmar99/marimg99.html, http://www.microscopy-uk.org.uk/mag/artmay99/jevimag.html
- This website is measuring quantities of coastal ciliates: http://www.liv.ac.uk/ciliate/
References
- Moe, M. A., (1997). Breeding the Orchid Dottyback, Pseudochromic fridmani: An aquarist’s journal. Green Turtle Publications, Islmorada FL. 285 pp.
- Pierce RW, Turner JT (1992) Ecology of planktonic ciliates in marine food webs. Reviews in Aquat Sci 6:139-181 Reid PC et al. (1991) Protozoa and their role in marine processes. NATO ASI publication, Springer, New York
- Reid PC (1987) Mass encystment of a planktonic oligotrich ciliate. Mar Biol 95:221-230




0 Comments