Growing corals from sexually produced larvae and planulae is a relatively new approach to farming corals for the aquarium trade. Although coral settlement and juvenile growth has been previously studied (Babcock 1985, Morse et al. 1991, Negri and Heyward 1999, Rinkevich 1979), there are few individuals, myself included, using this approach in an attempt to meet the demands of the aquarium trade. There are several reasons why this technique should be realized, with the conservation of our natural reefs leading the pack by a thousand fold. Colony morphology and genetic diversity within the trade are distant seconds, but important reasons none-the-less. However, as with any type of aquaculture, this form of coral farming is not without it challenges.
Corals have two modes of sexual reproduction (Richmond and Hunter 1990). Corals that release their gametes (egg and sperm packaged together called the egg/sperm bundle) into the water column for external fertilization are called broadcast spawners. Sperm is attached to the positively buoyant eggs and the bundle floats to the surface where it will collide with bundles released from other corals of similar species. This mode requires that all of the corals in the area use specific seasonal cues to ensure that their gametes will mix with gametes from other similar corals. The term ‘mass spawning’ was ascribed to this event as many corals will release their gametes at the same time (Harrison, et. al.). Further research has revealed that different species of corals on the same reef will release their gametes on the same night, but not at the same time. For instance, in Guam, Acropora surculosa will release gametes on the same nights as Acropora humulis. However, each species will release them atspecifically different times (Richmond, Pers. Comm.). This will help increase the chance of colliding with species specific gametes and further minimize the chance of cross-breeding. Fertilization of the eggs by the sperm results in the formation of larvae. Larvae further develop for a few days in the water column before they drift down towards the reef and settle. A brooding coral internally fertilizes the egg and sperm and releases developed planulae into the water. The planulae, like the larvae, will then seek a suitable substrate on which to settle and grow.
In either case, corals are capable of releasing anywhere from hundreds to millions of larvae or planulae per year. Some will release a only few planulae per day, as in the case of Leptoria purporea (Pers. Obs.) while others, like some Pocilloporids, can release thousands of planulae timed to each lunar cycle (Harrigan 1972, Richmond 1984, Rinkevich 1970). In the case of broadcast spawners, one specific night out of the year can trigger the release of millions of gametes into the water column (Richmond and Hunter 1990).
When the larvae and planulae are ready to search for a suitable substrate, they are deemed competent to settle. Corals can use biofilms, crustose coralline algae, or bacteria found on the reef substrate as cues to begin the transformation from the larvae into a benthic coral (Harrigan 1972, Iwao, et. al. 2002, Morse and Morse 1991, Negri, et. al. 2001). This natural process is the path that larvae and planulae would take to survive and grow on the reef. It is not much different than the steps I would have to perform to get the same results.
Collecting the larvae or planulae, consistently and quantitatively settling the coral larvae onto substrates, and growing the juveniles to adulthood represent these steps towards success. My challenge is infinitely harder than the just fulfilling these steps. As a farmer trying to raise enough corals to meet the demands the obstacle that I face is not in ushering some of them along the natural path towards adulthood, rather it’s that I need to usher all of them along this path. To do this successfully, I have to put them on a path that is un-natural and defies the strategy that corals have employed for millions of years.

The ultimate goal: a tray full of corals obtained from sexually produced larvae and planulae. Upper left hand corner: the finished product, Pocillopora damicornis.
r/K selection theory relates, in a general sense, to the two reproductive strategies that species are determined to take based on environmentally selective pressures (Pianka 1970, Heylighen 2000). The terms are familiar to theoretical ecologists who study population growth and derive from the equation:
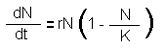
where ‘r‘ is growth rate of a given population (N) and K is the carrying capacity. ‘r‘ selective species or ‘r strategists’ are usually found in highly unstable and often competitive environments (Heylighen 2000). Resources that the individual use is often allocated towards adjusting to and surviving in their environment. Any chance to allocate resources to reproduction must be capitalized on before the conditions change again or external pressures require their immediate attention. r-strategists tend to have short life spans and are small in size. They reproduce quickly and with high fecundity (the number of offspring produced by an organism). As a function of their investment into the creation of many offspring, organisms invest only a minimal amount of resources into each offspring. Mortality rates among the offspring are exceedingly high, with the expectation that only a few will survive to adulthood. They also release them into the environment where they will be dispersed widely throughout their range (such as in the water column or in the air). Finally, no protection or resources from the parents are offered once the offspring are released. Examples of organisms using this strategy are frogs, weeds, clams, oysters, some insects and many fish. ‘K strategists’ have almost opposite considerations when producing offspring. They tend to reside in more stable environments, live longer and grow bigger. They invest highly in only a few offspring, and provide plenty of parental care and resources to the offspring. Since they are well adapted to a more stable environment, resources can be allocated to producing offspring that will also be well equipped to survive in the given environment. Examples are elephants, whales, birds, trees in an old growth forest, and of course, humans. Ultimately, the two strategies can be defined by the investment each organism makes into their perspective offspring. Organisms either invest in producing millions of ill-equipped offspring or a few well-equipped offspring.
Like many organisms, corals as a whole group do not fit neatly into either category however; they do tend to be r strategists. Corals exist in a highly competitive often unpredictable environment and demonstrate many of the
characteristics that are important qualifications for that of r-strategists. Even corals that are thought to be
K-strategists use many of the mechanisms for reproduction that are generally related to r-strategists. For example massive, long lived corals that reproduce once per year, like some Poritiids, release tens of thousands of gametes into the water column and do not equip their offspring with abundant internal resources nor give parental care once the gametes are released.
The selective pressures that drive corals to be r-strategists is further understood when their habitat is taken into consideration. Reasons for coral mortality, both as adults and more so as juveniles, are much more plentiful than reasons for their survival. Algal overgrowth, temperature changes, sedimentation (from storms or terrestrial run-off), diseases, predators and herbivores all contribute to creating a less than stable environment, especially for the offspring (Hunte and Wittenberg 1992, Kuffner, 2004, Sammarco 1980, Tanner 1995, Te 1992). Further, resources available to corals on the reef are at a premium and competing successfully againstother corals for a favorable position on the reef (availability to sunlight, water movement, and food) often requires a large amount of energy (Lang and Chornesky 1990). If resources went into producing only a few, but well developed offspring, it is possible that they would not have the resources to compete successfully with neighboring corals.

Faviid sp. One of the corals that opportunistically settled on my engineered chips. I will do further research into Faviids during the summer spawning seasons.
It is for these reasons that corals spend their reproduction resources on producing as many offspring as possible. It ensures that a small percentage out of the thousands of released larvae will make it past the open ocean predators; and that a small percentage of those who escape predation will find a spot on the reef that is suitable for settlement; and that a small percentage of those that find a spot on the reef will metamorphose quickly enough to grow and defend themselves against the myriad of potential life ending confrontations. Further, the ability to distribute offspring to a wide range of areas will ensure that a few may make it to areas that are temporarily more favorable than the area from which they were released (Richmond 1988). On the natural reef, the ‘r‘ strategy that corals employ is obvious. If we apply a simple mathematical model for exponential increase to Pocillopora damicornis it might look like this: A single colony releases 1000 planulae and half survive (opposing r-strategy and its selective pressures). If, in a few years of growth the survivors (including the original colony) release another 1000 planulae of which half survive, there would be 250,500 new and existing (the single colony who started it all) colonies. A few years later, keeping in proportion with the previous years, there would be 125,500,500 new and existing colonies. This is the math for only one initial colony and one species on the reef. It gets very obvious when we throw 300 different species of corals each with 20 local representatives into the mix. Regardless of the type of selective pressure, it’s the nature of the strategy that corals use that only a few offspring survive to adulthood.
As previously mentioned, my job as a researcher dedicated to this type of farming is to have as many offspring make it to adulthood (or, at least, marketable sizes) as possible. Another way to look at it is that I have to create a supportive habitat for larvae and planulae that were destined to die the moment they were released. I have to further rear them through their steps towards adulthood, which requires an understanding of their nutritional and environmental needs. If I rely on the natural percentages for offspring survival in corals, I may only have a handful of corals reach marketable sizes. This is further complicated by the limited spawning seasons for many of the corals popular in the aquarium trade. Would I be able to compete against other farms that currently fragment corals, meet financial obligations or convince other facilities to subscribe to this type of farming if I have only a dozen corals per annual season? The short answer is: No. At the very least, it is an extremely intimidating task; at most it borders on impossible. The task, however, is not without its positive role models. Another very popular animal that is cultured with great success are the Pacific White Shrimp (Litopenareus vannamei). These shrimp have been successfully grown in hatcheries, mostly for human consumption, for decades. Shrimp are also r-strategists. They produce millions of offspring with the hopes that a few survive to adulthood. The beginning of shrimp culture started out similar to that of the corals. Through exhaustive research over the years, hatcheries have gained the knowledge to be able to rear large percentages of shrimp into adulthood (Victor Camacho, Pers. Comm). I believe this is the next step towards successfully rearing corals from larvae. Research, research, and more research into all aspects of their requirements is the future of this method. At my lab, this is my goal. It’s a lofty goal but with benefits that my baby girl can enjoy in the future; beautiful and natural reefs worthy of her admiration.
When people ask me what the hardest challenge I face in my pursuit of this goal is, I simply state, “oh, it’s nothing. I just have to defy the strategy that corals use for reproduction and rear the animals past their extremely high trend towards fatality”. It’s easy…yeah ‘r‘ight.
Author Information
Lee Goldman currently divides his time between his Master’s work at the University of Guam and his research on growing corals from larvae for the aquarium trade. He will be speaking about his work at the upcoming MACNA XVIII conference in September.
References
- Babcock, R.C.1985. Growth and mortality in juvenile corals (Goniastrea, Platygyra and Acropora): The first year. Proc. Of the Fifth Inter. Coral Reef Congress Vol 4, Tahiti
- Harrigan, J. F.1972. The planulae larva of Pocillopora damicornis, lunar periodicity of swarming and substratum selection behavior. Ph.D. Dissertation, University of Hawaii
- Harrison, P.L., R.C. Babcock, G.D. Bull, J.K. Olive, C.C. Wallace, B.L. Willis. 1984. Mass spawning in tropical reef corals. Science 223: 1187-1189
- Heylighen, F., C. Joslyn and V. Turchin. 2000: r-K selection: the development-reproduction trade-off. Principia Cybernetica Web (Principia Cybernetica, Brussels), URL: http://pespmc1.vub.ac.be/REFERPCP.html.
- Heyward, A.J. and N.P. Negri. 1999. Natural inducers for coral larval metamorphosis. Coral Reefs 18:273-279
- Hunte, W., Wittenberg, M. 1992. Effects of eutrophication and sedimentation on juvenile corals: II Settlement. Mar. Bio. 114: 625-631
- Iwao, K., Fujisawa, T., Hatta, M. 2002. A cniadarian neuropeptide of GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 21: 127-129
- Kuffner, I.B., Paul, V.J. 2004. Effects of the benthic cyanobacterium Lyngbya majuscula on larval recruitment of the reef corals Acropora surculosa and Pocillopora damicornis. Coral Reefs 23: 455-458
- Lang, J.C. and E.A. Chornesky. 1990. Competition between scleractinian reef corals – A review of mechanisms and effects. Ecosystems of the World (Z. Dubinsky, ed.). Coral Reefs 25: 209-252
- Morse, D.E., A.N.C. Morse, P.T. Raimondi, N. Hooker. 1994. Morphogen-based chemical flypaper for Agaricia humulis coral larvae. Biol. Bull. 186: 172-181
- Morse, D.E. and A.N.C. Morse. 1991. Enzymatic characterization of the morphogen recognized by Agaricia humulis larvae. Biol. Bull 181: 104-122
- Negri, A.P., N.S. Webster, R.T. Hill, A.J. Heyward. 2001. Metamorphosis of broadcast spawning corals in response to bactera isolated from crustose algae. Mar. Ecol. Prog. Ser. Vol. 223: 121-131
- Pianka, E.R. 1970. On r and K selection. American Naturalist 104:592-597
- Richmond, R.H. 1988. Competency and dispersal potential of planulae larvae of a spawning versus a brooding coral. 1988. Proc of the 6th Inter’l Coral Reef Symposium. Vol. 2
- Richmond R.H. and C.L. Hunter. 1990. Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific and the Red Sea. Mar. Ecol. Prog. Ser. 60:185-203
- Richmond, R.H. and P.L. Jokiel. 1984. Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bull. Mar. Sci. 34 (2): 280-287
- Rinkevich, B and Y. Loya. 1979. The reproduction of the Red Sea coral Stylophora pistillata. I. Gonads and planulae. Mar. Ecol. Prog. Ser. Vol 1: 133-134
- Rinkevich, B and Y. Loya. 1970. The reproduction of the Red Sea coral Stylophora pistillata. II. Synchronization in breeding and seasonality of planulae shedding. Mar. Ecol. Prog. Ser. Vol 1: 146-152
- Sammarco, P.W. 1980. Diadema and its relationship to coral spat mortality: grazing, competition and biological disturbance. J. exp. Mar. Bio. Ecol. 45: 245-272
- Tanner, J.E. 1995. Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. J. Exp. Mar. Biol. Ecol. 190: 151-168
- Te, F.T. 1992. Response to higher sediment loads by Pocillopora damicornis larvae. Coral Reefs 11: 131-134







0 Comments